Abstract
The presence of the conceptus in uterine cavity necessitates an elaborate network of interactions between the implanting embryo and a receptive endometrial tissue. We believe that embryo-derived signals play an important role in the remodeling and the extension of endometrial receptivity period. Our previous studies provided original evidence that human Chorionic Gonadotropin (hCG) modulates and potentiates endometrial epithelial as well as stromal cell responsiveness to interleukin 1 (IL1), one of the earliest embryonic signals, which may represent a novel pathway by which the embryo favors its own implantation and growth within the maternal endometrial host. The present study was designed to gain a broader understanding of hCG impact on the modulation of endometrial cell receptivity, and in particular, cell responsiveness to IL1 and the acquisition of growth-promoting phenotype capable of receiving, sustaining, and promoting early and crucial steps of embryonic development. Our results showed significant changes in the expression of genes involved in cell proliferation, immune modulation, tissue remodeling, apoptotic and angiogenic processes. This points to a relevant impact of these embryonic signals on the receptivity of the maternal endometrium, its adaptation to the implanting embryo and the creation of an environment that is favorable for the implantation and the growth of this latter within a new and likely hostile host tissue. Interestingly our data further identified a complex interaction between IL1 and hCG, which, despite a synergistic action on several significant endometrial target genes, may encompass a tight control of endogenous IL1 and extends to other IL1 family members.
Introduction
Embryonic implantation and establishment of successful pregnancy require a dynamic process of interactions between the embryo and a receptive maternal endometrium. This embryo/maternal cross-talk involves an elaborate and coordinated network of communication via timely released embryonic and maternal-derived signals and well-targeted actions. Optimal receptivity of the human endometrium to the implantation of a competent blastocyst occurs during a limited period of time within the menstrual cycle called “implantation window”, which is generally believed to span d6–10 following luteinizing hormone (LH) peak in the normal menstrual cycle [1], [2]. Numerous studies showed major and specific changes arising within this specific time interval, which encompass adhesion, invasion, survival, growth, differentiation and immune-modulating factors that shape up endometrial receptivity. The dynamics of this transition from a non-receptive to a receptive endometrium are poorly understood, but the correct spatio-temporal synthesis and balance of various factors is thought to play an important role in human uterine preparation for implantation [3], [4], [5].
Indeed, under the influence of a developing embryo, endocrine factors, particularly ovarian hormones, play a critical role in the regulation of the molecular changes that occur. Embryonic human chorionic gonadotropin (hCG) maintains for instance the production of progesterone by the corpus luteum, which is critical to sustain early pregnancy. However, direct interactions at the fetal-maternal interface and appropriate coordination between embryonic and maternal signals at the implantation site are essential for providing the synergistic environment needed for the establishment of pregnancy [6], [7].
hCG is a major embryonic signal playing a key role in the initiation and maintenance of pregnancy [8]. It is transcribed as early as the 2-cell embryo stage [9] and is produced abundantly by the trophectodermal cells of the pre-implantation blastocyst [10]. Following implantation, hCG is produced by syncytiotrophoblast of the developing conceptus [11]. Recent evidence suggests that hCG is also produced in glandular and luminal epithelium of human endometrium, primarily during the secretory phase [12], [13]. hCG production by embryonic cells may directly regulate the expression of endometrial factors and extend the period during which the endometrium is receptive [2], [14].
hCG acts on the intrauterine environment via the luteinizing hormone (LH)/hCG receptor (hLHCGR), which was detected in various cell types including human uterus and decidua, placenta and fetal membranes [15], [16]. Synthesized early by the trophoblast, hCG may therefore have a wide spectrum of cell targets and biological actions that influence endometrial receptivity and embryo implantation. It promotes human endometrial stromal cell (ESC) decidualization [17] via functional differentiation resulting in an up-regulation of cyclooxygenase 2 (COX2) gene expression and increased production of prostaglandin (PG)E2 [18], possesses both direct and indirect angiogenic properties [19], induces tissue specific human uterine natural killer (uNK) cell proliferation [20] and regulates embryonic autocrine and maternal paracrine factors involved in embryo attachment, endometrial remodeling, antioxidant defense and immune mechanisms around the implanting blastocyst [2], [21], [22].
Several studies provide strong evidence that interleukin (IL1)B may play a pivotal role at the embryo-maternal interface and represents one of the earliest signals [23], [24], [25], [26], [27]. IL1 is synthesized by the human embryo during its initial stages, and the concentration of this cytokine has been positively correlated with successful implantation after in vitro fertilization and transfer to the uterine cavity [28], [29]. A key regulator of the inflammatory response, IL1 is currently recognized as a multifunctional cytokine with a wide spectrum of effects on numerous cell types (eg nervous system cells, immune cells, connective tissue cells, endometrial cells, hepatocyte, fibroblast and endothelial cells) [30], [31]. IL1 acts on human endometrial cells to induce the secretion of leukemia inhibitory factor (LIF) and PGE2 [32], [33], which play an important role in the implantation process [34], up-regulates the expression of integrin β3, a marker of uterine receptivity, in human endometrial epithelial cells [35] and stimulates the migration of human first-trimester villous cytotrophoblast cells via endometrium-derived factors [36]. Purified human cytotrophoblasts in culture release IL1B in the manner that parallels their invasive potential [37]. IL1B stimulates the release of human placental metalloproteinase (MMP)9 [37], proMMP3 expression in baboon stromal cells [38] and hCG by first trimester human trophoblastic cells [39].
The IL1 system is composed of two receptors (IL1R1 and IL1R2), one accessory protein (IL1 RAP) also called IL1R3, one receptor antagonist (IL1RN) and two agonists (IL1A and IL1B), which both trigger cell activation via the functional signaling IL1R1 [40]. IL1R2 rather acts as a negative regulator of IL1 action. Either the membrane-bound (mb) or the soluble (s) form of IL1R2, which is released by proteolysis from the cell surface, acts by capturing IL1, thereby inhibiting IL1-mediated cell activation [41].
Our previous studies pointed to new mechanisms by which the embryo may fine-tune the receptivity of the maternal endometrium, and revealed the ability of hCG to interact with different human endometrial cell types and modulate cell receptivity to IL1. Actually, hCG appeared to down-regulate the expression of the inhibitory IL1R2 in endometrial epithelial cells without affecting that of the activating IL1R1 [42]. Comparable effects were observed in ESCs during the implantation window with, interestingly, a concomitant up-regulation of IL1R1, a down-regulation of IL1RN, an increased angiogenic activity and a higher secretion of monocyte chemotactic protein1 (MCP1) [23]. First identified as a specific factor for macrophage recruitment and activation, MCP1 was later found to be endowed with various immune modulating, proangiogenic and growth-promoting properties [43], [44]. The aim of the present work was to gain a broader understanding of the global impact of hCG on the modulation of human endometrial cell responsiveness to IL1 and the acquisition of growth-promoting phenotype capable of sustaining active embryonic implantation and growth. Using micro-array analysis of hCG, IL1 and hCG/IL1-treated ESCs from the implantation window, our data identified several significantly regulated genes targeted by hCG/IL1 synergy and implicated in angiogenesis, proliferation, tissue remodeling, cell signaling and immune modulation, which is relevant to early embryo implantation process, and a wide spectrum of targets encompassing IL1 family members.
Materials and Methods
Subjects and tissue handling
Endometrial tissue specimens were obtained during the implantation window (days 19 to 24) from normal fertile women with a regular menstrual cycle, who were undergoing laparoscopy for tubal ligation and had not received hormonal or anti-inflammatory therapy for at least 3 months prior to surgery (mean age ± SD, 35.6±4.9 yr.; n = 7). Menstrual cycle day was determined according to the histological criteria of Noyes et al [45]. A written informed consent was obtained from participants under a study protocol approved by the Ethics Committee on Human Research of Laval University, Quebec, Canada. Collection of endometrial tissue biopsies was performed using a Pipelle (Unimar Inc., Prodimed, Neuilly-En-Thelle, France). Tissue samples were kept at 4°C in sterile Hank's balanced salt solution (HBSS) containing 100 U/mL penicillin, 100 µg/mL streptomycin and 0.25 µg/mL amphotericin B (Invitrogen Life Technologies, Burlington, ON, Canada) and immediately transported to the laboratory.
Cell culture and treatment
ESCs were isolated and characterized according to our previously described procedure [46]. Concisely, tissue was minced into small pieces, dissociated with collagenase before ESCs were separated by differential sedimentation and adhesion. The purity of primary ESC cultures was tested morphologically by light microscopy and immunocytochemically on parallel cultures, as previously described. Cultures were free of CD45−positive leukocytes and contamination by factor VIII-positive endothelial cells was generally less than 1%. ESCs were cultured at 37°C in DMEM∶F12 (1∶1) supplemented with 10% fetal bovine serum (FBS), insulin, transferrin, and a mix of antibiotics–antimycotics. Preconfluent cells were washed with HBSS, incubated overnight with charcoal-treated FBS-supplemented medium, washed with phenol red-free DMEM∶F12 (1∶1) and cultured with phenol red- and FBS-free medium containing hCG (100 ng/mL, recombinant protein expressed in a mouse cell line, 10,000 IU/mg; Sigma-Aldrich Co., St. Louis, MO) for 24 h. Cells were then incubated with a fresh phenol red- and FBS-free medium containing IL1B (0.1 ng/mL, R&D Systems, Minneapolis, MN) for additional 24 h. hCG and IL1B concentrations were determined based on our previous studies with human ESCs where different doses were used (Bourdiec A et al, Biol Reprod, 2012). hCG and IL1B concentrations are within the range of the molecules' physiological concentrations [28], [47].
RNA preparation and micro-array analysis
Total RNA of ESC cultures issued from 3 different women was extracted with Trizol according to the manufacturer's directions (Invitrogen). Then they were washed using the micro RNeasy Kit (Quiagen). Total RNA quantity was measured with Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies Inc., DE,USA) and RNA integrity was assessed by capillary electrophoresis using the Bioanalyzer 2100 (Agilent Technologies, Mississauga, Ontario, Canada). DNA micro-array analyses were carried out with Affymetrix Human Gene 1.0 ST at the Gene Expression Platform of the Research Centre of Laval University Hospital Centre, Quebec, Canada. The array interrogates 28,869 well-annotated genes with 764,885 distinct probes. The design of the Human Gene 1.0 ST Array was based on the March 2006 human genome sequence assembly (UCSC Hg18, NCBI build 36) with comprehensive coverage of RefSeq, Ensembl and putative complete CDS GenBank transcripts. Chips were processed according to the Affymetrix standard protocol. Briefly, total RNA (150 ng per sample) was labeled using the Ambion WT Expression kit and Affymetrix GeneChip® WT Terminal Labeling kit, and hybridized to the arrays as described by the manufacturer (Affymetrix, Santa Clara, CA). The cDNA hybridization cocktail was incubated overnight at 45°C while rotating in a hybridization oven. After 17±1 h of hybridization, the cocktail was removed and the arrays were washed and stained in an Affymetrix GeneChip fluidics station 450, according to Affymetrix protocol (http://media.affymetrix.com/support/downloads/manuals/wt_sensetarget_label_manual.pdf). The arrays were scanned using the Affymetrix GCS 3000 7G and the Affymetrix Expression Console Software (Affymetrix, Santa Clara, CA), to produce the intensity files.
