Abstract
Nicotinic acetylcholine receptors (nAChRs) containing the α9 subunit are expressed in a wide variety of non-neuronal tissues ranging from immune cells to breast carcinomas. The α9 subunit is able to assemble into a functional homomeric nAChR and also co-assemble with the α10 subunit into functional heteromeric nAChRs. Despite the increasing awareness of the important roles of this subunit in vertebrates, the study of human α9-containing nAChRs has been severely limited by difficulties in its expression in heterologous systems. In Xenopus laevis oocytes, functional expression of human α9α10 nAChRs is very low compared to that of rat α9α10 nAChRs. When oocytes were co-injected with cRNA of α9 and α10 subunits of human versus those of rat, oocytes with the rat α9 human α10 combination had an ∼-fold higher level of acetylcholine-gated currents (IACh) than those with the human α9 rat α10 combination, suggesting difficulties with human α9 expression. When the ratio of injected human α9 cRNA to human α10 cRNA was increased from 1∶1 to 5∶1, IACh increased 36-fold (from 142±23 nA to 5171±748 nA). Functional expression of human α9-containing receptors in oocytes was markedly improved by appending the 5′-untranslated region of alfalfa mosaic virus RNA4 to the 5′-leader sequence of the α9 subunit cRNA. This increased the functional expression of homomeric human α9 receptors by 70-fold (from 7±1 nA to 475±158 nA) and of human α9α10 heteromeric receptors by 80-fold (from 113±62 nA to 9192±1137 nA). These findings indicate the importance of the composition of the 5′ untranslated leader sequence for expression of α9-containing nAChRs.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are ACh-gated ion channels implicated in many physiological as well as pathophysiological processes. The role of nAChRs in mediating EPSPs at synapses in autonomic ganglia [1], [2] and at the skeletal neuromuscular junction is well established [3], [4]. In the CNS, nAChRs are involved in modulation of neurotransmitter release [5] and in attention and memory [6], [7]. The pathological conditions where involvement of nAChRs have been implicated include Alzheimer's and Parkinson's diseases [8], [9], nicotine addiction [10], [11] and schizophrenia [12], [13]. Seventeen vertebrate nAChR subunits have been cloned to date (α1 through α10, β1 through β4, γ, δ, and ε) [14]. The nAChR is formed from five subunits, either homomeric receptors (α7, α9) containing five identical subunits or heteromeric receptors (for example, α4β2, α3α5β4, α6α4β2β3, or α9α10).
α9-containing nAChRs are unique among neuronal nAChRs in that they are found mainly outside of the CNS [15], [16], [17], [18], [19]. Also, unlike other nAChRs, they are inhibited by nicotine [15], [20], [21]. α9-containing nAChRs play roles in pain [22], [23], [24], [25], [26], [27], inflammation, keratinocyte adhesion [28], and in mediating synaptic transmission between the efferent olivocochlear fibers and cochlear hair cells [29], [30].
With advances in molecular biology, it became possible to isolate and sequence the genes encoding nAChRs. α9 and α10 subunits were among the last nicotinic receptor subunits to be isolated and characterized. The clone encoding the α9 subunit was originally obtained from a rat olfactory epithelium cDNA library [15]. X. laevis oocytes injected solely with rat α9 cRNA yielded homomeric receptors that responded to 100 µM ACh with currents that ranged from 20 to 500 nA [15]. The clone encoding the rat α10 subunit was isolated from an adult rat cochlea cDNA library [31]. The coinjection of rat α9 and rat α10 cRNAs into oocytes resulted in oocytes with ∼100-fold larger ACh-gated currents (IACh) than oocytes injected solely with α9 cRNA. Subsequently, the sequences of human α9 and α10 subunits were determined from keratinocytes [28] and inner-ear neuroepithelium [32], respectively.
To study the pharmacological properties of nAChRs, heterologous expression systems are often used. Mammalian cell lines such as HEK293 and SH-EP1 cells are frequently used to characterize nAChRs [33], [34]. Besides mammalian cells, oocytes of Xenopus laevis, the African clawed frog, have been frequently used for heterologous expression. These oocytes provide several advantages for the study of receptors. They are large and thus easy to handle and to inject with RNA, have long life-times (several days) and can be maintained under relatively simple culture conditions. Oocytes are largely free of endogenous receptors that could interfere with the signals of exogenously expressed channels/receptors. Thus, oocytes have been extensively used to characterize the biophysical and pharmacological properties of nAChRs. They have also been used to study the stoichiometry of receptor subunits, the contribution of different subunits to the properties of receptors, and the structure-function relationships with various ligands. For most nAChRs, oocytes have worked extremely well as an expression host [35], [36], [37]. However, in some instances cRNA-injected oocytes have failed to yield readily detectable IACh. For instance, human α9 cRNA-injected oocytes have only small IACh compared to oocytes injected with its rat counterpart [38], [39]. There is no report to date of successful functional expression of human α9-containing receptors in mammalian cell lines and few reports of successful transfection of rat a9-containing receptors [40], [41].