Data analysis, background subtraction, and intensity normalization were performed using Robust Multiarray Analysis [48]. Differentially expressed genes and false discovery rate were estimated from t test (P<0.05) and corrected using Bayes approach [49]. Data analysis, hierarchical clustering, and ontology was performed using the OneChanelGUI to extend affylmGUI graphical interface capabilities [50] and Partek Genomics Suite, version 6.5 (Partek Inc., St. Louis, MO) with analysis of variance analysis.
Quantitative real time PCR
RNA was extracted using the standard Trizol® protocol (Invitrogen) as previously reported _ENREF_36[51]. Quantitative real time (qRT)-PCR was performed on the same 3 cultures used for microarray analysis and 4 more different cultures. An ABI 7000 Thermal Cycler (Applied Biosystems, Foster City, CA) was used. Each standard PCR reaction contained 2 µL reverse transcriptase (RT) product, 0.5 µL of primer (final concentration, 0.1 mM), 12.5 µL of SYBR Green PCR Master Mix (Invitrogen) consisting of Taq DNA polymerase reaction buffer, Taq DNA polymerase, SYBR green I, deoxynucleotide triphosphate mix and MgCl2. The reaction melting temperature (Tm) and the list of primers are reported in the Table 1. Primers were designed with Primer Premier 5 software to cross intron-exon boundaries. All samples were tested in duplicate and for each reaction negative controls without RNA and without reverse transcriptase were included.
Table 1. List of PCR primers.
| Gene | Primer | Tm°C |
| CCL2 | F-CTCTGCCGCCCTTCTGT | 60 |
| R-CTTCTTTGGGACACTTGCTG | ||
| CCL5 | F-CTCGCTGTCATCCTCA | 56 |
| R-CACTTGCCACTGGTGTA | ||
| CCL7 | F-GCCTCTGCAGCACTTCTGTG | 60 |
| R-CACTTCTGTGTGGGGTCAGC | ||
| CCL8 | F-CTTCAAGACCAAACGG | 52 |
| R-GAATCCCTGACCCAT | ||
| GAPDH | F- CAGGGCTGCTTTTAACTCTGG | 60 |
| R-TGGGTGGAATCATATTGGAACA | ||
| IL18R1 | F-CTGGAGGAGCTGTTGT | 60 |
| R-GATTAGTCTTCGGCTTT | ||
| IL1A | F-AAGACAGTTCCTCCAT | 52 |
| R-TTGCTACTACCACCAT | ||
| IL1B | F-ACAGTGGCAATGAGGATG | 58 |
| R-TGTAGTGGTGGTCGGAGA | ||
| IL1RL1 | F-CTGAGGACGCAGGTGA | 54 |
| R-CTCCGATTACTGGAAACA | ||
| IL18 | F-GCCAGCCTAGAGGTATG | 60 |
| R-GTTATCAGGAGGATTCATTT | ||
| IL33 | F-CAGGTGACGGTGTTG | 56 |
| R-TGTAGGACTCAGGGTTA | ||
| IL6 | F-GGAGACTTGCCTGGTGAA | 60 |
| R-GCATTTGTGGTTGGGTCA | ||
| KRT19 | F-CGACAATGCCCGTCTG | 58 |
| R-GCCTGTTCCGTCTCAAA | ||
| MMP10 | F-CAAGAGGCATCCATAC | 54 |
| R-AACCTTAGGCTCAACT | ||
| MMP9 | F-TTGACAGCGACAAGAAGTGG | 54 |
| R-CCCTCAGTGAAGCGGTACAT | ||
| PTGS2 | F-TCCCTTGGGTGTCAAAGGTAA | 60 |
| R-AAAACTGATGCGTGAAGTGCTG | ||
| TIMP3 | F-CTCCGACATCGTGATC | 54 |
| R-TCCTTTACCAGCTTCTT | ||
| VCAM1 | F-TGAAGGATGCGGGAGT | 58 |
| R-GCAGGTATTATTAAGGAGG | ||
| VEGFC | F-GCCAGCAACACTACCA | 58 |
| R-TTGAGTCATCTCCAGCAT |
Enzyme-Linked Immunosorbent Assay (ELISA)
CCL2 and CCL5 concentrations in the culture medium were measured using previously reported sandwich ELISAs [52], [53]. VEGFC, TIMP3, MMP9 and prolactin were measured using DuoSet kit (DuoSet, R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Statistical analysis
qRT-PCR and ELISA data followed a parametric distribution and were expressed as means ± SEM. Statistical analyses were performed with GraphPad Software Prism 4.0 (GraphPad Software, Inc., San Diego, CA, USA). The significance of statistical differences was determined using one way analysis of variance (ANOVA) followed by the Bonferroni test post hoc, for multiple comparisons, and the Student's t-test for the comparison of two groups.
Results
Gene expression profile for all experiments
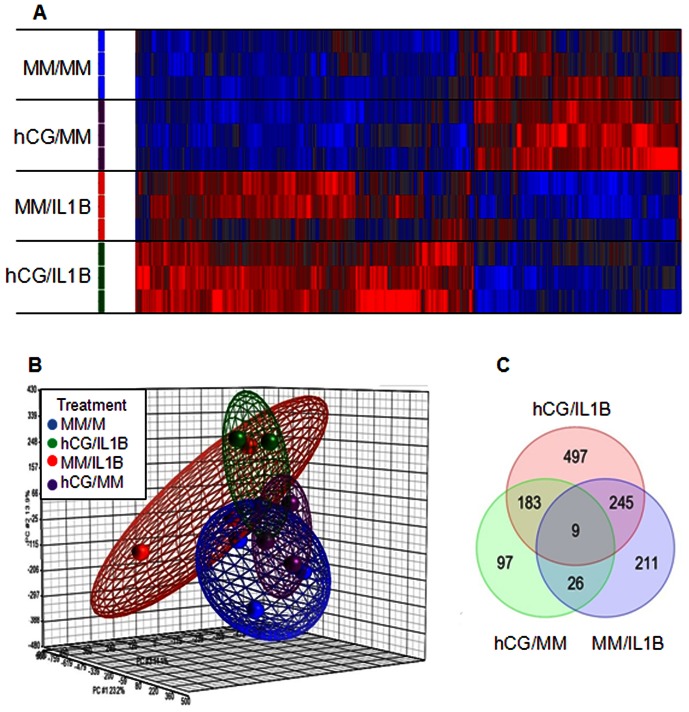
Total RNA was isolated from primary human ESCs treated with hCG (100 ng/mL) for 24 h before being stimulated or not with IL1B (0.1 ng/mL) for additional 24 h. RNA from all treated groups was compared by micro-array with the corresponding non-treated control using Affymetrix GeneChip Human Genome. Using Significance Analysis of Micro-arrays (SAM), all genes significantly regulated between treated versus the non-treated group were selected [fold change (FC) 1.5 and a false discovery rate (FDR) <5%]. The unsupervised hierarchical clustering analysis of the array data showed specific molecular signatures of the global gene expression for each group and a noticeable discrimination between IL1B-treated and IL1B-untreated cells with and without hCG pre-treatment (Figure 1A). Three dimensional Principal Component Analysis (PCA) further showed different patterns of gene expression and a clear segregation between the four groups included in this study [MM/MM (control minimal medium), hCG/MM, MM/IL1B and hCG/IL1B treatments). Also, samples from the same group were very tightly clustered together, which corroborates the robustness of the Affymetrix micro-arrays (Figure 1B).
Figure 1. Analysis of genes significantly modulated by each treatment.
A) Headmap of probe sets corresponding to genes significantly modulated (P<0.05) in each group. Increased signal intensities are displayed in red, whereas lower signal intensities are shown in blue. Cluster distances were evaluated by Spearman correlation on average linkage (Partek Genomics Suite). B) PCA scatter plot of all samples was generated to assess the variability of micro-array data. Each sphere represents a whole chip data. As shown in the legend, samples are colored by treatment and grouped by an ellipsoid that considers two standard deviations from the center of each group. C) Venn diagram of the respective gene lists showing the overlap of action between hCG/MM, MM/IL1B and hCG/IL1B. Data were obtained with ESC cultures issued from 3 different subjects.
Gene expression profile in ESCs is under embryonic stimuli
The different gene lists identified using SAM analysis (FC 1.5 and an FDR <5%) of treatment versus control groups were then intersected to determine their overlap. The results showed that 9 significantly regulated genes were common to all treatments, and 97, 211 and 497 genes were independently regulated by hCG, IL1B and hCG/IL1, respectively (Figure 1C), thereby suggesting highly specific expression profiles.
Genes ontology (GO) of biological processes
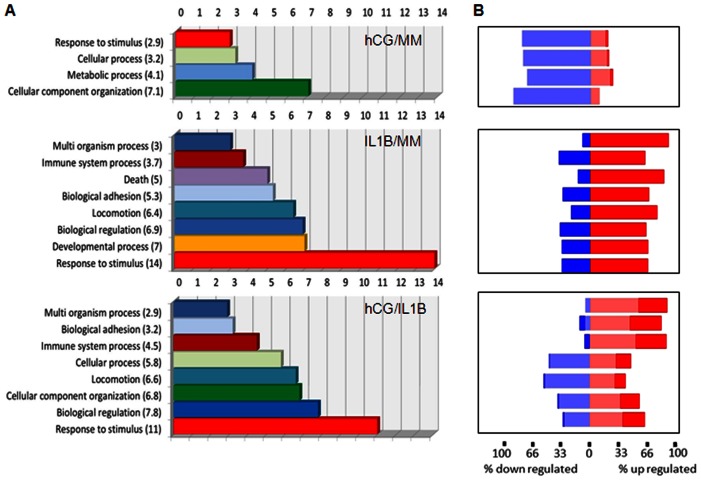
Gene ontology (GO) annotations were then used to explore the specific functional properties of the molecular signatures. A functional enrichment analysis was performed using Partek software (Figure 2). Only significant biological functions were reported. The molecular signature of hCG/MM-treated group was enriched in genes associated with the regulation of cell response to stimulus, cellular and metabolic processes and cellular component organization. Analysis of the IL1B-treated group identified several enriched GO categories that were linked to multi-organism process, immune system process, death, biological adhesion, locomotion, biological regulation, developmental process and response to stimulus, with a marked increase in the latter process. In hCG/IL1 treated group, most enriched GO categories were similar to those found in the IL1B-treated group, but the enrichment scores appeared to be different. The most perceptible increase over hCG and IL1B/hCG was related to cellular, biological regulation and immune system processes, whereas other processes such as biological adhesion and response to stimulus seemed to be lessened to some extent. As observed in the forest plot shown in Figure 2B, IL1B increased the percentage of differentially up- and down- regulated gene populations with a fold change above 2 in each of these biological processes, while co-exposure to hCG led globally to a more moderated regulation. These observations are noteworthy considering the possible involvement of these critical biological processes in the embryo-maternal crosstalk and the establishment of pregnancy.