The translational efficiency of nAChRs in oocytes is influenced by the structure of the injected cRNA [42], [43] including the Kozak sequence [44], the secondary structure [45], [46] and composition of untranslated regions [47], [48]. The 5′ leader sequence preceding the coding region plays an important role in the binding of cap-binding proteins and in facilitation of translation initiation [49]. One approach to improve the translation in oocytes is to flank the gene-encoding sequence with the untranslated regions of highly translatable proteins of X. laevis, such as β-globin [50], [51]. When 5′ and 3′untranslated regions (UTRs) of human interferon-β mRNA are replaced by those of X. laevis β-globin mRNA, the translation is increased as much as 20- and 300- fold in reticulocyte lysates and in X. laevis oocytes, respectively [52]. The X. laevis β-globin leader sequence exerts its facilitatory effect presumably by increasing translation initiation, and not by increasing the binding of limiting factors [50]. However, for human α9, the addition of the X. laevis β-globin sequence to the 5′ and 3′ UTRs is not sufficient to produce high expression levels.
In this report, we show that the human α9 subunit is the limiting factor in the expression of human α9α10 nAChRs in X. laevis oocytes. Furthermore, we found that this expression can be substantially improved by the insertion of the 5′ leader sequence of alfalfa mosaic virus RNA4 (AMV) to the human α9 5′ UTR.
Materials and Methods
Ethics Statement
Isolation of oocytes from X.laevis frogs was performed in accordance with and under approval of the Institutional Animal Care and Use Committee of the University of Utah.
cDNA constructs
cDNAs encoding α9 and α10 nAChR subunits from rat were provided by A. B. Elgoyhen (University of Buenos Aires, Argentina). The rat α9 cDNA was in a pGEMHE [51] vector between SmaI and EcoRI restriction sites, and the rat α10 cDNA was in a pSGEM vector (a modified pGEMHE vector) between EcoRI and XhoI restriction sites. cDNAs encoding human α9 and human α10 subunits, in the pGEM-11Zf(+) vector, were generously provided by L. Lustig (University of California San Francisco, San Francisco, CA). The cDNAs encoding human subunits were subsequently inserted into the pSGEM vector between EcoRI and XhoI restriction sites. The oligonucleotides encoding the 5′leader sequence of alfalfa mosaic virus RNA4 (AMV) were synthesized at the University of Utah core facility. The sequence of the synthesized oligonucleotides was as follows: sense- 5′ GGGTTTTTATTTTTAATTTTCTTTCAAATACTTCCACCG 3′; antisense-5′ AATTCGGTGGAAGTATTTGAAAGAAAATTAAAAATAAAAACCCGC 3′. The oligonucleotides were diluted in 10 mM Tris-Cl, pH 8.5 to a final concentration of 107 µM for sense oligonucleotide and 80 µM for antisense oligonucleotide. 20 µL of each oligonucleotide was mixed in an annealing reaction tube. The annealing reaction was as follows: exposure to 95°C for 10 minutes followed by cooling to 25°C over a period of 45 minutes. The annealed oligonucleotide was ligated into MCS of pSGEM vector between the SacII and EcoRI restriction sites.
cRNA synthesis
The NheI enzyme was used to linearize the vector encoding human α9 and human α10 subunits. In vitro transcription was performed using the mMessage mMachine T7 kit (Ambion, Austin, TX). The reaction was followed by DNase treatment. The cRNA was purified with a Qiagen RNeasy kit (Qiagen, Valencia, CA, USA). The cRNA concentration was determined by measuring absorbance at 260 nm on an Epoch spectrophotometer.
Oocyte isolation and injection
The isolation of the oocytes was performed as previously described [53]. Briefly, stage IV–V oocytes were isolated from anesthetized adult frog. The oocytes were kept at 17°C in ND96 (96 mM NaCl, 1.8 mM CaCl2, 2.0 mM KCl, 1.0 mM MgCl2, 5 mM HEPES, pH 7.1–7.5) supplemented with antibiotics (50 U/mL penicillin, 50 µg/mL streptomycin, 50 µg/mL gentamicin). The oocytes were injected with 50.6 nL of cRNA and incubated for 1–3 days before recording. The amount of cRNA injected into each oocyte varied in different experiments. To compare levels of expression of human and rat α9α10 receptors, 3.3 ng cRNA of each nAChR subunit was injected into individual oocytes. To compare the level of expression of human receptors formed from subunits injected at different ratios, 4.4 ng cRNA of each nAChR subunit was injected into individual oocytes when a ratio of (1) is indicated and 22 ng cRNA was injected when a ratio of (5) is indicated. For all other experiments, 14.4–32 ng cRNA of each subunit was injected.