Figure 2. Enrichment score of biological processes.
A) GO analysis was used to identify the main biological processes targeted by gene lists and significantly modulated by each treatment. Each functional group was assigned with a GO enrichment score that was calculated using a chi2 test. B) A forest plot using the same gene list was also generated to show the percentage of differentially expressed genes that were up-regulated (red) or down-regulated (blue) for each biological process. Light color: gene populations with a fold change ranging from 1.5 to 2. Dark color: gene populations with a fold change above 2. Data were obtained with ESC cultures issued from 3 different subjects.
Identification of differentially expressed genes implicated in early embryo implantation
Tables 2–5 summarize significantly up- and down-regulated genes in ESCs in response to hCG, IL1 and hCG/IL1 compared to untreated control cells. Genes were ranked according to the average of the fold change. To validate changes in the level of RNA transcripts, we have selected some genes found to be significantly regulated upon hCG, IL1B or hCG/IL1B treatment by micro-array and known for being involved in proliferation, immune modulation, tissue remodeling, cell signaling, apoptosis and angiogenesis, which are crucial for early embryo implantation. qRT-PCR was performed for the MCPs [chemokine C-C motif ligand (CCL)2 or MCP1, CCL8 or MCP2 and CCL7 or MCP3], vascular cell adhesion molecule 1(VCAM1), IL6, CCL5 or regulated and normal T cell expressed and secreted (RANTES), PG synthase (PTGS)2 or COX2, vascular endothelial growth factor C (VEGFC), MMP9, tissue inhibitor of metalloproteinase 3 (TIMP3) and keratin 19 (KRT19), and for IL1 family members IL1R-like 1 (IL1RL1) or IL33R, IL18R1, their respective ligands (IL33 and IL18) and both IL1 isoforms A and B.
Table 2. Genes up-regulated after in vitro treatment of human endometrium stromal cells.
| Gene | p-value hCG/IL1 | FC hCG/IL1 | p-value MM/IL1 | FC MM/IL1 | p-value hCG/MM | FC hCG/MM | IPA Pf. | IPA Im. | IPA Rm. | IPA CS. | IPA Ap. | IPA Ag. |
| CXCL6 | 0.00001 | 35.52 | 0.00002 | 21.51 | X | X | ||||||
| IL6á | 0.00088 | 15.06 | 0.00147 | 12.16 | X | X | X | X | X | X | ||
| CXCL1 | 0.00067 | 13.94 | 0.00090 | 12.35 | X | X | X | X | ||||
| TNFAIP3 | 0.00005 | 13.72 | 0.00027 | 8.03 | X | X | X | |||||
| IL1Bá | 0.00076 | 13.13 | 0.00020 | 23.61 | X | X | X | X | X | |||
| CYP7B1 | 0.00000 | 12.96 | 0.00001 | 5.09 | 0.02288 | 1.60 | X | X | ||||
| TNFAIP6 | 0.00013 | 11.79 | 0.00448 | 4.08 | X | |||||||
| PTGS2á | 0.00203 | 10.48 | 0.00368 | 8.33 | X | X | X | X | X | |||
| CCL5áâ | 0.01045 | 9.57 | 0.03962 | 5.30 | X | X | X | X | ||||
| IL8 | 0.00318 | 9.27 | 0.00227 | 10.59 | X | X | X | X | ||||
| C3 | 0.00337 | 8.42 | 0.01599 | 4.83 | X | X | X | X | ||||
| VCAM1á | 0.00099 | 8.08 | X | X | X | |||||||
| ITGB8 | 0.00002 | 8.07 | 0.00079 | 3.39 | X | X | ||||||
| CXCL2 | 0.00077 | 7.25 | 0.00343 | 4.69 | X | X | X | |||||
| TNFAIP2 | 0.00004 | 7.10 | ||||||||||
| CCL2áâ | 0.00249 | 6.74 | 0.01108 | 4.25 | X | X | X | X | ||||
| CTSS | 0.00014 | 6.68 | 0.00064 | 4.57 | X | X | X | |||||
| IRAK3 | 0.00001 | 6.56 | 0.00023 | 3.58 | X | X | X | |||||
| CFB | 0.00235 | 6.14 | X | X | ||||||||
| EPSTI1 | 0.00639 | 5.55 | ||||||||||
| IL24 | 0.00217 | 5.26 | 0.00063 | 7.59 | X | X | X | X | ||||
| IL13RA2 | 0.01786 | 5.17 | 0.03330 | 4.14 | X | |||||||
| MMP12 | 0.01798 | 4.49 | 0.01483 | 4.79 | ||||||||
| NFKBIZ | 0.00003 | 4.14 | 0.00005 | 3.84 | X | |||||||
| CX3CL1 | 0.02049 | 3.95 | X | X | X | X | ||||||
| IL7R | 0.00167 | 3.92 | 0.00162 | 3.95 | X | X | X | X | X | |||
| FGF7 | 0.00367 | 3.87 | 0.02245 | 2.57 | X | X | X | X | ||||
| GNA15 | 0.00250 | 3.85 | X | |||||||||
| CXCL3 | 0.00228 | 3.81 | X | X | X | |||||||
| CSF1 | 0.00613 | 3.60 | X | X | X | X | X | |||||
| CCL8á | 0.00031 | 3.47 | X | X | ||||||||
| ZC3H12A | 0.00003 | 3.23 | 0.00010 | 2.76 | ||||||||
| ICAM1 | 0.00001 | 3.13 | 0.00002 | 2.94 | X | X | X | X | X | |||
| IL32 | 0.00011 | 3.07 | 0.00285 | 1.97 | X | X | X | X | ||||
| ANK2 | 0.00002 | 3.05 | 0.00215 | 1.73 | ||||||||
| CXCL5 | 0.01034 | 3.02 | 0.01015 | 3.04 | X | X | ||||||
| IL15RA | 0.00093 | 2.94 | X | X | X | X | ||||||
| IL1Aá | 0.00012 | 2.88 | 0.00001 | 4.43 | X | X | X | X | X | |||
| PTGES | 0.00004 | 2.84 | X | X | X | X | ||||||
| ITGA8 | 0.01123 | 2.76 | ||||||||||
| IRAK2 | 0.01232 | 2.67 | 0.00250 | 3.75 | X | X | ||||||
| NFKBIA | 0.00003 | 2.60 | 0.00007 | 2.37 | X | X | X | X | ||||
| WNT2 | 0.00299 | 2.54 | 0.00760 | 2.20 | X | X | ||||||
| LIF | 0.00019 | 2.37 | 0.00078 | 2.01 | X | X | X | X | X | |||
| CYP1B1 | 0.01872 | 2.35 | X | X | X | |||||||
| BAMBI | 0.04533 | 2.28 | X | |||||||||
| IL1RL1á | 0.01807 | 2.05 | X | X | X | X | ||||||
| FGF2 | 0.00006 | 1.93 | 0.00006 | 1.93 | X | X | X | |||||
| HLA-DOB | 0.00425 | 1.89 | ||||||||||
| C1R | 0.00242 | 1.83 | ||||||||||
| CCL7á | 0.03055 | 1.80 | X | X |
Pf, proliferation; Im, immune functions; Rm, tissue remodeling; CS, cell signaling; Ap, apoptosis; Ag, angiogenesis
Real-time PCR/ELISA validation performed
Genes up-regulated after in vitro treatment of human endometrium stromal cells
Table 5. Name of down-regulated genes.
| RefSeq | Gene symbol | Genes name |
| NM_004956 | ETV1 | ets variant 1 |
| NM_002153 | HSD17B2 | hydroxysteroid (17-beta) dehydrogenase 2 |
| NM_032211 | LOXL4 | lysyl oxidase-like 4 |
| NM_001786 | CDK1 | cyclin-dependent kinase 1 |
| NM_000210 | ITGA6 | integrin, alpha 6 |
| NM_001946 | DUSP6 | dual specificity phosphatase 6 |
| NM_005415 | SLC20A1 | solute carrier family 20 (phosphate transporter), member 20 |
| NM_002692 | POLE2 | polymerase (DNA directed), epsilon 2 (p59 subunit) |
| NM_001237 | CCNA2 | cyclin A2 |
| NM_005915 | MCM6 | minichromosome maintenance complex component 6 |
| NM_002276 | KRT19 | keratin 19 |
| NM_002915 | RFC3 | replication factor C (activator 1) 3, 38kDa |
| NM_021013 | KRT34 | keratin 34 |
| NM_203394 | E2F7 | E2F transcription factor 7 |
| NR_027676 | BRCA1 | breast cancer 1, early onset |
| NM_001128620 | PAK1 | p21 protein (Cdc42/Rac)-activated kinase 1 |
| NM_018063 | HELLS | helicase, lymphoid-specific |
| NM_139314 | ANGPTL4 | angiopoietin-like 4 |
| NM_000885 | ITGA4 | integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA-4 receptor) |
| NM_001141945 | ACTA2 | actin, alpha 2, smooth muscle, aorta |
| NM_053056 | CCND1 | cyclin D1 |
| NM_005523 | HOXA11 | homeobox A11 |
| NM_002204 | ITGA3 | integrin, alpha 3 (antigen CD49C, alpha 3 subunit of VLA-3 receptor) |
| NM_003505 | FZD1 | frizzled homolog 1 (Drosophila) |
| NM_002689 | POLA2 | polymerase (DNA directed), alpha 2 (70kD subunit) |
| NM_002213 | ITGB5 | integrin, beta 5 |
| NM_001153 | ANXA4 | annexin A4 |
| NM_000362 | TIMP3 | TIMP metallopeptidase inhibitor 3 |
| NM_020396 | BCL2L10 | BCL2-like 10 (apoptosis facilitator) |
| NM_001127217 | SMAD9 | SMAD family member 9 |
| NM_006547 | IGF2BP3 | insulin-like growth factor 2 mRNA binding protein 3 |
| NM_001735 | C5 | complement component 5 |
| NM_003243 | TGFBR3 | transforming growth factor, beta receptor III |
| NM_012098 | ANGPTL2 | angiopoietin-like 2 |
| NM_000043 | FAS | Fas (TNF receptor superfamily, member 6) |
| NM_002291 | LAMB1 | laminin, beta 1 |
| NM_148957 | TNFRSF19 | tumor necrosis factor receptor superfamily, member 19 |
| NM_025208 | PDGFD | platelet derived growth factor D |
| NM_002425 | MMP10 | matrix metallopeptidase 10 (stromelysin 2) |
| NM_000640 | IL13RA2 | interleukin 13 receptor, alpha 2 |
| NM_057749 | CCNE2 | cyclin E2 |
| NM_002658 | PLAU | plasminogen activator, urokinase |
| NM_014791 | MELK | maternal embryonic leucine zipper kinase |
| NM_002203 | ITGA2 | integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) |
| NM_001178010 | CDC45 | cell division cycle 45 homolog (S. cerevisiae) |
| NM_006461 | SPAG5 | sperm associated antigen 5 |
| NM_000059 | BRCA2 | breast cancer 2, early onset |
| NM_024680 | E2F8 | E2F transcription factor 8 |
| NM_002422 | MMP3 | matrix metallopeptidase 3 (stromelysin 1, progelatinase) |
| NM_006739 | MCM5 | minichromosome maintenance complex component 5 |
| NM_002388 | MCM3 | minichromosome maintenance complex component 3 |
| NM_004153 | ORC1 | origin recognition complex, subunit 1 |
| NM_002592 | PCNA | proliferating cell nuclear antigen |
| NM_005225 | E2F1 | E2F transcription factor 1 |
| NM_000575 | IL1A | interleukin 1, alpha |
| NM_005438 | FOSL1 | FOS-like antigen 1 |
Table 3. Name of up-regulated genes.
| RefSeq | Gene symbol | Genes name |
| NM_002993 | CXCL6 | chemokine (C-X-C motif) ligand 6 |
| NM_000600 | IL6 | interleukin 6 (interferon, beta 2) |
| NM_001511 | CXCL1 | chemokine (C-X-C motif) ligand 1 |
| NM_006290 | TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 |
| NM_000576 | IL1B | interleukin 1, beta |
| NM_004820 | CYP7B1 | cytochrome P450, family 7, subfamily B, polypeptide 1 |
| NM_007115 | TNFAIP6 | tumor necrosis factor, alpha-induced protein 6 |
| NM_000963 | PTGS2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| NM_002985 | CCL5 | chemokine (C-C motif) ligand 5 |
| NM_000584 | IL8 | interleukin 8 |
| NM_000064 | C3 | complement component 3 |
| NM_001078 | VCAM1 | vascular cell adhesion molecule 1 |
| NM_002214 | ITGB8 | integrin, beta 8 |
| NM_002089 | CXCL2 | chemokine (C-X-C motif) ligand 2 |
| NM_006291 | TNFAIP2 | tumor necrosis factor, alpha-induced protein 2 |
| NM_002982 | CCL2 | chemokine (C-C motif) ligand 2 |
| NM_004079 | CTSS | cathepsin S |
| NM_007199 | IRAK3 | interleukin-1 receptor-associated kinase 3 |
| NM_001710 | CFB | complement factor B |
| NM_001002264 | EPSTI1 | epithelial stromal interaction 1 (breast) |
| NM_006850 | IL24 | interleukin 24 |
| NM_000640 | IL13RA2 | interleukin 13 receptor, alpha 2 |
| NM_002426 | MMP12 | matrix metallopeptidase 12 (macrophage elastase) |
| NM_031419 | NFKBIZ | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta |
| NM_002996 | CX3CL1 | chemokine (C-X3-C motif) ligand 1 |
| NM_002185 | IL7R | interleukin 7 receptor |
| NM_002009 | FGF7 | fibroblast growth factor 7 |
| NM_002068 | GNA15 | guanine nucleotide binding protein (G protein), alpha 15 |
| NM_002090 | CXCL3 | chemokine (C-X-C motif) ligand 3 |
| NM_000757 | CSF1 | colony stimulating factor 1 (macrophage) |
| NM_005623 | CCL8 | chemokine (C-C motif) ligand 8 |
| NM_025079 | ZC3H12A | zinc finger CCCH-type containing 12A |
| NM_000201 | ICAM1 | intercellular adhesion molecule 1 |
| NM_001012631 | IL32 | interleukin 32 |
| NM_001148 | ANK2 | ankyrin 2, neuronal |
| NM_002994 | CXCL5 | chemokine (C-X-C motif) ligand 5 |
| NM_002189 | IL15RA | interleukin 15 receptor, alpha |
| NM_000575 | IL1A | interleukin 1, alpha |
| NM_004878 | PTGES | prostaglandin E synthase |
| NM_003638 | ITGA8 | integrin, alpha 8 |
| NM_001570 | IRAK2 | interleukin-1 receptor-associated kinase 2 |
| NM_020529 | NFKBIA | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| NM_003391 | WNT2 | wingless-type MMTV integration site family member 2 |
| NM_002309 | LIF | leukemia inhibitory factor |
| NM_000104 | CYP1B1 | cytochrome P450, family 1, subfamily B, polypeptide 1 |
| NM_012342 | BAMBI | BMP and activin membrane-bound inhibitor homolog |
| NM_016232 | IL1RL1 | interleukin 1 receptor-like 1 |
| NM_002006 | FGF2 | fibroblast growth factor 2 (basic) |
| NM_002120 | HLA-DOB | major histocompatibility complex, class II, DO beta |
| NM_001733 | C1R | complement component 1, r subcomponent |
| NM_006273 | CCL7 | chemokine (C-C motif) ligand 7 |
| NM_201442 | C1S | complement component 1, s subcomponent |
| NM_001098479 | HLA-F | major histocompatibility complex, class I, F |
| NM_018724 | IL20 | interleukin 20 |
| NM_032682 | FOXP1 | forkhead box P1 |
| NM_003855 | IL18R1 | interleukin 18 receptor 1 |
| NM_000416 | IFNGR1 | interferon gamma receptor 1 |
| NM_000063 | C2 | complement component 2 |
| NM_003114 | SPAG1 | sperm associated antigen 1 |
| NM_000880 | IL7 | interleukin 7 |
| NM_001066 | TNFRSF1B | tumor necrosis factor receptor superfamily, member 1B |
| NM_018725 | IL17RB | interleukin 17 receptor B |
Name of up-regulated genes.
Our previous studies showed that hCG acts on ESCs, in synergy with IL1B, to stimulate the expression of CCL2 (MCP1), a monocyte/macrophage chemotactic factor with potent angiogenic properties, via the creation of an imbalance between IL1R1 and IL1R2 expression [23]. Micro-array analysis and qRT-PCR validation indicated that not only CCL2, but CCL8 (MCP2) and CCL7 (MCP3) were significantly upregulated by hCG/IL1 as well (P<0.001, P<0.05 and P<0.001, respectively). Furthermore, a significant increase of CCL2, CCL8 and CCL7 mRNA transcripts in cells exposed to hCG/IL1B compared to hCG was noted (P<0.001, P<0.05 and P<0.001, respectively), whereas only CCL2 and CCL7 mRNA transcripts were significantly increased in cells treated with hCG/IL1B compared to IL1B (P<0.05) (Figure 3 A, B and C).
Figure 3. hCG modulates MCPs' mRNA expression in ESCs.
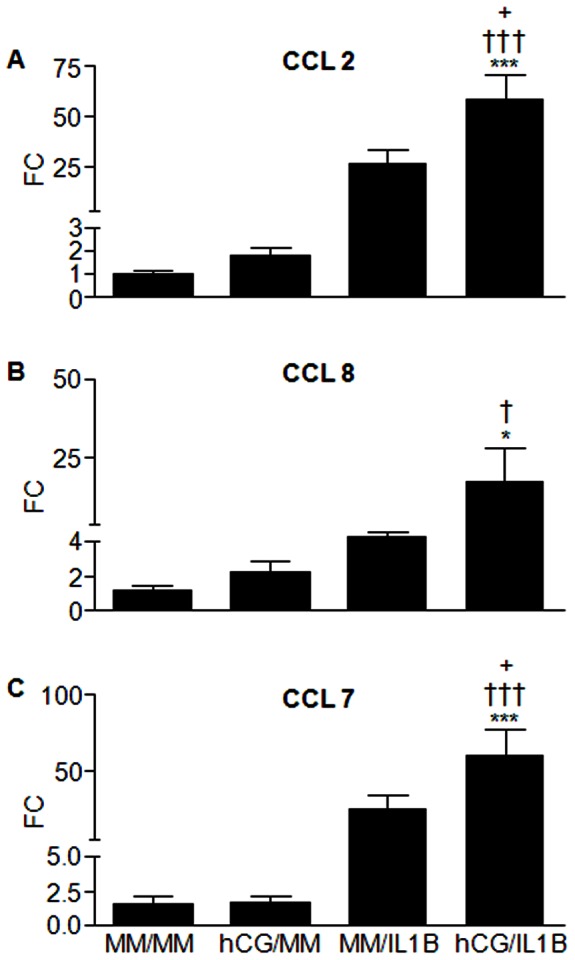
Confluent ESC cultures were incubated with minimal medium (MM) (control) or hCG (100 ng/mL) for 24 h before being exposed or not to IL1B (0.1 ng/mL) for additional 24 h. Total RNA was extracted and reverse transcribed. CCL2, CCL8, CCL7 and GAPDH (internal control) mRNA levels were quantified by real-time PCR. CCL2 (A), CCL8 (B) and CCL7 (C) mRNA ratio was then determined following normalization to GAPDH mRNA. Data were from ESC cultures issued from 7 different subjects and expressed as fold change (FC) over control (ratio of CCL2, CCL8 or CCL7 mRNA levels found in cells incubated with IL1B, hCG or hCG/ILB to those found in cells incubated with MM for an equivalent period of time). *P<0.05, *** P<0.001 relative to MM; †P<0.05, †††P<0.001 relative to cells stimulated with an equivalent concentration of hCG; +P<0.05 relative to cells stimulated with an equivalent concentration of IL1B. Data were obtained with ESC cultures issued from 7 different subjects (the 3 cultures used for microarray analysis and 4 additional cultures).
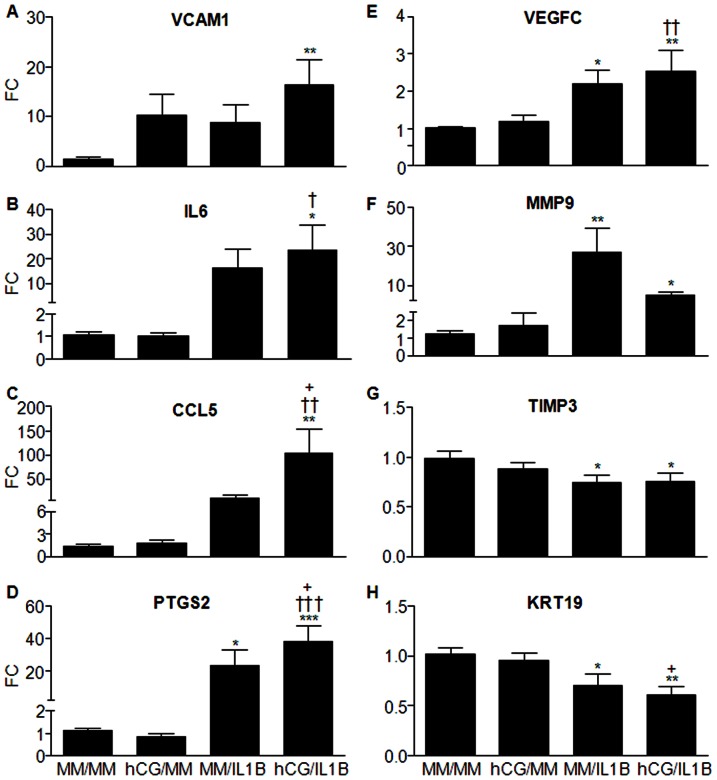
Many other cytokines and growth factors known for being involved in the regulation of immune responses, adhesion, cell proliferation and angiogenesis were also found to be targeted by hCG and IL1B synergistic action. Vascular cell adhesion protein 1, which mediates leukocyte adhesion to vascular endothelium, was significantly up-regulated by hCG/IL1B compared to the control minimal medium (MM) (P<0.01), but neither hCG nor IL1B had a statistically significant stimulatory effect (Figure 4A). IL6 and CCL5 mRNA levels were significantly increased by hCG/IL1B compared to MM (P<0.05 and P<0.01, respectively) or to hCG (P<0.05 and P<0.01, respectively). CCL5 levels were also significantly increased in cells treated with hCG/IL1B compared to IL1B (P<0.05) (Figure 4B, C).