Two-electrode voltage clamp recording
ACh-gated currents were recorded from oocytes as previously described [53]. Briefly, an oocyte was placed in ∼30 µL chamber (4 mm diameter ×2 mm deep) fabricated from Sylgard and gravity-perfused with ND96 at a constant flow rate (∼2 mL/min). The oocyte's membrane potential was held at −70 mV using an OC-725B two-electrode voltage clamp amplifier (Warner Instrument Corp., Hamden, CT). To evoke IACh, the perfusion of ND96 was replaced for one-second with ND96 containing100 µM ACh; such a pulse of ACh was applied once per minute. The peak of the ACh-gated current (IACh) was measured and the average of six consecutive IACh responses served as the control current response.
To minimize potential batch-to-batch variability, oocytes from the same isolation were used to compare the expression of receptors formed from unmodified and modified nAChR subunits. Furthermore, all recordings for a given comparison were performed on the same day.
Data analysis
Data are expressed as mean ± SEM. Statistical comparisons between two groups were done using Student's t-tests, and those between multiple groups were done using ANOVA test with Tukey's post-hoc comparison.
Results
Human α9α10 nAChRs express poorly in X. laevis oocytes
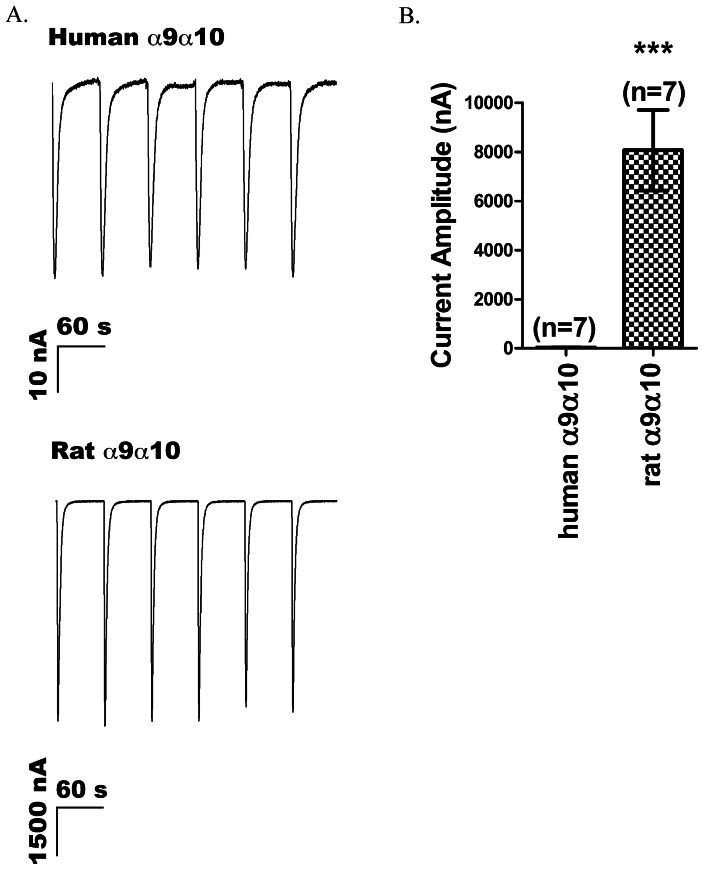
Previous investigations of human and rat α9-containing receptors reported difficulties in the expression of human α9-containing receptors [38], [39]. Consistent with these reports, when cRNAs encoding human α9 and human α10 subunits of nAChRs were co-injected into oocytes at a 1∶1 molar ratio, 100 µM ACh produced small currents (Fig. 1A top), which on average were 30±3 nA (Fig. 1B). Currents of this low magnitude are difficult to utilize for medium throughput pharmacological testing. In contrast, co-injection of rat α9 and rat α10 subunits yielded large currents (Fig. 1A bottom) with an average amplitude of 8067±1638 nA (Fig. 1B). The difference in functional expression between rat α9α10 and human α9α10 nAChRs might be due to the inefficient translation of the human α9 or human α10 subunit or both, and this was explored in experiments described below.
Figure 1. Comparison between the levels of exogenous expression of rat and human α9-containing nAChRs in X. laevis oocytes.