Figure 4. hCG modulates the IL1B-mediated mRNA expression of immune modulating, adhesion, growth, angiogenic and tissue remodeling factors in ESCs.
Confluent ESC cultures were incubated with minimal medium (MM) (control) or hCG (100 ng/mL) for 24 h before being exposed or not to IL1B (0.1 ng/mL) for additional 24 h. Total RNA was extracted and reverse transcribed, and mRNA levels were then quantified by qRT-PCR. VCAM1 (A), IL6 (B), CCL5 (C), PTGS2 (D), VEGFC (E), MMP9 (F), TIMP3 (G) and KRT19 (H) mRNA ratio was then determined following normalization to GAPDH mRNA (internal control). Data were from ESC cultures issued from 7 different subjects and expressed as fold change (FC) over control (ratio of VCAM1, IL6, CCL5, PTGS2, VEGFC, MMP9, TIMP3 or KRT19 mRNA levels found in cells incubated with IL1B, hCG or hCG/ILB to those found in cells incubated with MM for an equivalent period of time). *P<0.05, **P<0.01, *** P<0.001 relative to MM; †P<0.05, ††P<0.01, †††P<0.001 relative to cells stimulated with an equivalent concentration of hCG; +P<0.05 relative to cells stimulated with an equivalent concentration of IL1B. Data were obtained with ESC cultures issued from 7 different subjects (the 3 cultures used for microarray analysis and 4 additional cultures).
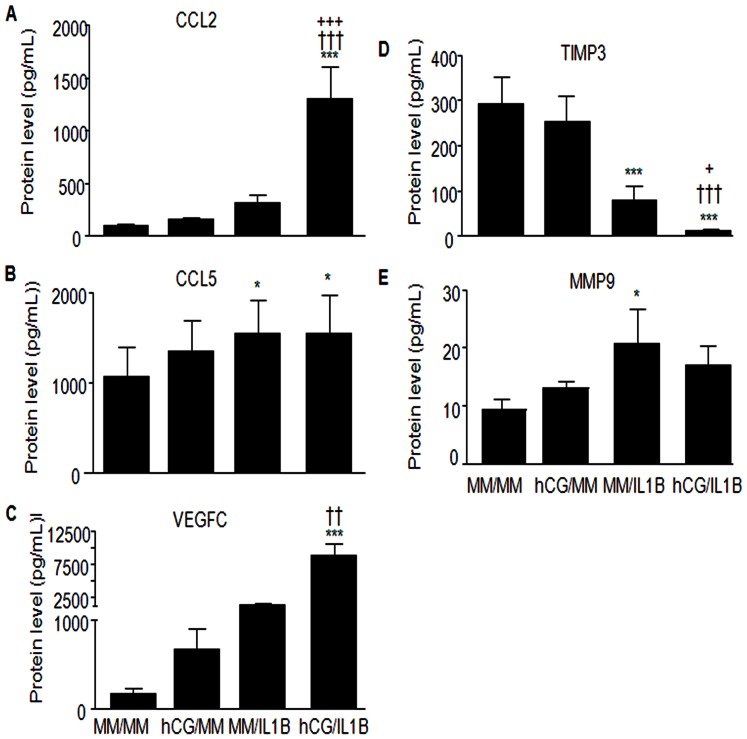
PTGS2, a major rate-limiting enzyme involved in PG synthesis, and VEGFC, an isoform of a potent angiogenic factor, also showed an increased mRNA expression in cells treated with hCG/IL1B compared to cells incubated with MM (P<0.001 and P<0.01, respectively) or with hCG alone (P<0.001 and P<0.01, respectively). IL1B stimulated PTGS2 and VEGFC mRNA synthesis as well (P<0.05), but cell exposure to hCG significantly stimulated in the IL1B-induced PTGS2 expression (P<0.05) (Figure 4D, E).
Also found to be regulated were some molecules shown to participate in endometrial tissue remodeling such as MMP9, TIMP3 and KRT19 (Figure4F, G, H). MMP9 was significantly up-regulated by IL1B and hCG/IL1B (P<0.01 and P<0.05, respectively). By itself, hCG had no statistically significant effect on MMP9 expression, but it moderated the IL1B-induced effect. TIMP3, a natural tissue inhibitor of MMPs, was down-regulated by IL1B either in the presence or the absence of hCG (P<0.05). Cytokeratin 19 (KRT19), an intermediate filament protein associated with embryonic placenta development [54], was found to be significantly inhibited by IL1B (P<0.05) and more by IL1B combined with hCG (P<0.01).
hCG modulates IL1B effects on the expression of IL1 family members in ESCs
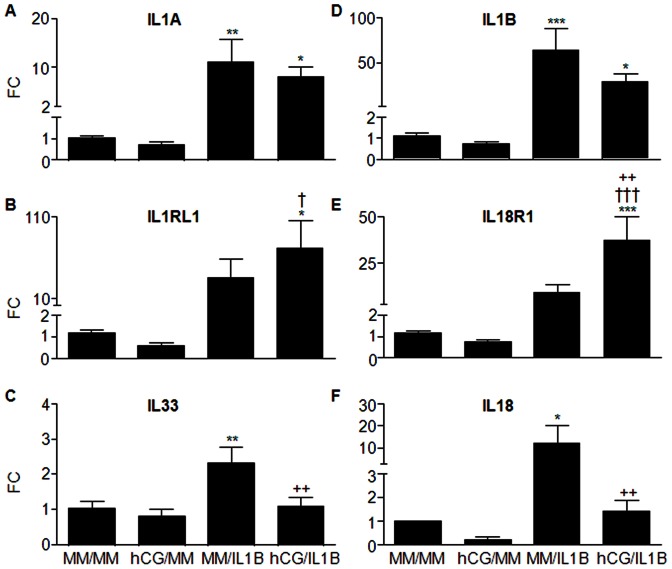
Our previous studies showed that hCG down-regulates the IL1B-induced increase of IL1R2 and IL1RN in ESCs and amplifies the IL1B-induced increase of IL1R1 [23]. Our current micro-array analysis further revealed that hCG/IL1B interaction may affect ESC responsiveness to other components of IL1 family including IL1 isoforms A and B as well as IL1RL1 and IL18R1 (Tables 2 and 4). To validate these findings, qRT-PCR analysis was performed. Our results showed that IL1B induced both ILA and IL1B in ESCs (P<0.01 and P<0.001, respectively), but hCG moderated that endogenous IL1B-mediated expression (Figure 5 A, D). However, both IL1 isoforms remained significantly up-regulated compared to the control medium (P<0.05) despite the down-regulatory effect of hCG. Furthermore, the micro-array data predicted a synergistic interaction between hCG and IL1B, resulting in the induction of IL18R1 and IL1R1L expression (Figure 5 B, E). This was confirmed by qRT-PCR analysis, which showed a significant up-regulation of these two receptors as compared to control (P<0.05 and P<0.001, respectively) and to hCG (P<0.05 and P<0,001, respectively). However, despite a noticeable increase of IL1RL1 and IL1R18 in hCG/IL1B-treated compared to IL1B-treated cells, this increase was statistically significant only for IL18R1 (P<0.01), but did not reach statistical significance for IL1RL1 with these small groups.
Table 4. Genes down-regulated after in vitro treatment of human endometrium stromal cells.
| Gene | p-value hCG/IL1 | FC hCG/IL1 | p-value MM/IL1 | FC MM/IL1 | p-value hCG/MM | FC hCG/MM | IPA Pf. | IPA Im. | IPA Rm. | IPA CS. | IPA Ap. | IPA Ag. |
| ETV1 | 0.02017 | −5.26 | ||||||||||
| HSD17B2 | 0.03976 | −5.06 | ||||||||||
| LOXL4 | 0.00528 | −4.74 | 0.00435 | −5.01 | ||||||||
| CDK1 | 0.03192 | −4.25 | X | X | ||||||||
| ITGA6 | 0.00651 | −3.82 | 0.01105 | −3.35 | X | X | X | |||||
| DUSP6 | 0.00051 | −3.56 | 0.01223 | −2.08 | X | X | ||||||
| SLC20A1 | 0.00306 | −3.08 | X | |||||||||
| POLE2 | 0.00645 | −2.86 | 0.01939 | −2.31 | ||||||||
| CCNA2 | 0.02166 | −2.66 | X | X | X | |||||||
| MCM6 | 0.00275 | −2.54 | ||||||||||
| KRT19á | 0.00000 | −2.50 | 0.00001 | −2.26 | ||||||||
| RFC3 | 0.00785 | −2.49 | ||||||||||
| KRT34 | 0.00247 | −2.45 | ||||||||||
| E2F7 | 0.04905 | −2.38 | X | |||||||||
| BRCA1 | 0.01466 | −2.25 | 0.02641 | −2.04 | X | X | ||||||
| PAK1 | 0.00399 | −2.24 | 0.02263 | −1.77 | X | X | X | X | ||||
| HELLS | 0.04234 | −2.22 | X | |||||||||
| ANGPTL4 | 0.01955 | −2.09 | X | X | ||||||||
| ITGA4 | 0.00435 | −2.03 | 0.00371 | −2.08 | X | X | X | X | ||||
| ACTA2 | 0.01071 | −1.91 | 0.02904 | −1.68 | X | |||||||
| CCND1 | 0.00041 | −1.90 | X | X | X | |||||||
| HOXA11 | 0.00279 | −1.88 | 0.00047 | −2.31 | ||||||||
| ITGA3 | 0.00146 | −1.69 | X | X | X | |||||||
| FZD1 | 0.00697 | −1.58 | 0.00048 | −2.06 | X | |||||||
| POLA2 | 0.02091 | −1.51 | 0.00795 | −1.66 | ||||||||
| ITGB5 | 0.03518 | −1.53 | X | X | X | X | ||||||
| ANXA4 | 0.02022 | −1.53 | X | |||||||||
| TIMP3áâ | 0.01839 | −1.57 | X | X | X | X | ||||||
| BCL2L10 | 0.00814 | −1.58 | X | |||||||||
| SMAD9 | 0.01991 | −1.61 | ||||||||||
| IGF2BP3 | 0.02094 | −1.62 | X | |||||||||
| C5 | 0.01257 | −1.68 | X | X | X | X | X | |||||
| TGFBR3 | 0.04598 | −1.82 | X | X | X | X | ||||||
| ANGPTL2 | 0.02817 | −1.84 | ||||||||||
| FAS | 0.03925 | −1.85 | X | X | X | X | X | |||||
| LAMB1 | 0.03736 | −1.91 | X | X | ||||||||
| TNFRSF19 | 0.02307 | −2.26 | X | |||||||||
| PDGFD | 0.01484 | −3.19 | X | X | ||||||||
| MMP10á | 0.01030 | −3.91 | X | |||||||||
| IL13RA2 | 0.04783 | −3.64 | X | |||||||||
| CCNE2 | 0.01264 | −2.78 | X | X | ||||||||
| PLAU | 0.00713 | −2.42 | X | X | X | X | ||||||
| MELK | 0.01473 | −2.41 | ||||||||||
| ITGA2 | 0.01329 | −2.37 | X | X | X | X | ||||||
| CDC45 | 0.01108 | −2.30 | X | X | ||||||||
| SPAG5 | 0.03517 | −2.16 | ? | |||||||||
| BRCA2 | 0.03864 | −1.96 | X | X | X | |||||||
| E2F8 | 0.02061 | −1.85 | X | |||||||||
| MMP3 | 0.02542 | −1.84 | X | X | X | |||||||
| MCM5 | 0.01884 | −1.79 | ||||||||||
| MCM3 | 0.02412 | −1.62 | ||||||||||
| ORC1 | 0.00293 | −1.60 | ||||||||||
| PCNA | 0.02189 | −1.59 | X | X | ||||||||
| E2F1 | 0.00984 | −1.55 | X | X | X | X | ||||||
| IL1Aá | 0.02429 | −1.52 | X | X | X | X | X | X | ||||
| FOSL1 | 0.01092 | −1.50 | X | X | X |
Real-time PCR/ ELISA validation performed
Pf, proliferation; Im, immune functions; Rm, tissue remodeling; CS, cell signaling; Ap, apoptosis; Ag, angiogenesis
Figure 5. hCG modulates IL1B effects on the expression of IL1 family members in ESCs.
Confluent ESC cultures were incubated with minimal medium (MM) or hCG (100 ng/mL) for 24 h before being exposed or not to IL1B (0.1 ng/mL) for additional 24 h. Total RNA was extracted and reverse transcribed, and mRNA levels were then quantified by qRT-PCR. IL1A (A), IL1B (D), IL1RL1 (B), IL18R1 (E), IL33 (C) and IL18 (F) mRNA ratio was then determined following normalization to GAPDH mRNA (internal control). Data were from ESC cultures issued from 7 different subjects and expressed as fold change (FC) over control (ratio of IL1A, IL1B, IL1RL1, IL18R1, IL33 or IL18 mRNA levels found in cells incubated with IL1B, hCG or hCG/ILB to those found in cells incubated with MM for an equivalent period of time). *P<0.05, **P<0.01, *** P<0.001 relative to MM; †P<0.05, †††P<0.001 relative to cells stimulated with an equivalent concentration of hCG; ++P<0.01 relative to cells stimulated with an equivalent concentration of IL1B. Data were obtained with ESC cultures issued from 7 different subjects (the 3 cultures used for microarray analysis and 4 additional cultures).
Because IL18R1 and IL1RL1 were targeted by hCG/IL1B, we have then investigated their ligands (IL18, IL33) by qRT-PCR. Our data showed that like IL1A and IL1B, both IL33 and IL18 were up-regulated in cells treated with IL1B (P<0.01 and P<0.05, respectively), but they were down-regulated in cells treated with hCG and IL1B compared to IL1B alone (P<0.01) (Figure 5 C, F).
Validation of selected soluble proteins
To confirm the gene expression changes at the protein level, we have selected some genes found to be significantly regulated upon hCG, IL1B or hCG/IL1B treatment and known for being involved in immune modulation, tissue remodeling and angiogenesis. Results indicated that CCL2 was significantly upregulated by hCG/IL1B (P<0.001), which corroborates the microarray data (Figure 6A). CCL5 secretion was upregulated either by IL1B or hCG/IL1B (P<0.05), but hCG/IL1B synergism was not perceptible at the protein level (Figure 6 B). hCG/IL1B treatment further appeared to upregulate VEGFC (P<0.001) and downregulate TIMP3 secretion (P<0.001) (Figure 6 C, D). MMP9 was significantly up-regulated by IL1B (P<0.05), but hCG/IL1 did not show a statistically significant stimulatory effect (Figure 6E).
Figure 6. hCG modulates the expression of immune, angiogenic and tissue remodeling factors in ESCs at the protein level.
Confluent ESC cultures were incubated with minimal medium (MM) or hCG (100 ng/mL) for 24 h before being exposed or not to IL1B (0.1 ng/mL) for additional 24 h. Supernatant was collected and soluble proteins CCL2 (A) CCL5 (B) VEGFC (C) TIMP3 (D) MMP9 (E) were then quantified by ELISA. Data were from ESC cultures issued from 4 different subjects and expressed in pg/mL. *P<0.05, *** P<0.001 relative to MM; ††P<0.01, †††P<0.001 relative to cells stimulated with an equivalent concentration of hCG; +P<0.05, +++P<0.001 relative to cells stimulated with an equivalent concentration of IL1B.
Discussion
Cumulating evidences point to an important role for embryo-endometrial dialogue in mediating the adhesion, invasion and growth of the embryo during implantation and early development. Well-known for rescuing the corpus luteum and maintaining the production of progesterone, embryo-derived signals such as hCG seem to orchestrate endometrial adaptation to the implantation of the newly formed embryo as well [22], but little is known about the involved pathways and the underlying mechanisms. Human CG is quite known, for instance, for stimulating cytokine/chemokine production by endometrial epithelial and stromal cells and playing direct and indirect roles in human ESC decidualization [17] and angiogenesis in human endothelial cells [19], [23], [55]. It is unclear if hCG is involved in blastocyst attachment in humans. However, it may have an indirect role, as suggested by our and other studies, and act via the modulation of human epithelial cell receptivity/responsiveness to other major embryonic signals such as IL1 [56]. A recent study demonstrated that the expression of β3-integrin-subunit, a cell adhesion mediator and marker of uterine receptivity [57], on the surface of human endometrial epithelial cells could be up-regulated by coculture with a human preimplantation embryo and blocked by anti-IL1 antibody [35]. Another study showed that the expression of trophinin, which mediates cell adhesion by homophilic binding, and the ability for apical cell adhesion with trophinin-expressing human trophoblastic cells are increased in presence of hCG associated with IL1β [58].
The presence of LHCG receptor in various endometrial cell types [15], [16] makes plausible that hCG has a broad spectrum of endometrial cell targets. After the intrusion of the embryo through the luminal endometrial epithelium, trophoblastic cells are in close contact with different maternal stromal cell types [59]. To achieve a successful pregnancy, an appropriate cross-talk between embryonic and maternal cells must therefore take place, where numerous embryo- as well as maternal-derived factors including steroid hormones, matrix degrading enzymes, integrins, cytokines, chemokines and growth factors could be involved [60], [61].
Our previous studies revealed a new mechanism by which hCG can target different human endometrial cell types, including epithelial and stromal cells, to modulate their receptivity to IL1, an early potent embryonic signal, and amplify thereby the release of immune and angiogenic factors [23], [42]. In the present study, we further showed that hCG acts on ESCs, either alone or via the modulation of the IL1-mediated cell responsiveness, to regulate numerous relevant genes involved in cell signaling, proliferation, apoptosis, immune modulation, tissue remodeling and angiogenesis, which are highly relevant mechanisms underlying the implantation process and the modulation of the immune response around the implanting embryo. Some of the genes were known for playing important roles in the various embryo implantation stages, but many genes were not known for being possibly involved and regulated by hCG or hCG/IL1B synergism.
MCPs were among the most significantly induced immune factors in ESCs in response to hCG and IL1B. hCG amplified the IL1B-induced expression of MCP1, 2 and 3. These chemokines are involved in the recruitment of monocytes/macrophages, T cells and NK cells into inflammatory sites [62], [63]. Moreover, MCPs stimulate angiogenesis, either directly via MCP1-induced protein (MCPIP) or indirectly via their activation of immune cells such as NK cells and macrophages, which are known for releasing growth and angiogenic factors [64]. Interestingly, the current micro-array data are in keeping with our previous findings of an increased IL1B-mediated secretion of MCP1 in human ESCs from the implantation window following hCG treatment [23] and consistent with a possible role for MCPs in embryo implantation. Actually, macrophages contribute to decidualization and implantation and remain abundant at the implantation site throughout pregnancy [65], [66], and trophoblastic cells were shown to regulate human monocyte migration and differentiation [67]. However, uterine macrophages do not appear to impair the growth of the semi-allogeneic embryo. They rather seem to play a protective role against possible infections, maintain immune tolerance toward trophoblastic antigens, mediate trophoblast invasion and support embryonic growth [68]. This strengthens the relevance of our findings and broadens the spectrum of hCG's impact on early embryonic growth and development.
VCAM1, an adhesion molecule of endothelial cells playing an important role in immune cell trafficking [69], appeared to be up-regulated by hCG or IL1B, but significantly by hCG and IL1B in ESCs. During pregnancy, the few available reports suggest a possible role for VCAM1. The expression of this adhesion molecule is strongly induced in the endothelium of early pregnant sheep endometrium [70] and decreases in fetal membranes with advancing gestational age [71]. However, its role in human pregnancy and during embryo implantation remains to be elucidated.
Many other cytokines including IL6, CCL5 (RANTES) and VEGFC appeared to be targeted by hCG and IL1B synergistic action. These pluripotent factors are quite known for being involved in the regulation of immune response, cell proliferation, tissue remodeling and angiogenesis. First identified as a promoter of B-cell differentiation and antibody production, IL6 is nowadays known as a pleiotropic cytokine that regulates cell growth, angiogenesis, inflammation and hematopoiesis [72]. IL6 expression was described in human granulosa and theca cells, endometrium and pre-implantation embryo [73]. Also, habitual abortion in women is associated with a decrease in expression of IL1B and IL6 [74], suggesting a role for these cytokines in the maintenance of pregnancy. A growing body of evidence implicates CCL5 in the induction of tolerance at immune-privileged sites. This cytokine seems to suppress maternal allogeneic responses, which is necessary for successful implantation [75], [76], [77], [78]. VEGFC is primarily a potent angiogenic growth factor and may play an important role in embryonic cell growth. However, it was described recently as an immune modulator that induces immune tolerance in murine tumor cells [79]. Therefore, these hCG/IL1B-induced biological properties in endometrial cells may represent a relevant mechanism involved in the immune tolerance of the implanting embryo within the uterine maternal host. This is in keeping with a previous study reporting that in vivo infusion of IL1B and hCG induces endometrial changes that mimic early pregnancy events in the baboon and lead to the development of an immunotolerant environment [80].
Invasion of the trophoblast into the endometrium requires a delicate balance between tissue degradation and maintenance. Up-regulation of MMP9 expression in endometrial cells by IL1B in human endometrial cells [81] and secretion by cultured first trimester human trophoblastic cells and fibroblasts has been demonstrated [82]. In addition, the expression of MMPs correlated with the invasive potential of human trophoblast cells [82]. Our micro-array data and qRT-PCR validation revealed the regulation of several tissue remodeling mediators such as MMP9, TIMP3 and KRT19. MMP9 was significantly up-regulated by IL1B in ESCs and hCG seemed to moderate this action, which, however, remained significant compared to non-stimulated cells. TIMP3, a natural tissue inhibitor of MMPs [83] was down-regulated by IL1B either in the presence or the absence of hCG. The reduction of TIMP3 expression levels, combined with the increased expression of MMP9, may create an imbalance that favors tissue matrix proteolysis and embryo implantation. However, the recent literature reporting that TIMP3 induces apoptosis, inhibits angiogenesis and impedes cell migration [84] makes highly relevant our present findings, considering the crucial importance of embryonic cell survival, proliferation and migration for the establishment of early pregnancy. Interestingly, KRT19, a molecule associated with embryonic placenta development [54] was found to be significantly inhibited by IL1B and more by IL1B combined with hCG. KRT19 is an intermediate filament protein and an epigenetically regulated tumor suppressor gene down-regulated in several cancerous tumors [85]. Also, down-regulation of KRT19 in human oral squamous cell carcinoma lines increases the invasive potential [86], but no other previous studies showed any eventual relationship with the invasive capacity of embryonic cells.
Micro-array and qRT-PCR validation data further revealed a synergistic interaction between hCG and IL1B to induce PTGS2, which is a rate limiting enzyme for PG synthesis. This is quite relevant considering the well-documented role of PGs as key regulators of female reproductive tract functions, including ovulation, menstruation and myometrial contractility, and vascular permeability and angiogenesis at the implantation site [87], [88], [89].
Interestingly, validation of the expression of some major selected genes at the protein level corroborates the combined role of hCG and IL1B in the modulation of angiogenic, immune and tissue remodeling functions of ESCs. Actually, assessment of protein secretion showed that hCG and IL1B synergistically induced CCL2 and VEGFC, inhibited TIMP3 and moderate the IL1B-induced MMP9.
Our previous studies showed that hCG down-regulates the IL1B-induced increase in IL1R2 and IL1RN in human ESCs and further enhances the IL1B-induced increase in IL1R1, thereby amplifying in vitro the release of angiogenic activity [23]. Surprisingly, the results of the current micro-array analysis showed a broader spectrum of regulation encompassing other IL1 family members, and suggest a possible modulation of endometrial cell responsiveness to IL1 family. In fact, hCG appeared to potentiate the IL1B-induced expression of IL1R1L (IL33R) and IL18R and to moderate, on the other hand, the expression of endogenous IL33, IL18, IL1A and IL1B in ESCs. Nonetheless, the expression of IL1 isoforms was still up-regulated despite the down-regulatory effect of hCG. These results indicate a possible mechanism by which hCG may induce immunotropism and prevent undue local expression of proinflammatory cytokines, as excessive production levels may be associated with repeated miscarriage and fetal growth retardation [90].
It is noteworthy that according to the recent literature, hCG has been shown to be produced by human endometrial epithelial cells in the luteal phase [12]. Indeed, endogenous endometrial hCG may, though produced at low quantities, have a role in in embryo implantation and the hCG-mediated growth promoting effects, but this is still to be demonstrated.
In conclusion, our study showed that hCG induces major changes in human ESC phenotype and deeply modulates their responsiveness to a proinflammatory, but a growth mediator and a potent embryonic signal such as IL1B. Generally via synergistic stimulatory or inhibitory mechanisms, hCG induces significant alterations in the expression of genes known for being involved or having the potential to play an important role in embryonic implantation and growth and the modulation of the immune response around the implanting blastocyst. Furthermore, our study revealed that the modulation of endometrial cell receptivity via hCG is not limited to IL1 receptors' agonists and antagonists, but also extends to other IL1 family members, which share numerous growth-promoting, immune-modulating and signaling pathways. This, together with our previous data showing that hCG can similarly target different endometrial cell types, strengthens the relevance of such a modulatory mechanism for implantation and early embryonic growth within the host maternal endometrial tissue and further suggests that hCG plays an important role in the establishment of a receptive endometrial phenotype.
Acknowledgments
We thank Dr M. Al-Akoum and Nathalie Bourcier for technical assistance in statistical analyses.
Funding Statement
Supported by NSERC Discovery Grant to AA, Chercheur National, Fonds de la Recherche en Santé du Québec. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wilcox AJ, Baird DD, Weinberg CR (1999) Time of implantation of the conceptus and loss of pregnancy. The New England journal of medicine 340: 1796–1799. [DOI] [PubMed] [Google Scholar]
- 2. Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, et al. (2009) Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23: 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, et al. (2000) Embryo implantation. Dev Biol 223: 217–237. [DOI] [PubMed] [Google Scholar]
- 5. Giudice LC (2003) Elucidating endometrial function in the post-genomic era. Hum Reprod Update 9: 223–235. [DOI] [PubMed] [Google Scholar]
- 6. Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, et al. (2010) Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Molecular human reproduction 16: 135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisert R, Fazleabas A, Lucy M, Mathew D (2012) Interaction of the conceptus and endometrium to establish pregnancy in mammals: role of interleukin 1beta. Cell and tissue research. [DOI] [PMC free article] [PubMed]
- 8. Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao Ch V, et al. (2005) Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertil Steril 84: 275–284. [DOI] [PubMed] [Google Scholar]
- 9. Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF (1999) Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Human reproduction 14: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 10. Lopata A, Hay DL (1989) The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Human reproduction 4: 87–94. [DOI] [PubMed] [Google Scholar]
- 11. Hoshina M, Boothby M, Hussa R, Pattillo R, Camel HM, et al. (1985) Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta 6: 163–172. [DOI] [PubMed] [Google Scholar]
- 12. Zimmermann G, Ackermann W, Alexander H (2009) Epithelial human chorionic gonadotropin is expressed and produced in human secretory endometrium during the normal menstrual cycle. Biology of reproduction 80: 1053–1065. [DOI] [PubMed] [Google Scholar]
- 13. Zimmermann G, Ackermann W, Alexander H (2012) Expression and production of human chorionic gonadotropin (hCG) in the normal secretory endometrium: evidence of CGB7 and/or CGB6 beta hCG subunit gene expression. Biology of reproduction 86: 87. [DOI] [PubMed] [Google Scholar]
- 14. Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao Ch V, et al. (2005) Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertility and sterility 84: 275–284. [DOI] [PubMed] [Google Scholar]
- 15. Bernardini L, Moretti-Rojas I, Brush M, Rojas FJ, Balmaceda JP (1995) Status of hCG/LH receptor and G proteins in human endometrium during artificial cycles of hormone replacement therapy. J Soc Gynecol Investig 2: 630–635. [DOI] [PubMed] [Google Scholar]
- 16. Reshef E, Lei ZM, Rao CV, Pridham DD, Chegini N, et al. (1990) The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab 70: 421–430. [DOI] [PubMed] [Google Scholar]
- 17. Tang B, Gurpide E (1993) Direct effect of gonadotropins on decidualization of human endometrial stroma cells. J Steroid Biochem Mol Biol 47: 115–121. [DOI] [PubMed] [Google Scholar]
- 18. Han SW, Lei ZM, Rao CV (1999) Treatment of human endometrial stromal cells with chorionic gonadotropin promotes their morphological and functional differentiation into decidua. Mol Cell Endocrinol 147: 7–16. [DOI] [PubMed] [Google Scholar]
- 19. Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, et al. (2002) Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab 87: 5290–5296. [DOI] [PubMed] [Google Scholar]
- 20. Kane N, Kelly R, Saunders PT, Critchley HO (2009) Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 150: 2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao CV, Lei ZM (2007) The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol Cell Endocrinol 269: 2–8. [DOI] [PubMed] [Google Scholar]
- 22. Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, et al. (2007) Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 148: 618–626. [DOI] [PubMed] [Google Scholar]
- 23.Bourdiec A, Shao R, Rao CV, Akoum A (2012) Human Chorionic Gonadotropin Triggers Angiogenesis via the Modulation of Endometrial Stromal Cell Responsiveness to Interleukin 1: A New Possible Mechanism Underlying Embryo Implantation. Biology of reproduction. [DOI] [PubMed]
- 24. Paulesu L, Jantra S, Ietta F, Brizzi R, Bigliardi E (2008) Interleukin-1 in reproductive strategies. Evolution & development 10: 778–788. [DOI] [PubMed] [Google Scholar]
- 25. Simon C, Frances A, Piquette G, Hendrickson M, Milki A, et al. (1994) Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. The Journal of clinical endocrinology and metabolism 78: 847–854. [DOI] [PubMed] [Google Scholar]
- 26. Simon C, Frances A, Piquette GN, el Danasouri I, Zurawski G, et al. (1994) Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 134: 521–528. [DOI] [PubMed] [Google Scholar]
- 27. Krussel JS, Bielfeld P, Polan ML, Simon C (2003) Regulation of embryonic implantation. European journal of obstetrics, gynecology, and reproductive biology 110 Suppl 1 S2–9. [DOI] [PubMed] [Google Scholar]
- 28. Baranao RI, Piazza A, Rumi LS, Polak de Fried E (1997) Determination of IL-1 and IL-6 levels in human embryo culture-conditioned media. American journal of reproductive immunology 37: 191–194. [DOI] [PubMed] [Google Scholar]
- 29. Sheth KV, Roca GL, al-Sedairy ST, Parhar RS, Hamilton CJ, et al. (1991) Prediction of successful embryo implantation by measuring interleukin-1-alpha and immunosuppressive factor(s) in preimplantation embryo culture fluid. Fertil Steril 55: 952–957. [DOI] [PubMed] [Google Scholar]
- 30. Bankers-Fulbright JL, Kalli KR, McKean DJ (1996) Interleukin-1 signal transduction. Life sciences 59: 61–83. [DOI] [PubMed] [Google Scholar]
- 31. Rossi M, Sharkey AM, Vigano P, Fiore G, Furlong R, et al. (2005) Identification of genes regulated by interleukin-1beta in human endometrial stromal cells. Reproduction 130: 721–729. [DOI] [PubMed] [Google Scholar]
- 32. Sawai K, Matsuzaki N, Okada T, Shimoya K, Koyama M, et al. (1997) Human decidual cell biosynthesis of leukemia inhibitory factor: regulation by decidual cytokines and steroid hormones. Biology of reproduction 56: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 33. Tabibzadeh S, Kaffka KL, Satyaswaroop PG, Kilian PL (1990) Interleukin-1 (IL-1) regulation of human endometrial function: presence of IL-1 receptor correlates with IL-1-stimulated prostaglandin E2 production. The Journal of clinical endocrinology and metabolism 70: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 34. Dey SK, Lim H, Das SK, Reese J, Paria BC, et al. (2004) Molecular cues to implantation. Endocrine reviews 25: 341–373. [DOI] [PubMed] [Google Scholar]
- 35. Simon C, Gimeno MJ, Mercader A, O′Connor JE, Remohi J, et al. (1997) Embryonic regulation of integrins beta 3, alpha 4, and alpha 1 in human endometrial epithelial cells in vitro. The Journal of clinical endocrinology and metabolism 82: 2607–2616. [DOI] [PubMed] [Google Scholar]
- 36. Hirota Y, Osuga Y, Hasegawa A, Kodama A, Tajima T, et al. (2009) Interleukin (IL)-1beta stimulates migration and survival of first-trimester villous cytotrophoblast cells through endometrial epithelial cell-derived IL-8. Endocrinology 150: 350–356. [DOI] [PubMed] [Google Scholar]
- 37. Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, et al. (1994) Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. The Journal of biological chemistry 269: 17125–17131. [PubMed] [Google Scholar]
- 38. Strakova Z, Szmidt M, Srisuparp S, Fazleabas AT (2003) Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology 144: 5339–5346. [DOI] [PubMed] [Google Scholar]
- 39. Yagel S, Lala PK, Powell WA, Casper RF (1989) Interleukin-1 stimulates human chorionic gonadotropin secretion by first trimester human trophoblast. The Journal of clinical endocrinology and metabolism 68: 992–995. [DOI] [PubMed] [Google Scholar]
- 40. Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, et al. (1995) Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem 270: 13757–13765. [DOI] [PubMed] [Google Scholar]
- 41. Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, et al. (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261: 472–475. [DOI] [PubMed] [Google Scholar]
- 42. Herrmann-Lavoie C, Rao CV, Akoum A (2007) Chorionic gonadotropin down-regulates the expression of the decoy inhibitory interleukin 1 receptor type II in human endometrial epithelial cells. Endocrinology 148: 5377–5384. [DOI] [PubMed] [Google Scholar]
- 43. Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem 283: 14542–14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hong KH, Ryu J, Han KH (2005) Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 105: 1405–1407. [DOI] [PubMed] [Google Scholar]
- 45. Noyes RW, Hertig AT, Rock J (1975) Dating the endometrial biopsy. American journal of obstetrics and gynecology 122: 262–263. [DOI] [PubMed] [Google Scholar]
- 46. Akoum A, Lemay A, Brunet C, Hebert J (1995) Cytokine-induced secretion of monocyte chemotactic protein-1 by human endometriotic cells in culture. The Groupe d'Investigation en Gynecologie. Am J Obstet Gynecol 172: 594–600. [DOI] [PubMed] [Google Scholar]
- 47. Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reproductive biology and endocrinology : RB&E 8: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- 49. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I (2001) Controlling the false discovery rate in behavior genetics research. Behavioural brain research 125: 279–284. [DOI] [PubMed] [Google Scholar]
- 50. Wettenhall JM, Simpson KM, Satterley K, Smyth GK (2006) affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22: 897–899. [DOI] [PubMed] [Google Scholar]
- 51. Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A (2009) Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology 150: 3128–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bellehumeur C, Blanchet J, Fontaine JY, Bourcier N, Akoum A (2009) Interleukin 1 regulates its own receptors in human endometrial cells via distinct mechanisms. Human reproduction 24: 2193–2204. [DOI] [PubMed] [Google Scholar]
- 53. Akoum A, Lemay A, Maheux R (2002) Estradiol and interleukin-1beta exert a synergistic stimulatory effect on the expression of the chemokine regulated upon activation, normal T cell expressed, and secreted in endometriotic cells. The Journal of clinical endocrinology and metabolism 87: 5785–5792. [DOI] [PubMed] [Google Scholar]
- 54. Maurer J, Nelson B, Cecena G, Bajpai R, Mercola M, et al. (2008) Contrasting expression of keratins in mouse and human embryonic stem cells. PloS one 3: e3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Islami D, Bischof P, Chardonnens D (2003) Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol Hum Reprod 9: 395–398. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann-Lavoie C, Rao CV, Akoum A (2007) Chorionic Gonadotropin Downregulates the Expression of the Decoy Inhibitory Interleukin 1 Receptor Type II in Human Endometrial Epithelial Cells. Endocrinology. [DOI] [PubMed]
- 57. Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, et al. (1994) Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 79: 643–649. [DOI] [PubMed] [Google Scholar]
- 58. Sugihara K, Kabir-Salmani M, Byrne J, Wolf DP, Lessey B, et al. (2008) Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS letters 582: 197–202. [DOI] [PubMed] [Google Scholar]
- 59. Norwitz ER, Schust DJ, Fisher SJ (2001) Implantation and the survival of early pregnancy. The New England journal of medicine 345: 1400–1408. [DOI] [PubMed] [Google Scholar]
- 60.Dominguez F, Pellicer A, Simon C (2003) The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface–a review. Placenta24 Suppl B: : S48–55. [DOI] [PubMed] [Google Scholar]
- 61. Castro-Rendon WA, Castro-Alvarez JF, Guzman-Martinez C, Bueno-Sanchez JC (2006) Blastocyst-endometrium interaction: intertwining a cytokine network. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 39: 1373–1385. [DOI] [PubMed] [Google Scholar]
- 62. Proost P, Wuyts A, Van Damme J (1996) Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. Journal of leukocyte biology 59: 67–74. [DOI] [PubMed] [Google Scholar]
- 63. Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, et al. (1997) In vivo properties of monocyte chemoattractant protein-1. Journal of leukocyte biology 62: 577–580. [DOI] [PubMed] [Google Scholar]
- 64. Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE (2008) Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). The Journal of biological chemistry 283: 14542–14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Mourik MS, Macklon NS, Heijnen CJ (2009) Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. Journal of leukocyte biology 85: 4–19. [DOI] [PubMed] [Google Scholar]
- 66. Chen SJ, Liu YL, Sytwu HK (2012) Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clinical & developmental immunology 2012: 258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, et al. (2007) Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. American journal of reproductive immunology 57: 55–66. [DOI] [PubMed] [Google Scholar]
- 68. Houser BL (2012) Decidual macrophages and their roles at the maternal-fetal interface. The Yale journal of biology and medicine 85: 105–118. [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S, Yoon IH, Yoon A, Cook-Mills JM, Park CG, et al.. (2012) An Antibody to the Sixth Ig-like Domain of VCAM-1 Inhibits Leukocyte Transendothelial Migration without Affecting Adhesion. Journal of immunology. [DOI] [PubMed]
- 70. Rahman AN, Snibson KJ, Lee CS, Meeusen EN (2004) Effects of implantation and early pregnancy on the expression of cytokines and vascular surface molecules in the sheep endometrium. Journal of reproductive immunology 64: 45–58. [DOI] [PubMed] [Google Scholar]
- 71. Vega-Sanchez R, de Jesus-Torres E, Arenas-Hernandez M, Beltran-Montoya J, Maida-Claros R, et al. (2010) [Expression of cell adhesion molecules in the maternal fetal period of human gestation]. Ginecologia y obstetricia de Mexico 78: 677–684. [PubMed] [Google Scholar]
- 72. Altun T, Jindal S, Greenseid K, Shu J, Pal L (2011) Low follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. Journal of assisted reproduction and genetics 28: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tabibzadeh S, Kong QF, Babaknia A, May LT (1995) Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Human reproduction 10: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 74. von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, et al. (2000) Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Molecular human reproduction 6: 627–634. [DOI] [PubMed] [Google Scholar]
- 75. Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN (2001) Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Molecular human reproduction 7: 163–168. [DOI] [PubMed] [Google Scholar]
- 76. Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, et al. (2006) Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. Journal of reproductive immunology 72: 60–73. [DOI] [PubMed] [Google Scholar]
- 77. Ramhorst R, Patel R, Corigliano A, Etchepareborda JJ, Fainboim L, et al. (2006) Induction of maternal tolerance to fetal alloantigens by RANTES production. American journal of reproductive immunology 56: 302–311. [DOI] [PubMed] [Google Scholar]
- 78. Ramhorst R, Gutierrez G, Corigliano A, Junovich G, Fainboim L (2007) Implication of RANTES in the modulation of alloimmune response by progesterone during pregnancy. American journal of reproductive immunology 57: 147–152. [DOI] [PubMed] [Google Scholar]
- 79. Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, et al. (2012) VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell reports 1: 191–199. [DOI] [PubMed] [Google Scholar]
- 80. Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, et al. (2005) In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 146: 4097–4104. [DOI] [PubMed] [Google Scholar]
- 81.Guay S, Akoum A (2007) Stable inhibition of interleukin, 1 receptor type II in Ishikawa cells augments secretion of matrix metalloproteinases: possible role in endometriosis pathophysiology. Reproduction (accepted with modifications). [DOI] [PubMed]
- 82. Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, et al. (2011) Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Molecular human reproduction 17: 637–652. [DOI] [PubMed] [Google Scholar]
- 83. Butler GS, Apte SS, Willenbrock F, Murphy G (1999) Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions. The Journal of biological chemistry 274: 10846–10851. [DOI] [PubMed] [Google Scholar]
- 84. Black RA (2004) TIMP3 checks inflammation. Nature genetics 36: 934–935. [DOI] [PubMed] [Google Scholar]
- 85. Caren H, Djos A, Nethander M, Sjoberg RM, Kogner P, et al. (2011) Identification of epigenetically regulated genes that predict patient outcome in neuroblastoma. BMC cancer 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Crowe DL, Milo GE, Shuler CF (1999) Keratin 19 downregulation by oral squamous cell carcinoma lines increases invasive potential. Journal of dental research 78: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 87. Sales KJ, Grant V, Catalano RD, Jabbour HN (2011) Chorionic gonadotrophin regulates CXCR4 expression in human endometrium via E-series prostanoid receptor 2 signalling to PI3K-ERK1/2: implications for fetal-maternal crosstalk for embryo implantation. Molecular human reproduction 17: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jabbour HN, Kelly RW, Fraser HM, Critchley HO (2006) Endocrine regulation of menstruation. Endocrine reviews 27: 17–46. [DOI] [PubMed] [Google Scholar]
- 89. Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, et al. (2002) Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. The Journal of biological chemistry 277: 29260–29267. [DOI] [PubMed] [Google Scholar]
- 90. Kauma S, Matt D, Strom S, Eierman D, Turner T (1990) Interleukin-1 beta, human leukocyte antigen HLA-DR alpha, and transforming growth factor-beta expression in endometrium, placenta, and placental membranes. American journal of obstetrics and gynecology 163: 1430–1437. [DOI] [PubMed] [Google Scholar]