ACh-gated currents were measured in voltage-clamped oocytes as described in Methods. (A) Representative traces from an oocyte injected with human α9 and human α10 cRNA (top) and rat α9 and rat α10 cRNA (bottom). Robust currents were observed with rat cRNA; but only small currents were observed with human cRNA. (B) Comparison of the averaged current responses evoked by 100 µM ACh applications from oocytes expressing human α9α10 and rat α9α10 receptors. The mean current amplitude was 30±3 nA (n = 7 oocytes) for human α9α10 and 8067±1638 nA (n = 7) for rat α9α10, p<0.005. Error bars indicate SEM.
Functional expression of α9 versus α10 subunitsIn order to assess the influence of α9 vs
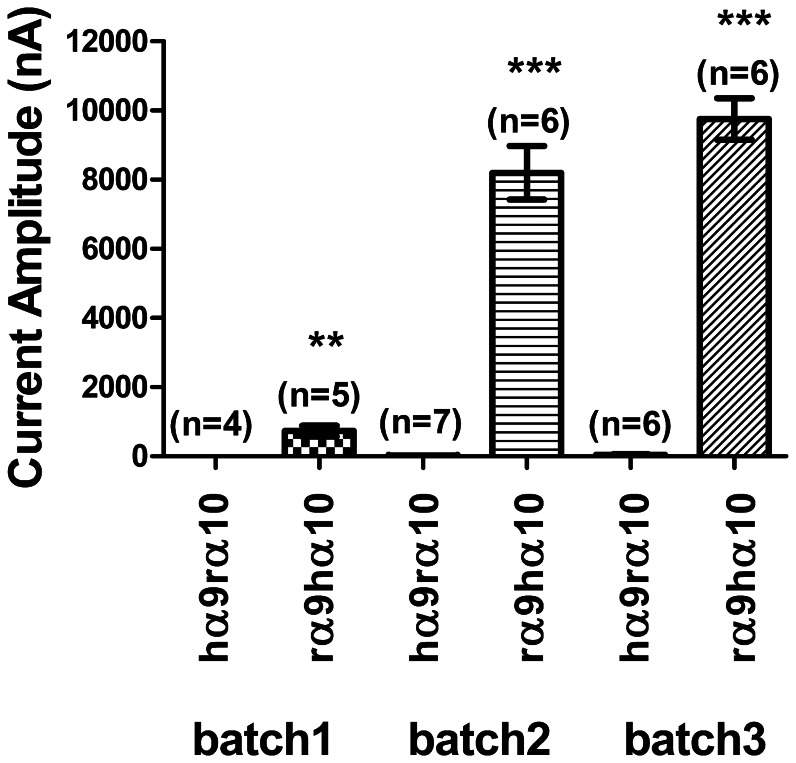
α10 subunits on the functional expression of α9α10 nAChRs, we injected cRNA encoding subunits from different species (i.e., rat versus human) at a 1∶1 ratio. When human α9 was co-expressed with rat α10, the current amplitude was invariably low in all three batches of oocytes tested, averaging from 5±1 nA to 50±15 nA (Fig. 2 and Table 1). When rat α9 was co-expressed with human α10, the current was readily detectable (Fig. 2 and Table 1) and at a level similar to that seen after co-injection of rat α9 with rat α10 subunits (Fig. 1A bottom and Fig. 1B); the average current amplitude ranged between 732±155 nA and 9755±596 nA, depending which of three batches of oocytes was used. There are at least two possible reasons for the low functional expression: A) rat α10 co-expressed with human α9 produced functionally impaired receptors or B) human α9 subunits are not translated efficiently in oocytes.
Figure 2. Comparison between the level of expression of human α9/rat α10 (hα9rα10) and rat α9/human α10 (rα9hα10) receptors.
Receptors assembled from injection of cRNAs encoding subunits from different species have different levels of functional expression. hα9rα10 nAChRs were expressed with low efficiency compared to rα9hα10. Results from three batches of oocytes are shown. All oocytes of a given batch were injected on the same day and recordings performed 2 days later. Values of mean current amplitudes are given in Table 1. **p<0.01. Error bars indicate SEM.
Table 1. Comparison of the functional expression of receptors following co-injection of cRNA for subunits of different species.a .
| Oocyte Batch # | Receptor | Mean current amplitude (nA) | SEM | n |
| 1 | hα9rα10 | 5 | 1 | 4 |
| 1 | rα9hα10 | 732 | 155 | 5 |
| 2 | hα9rα10 | 21 | 7 | 7 |
| 2 | rα9hα10 | 8200 | 774 | 6 |
| 3 | hα9rα10 | 50 | 15 | 6 |
| 3 | rα9hα10 | 9755 | 596 | 6 |
Graphical representations of these results are provided in Fig. 2.
Inefficient translation of the human α9 subunit appears to limit assembly of functional human α9/human α10 receptors
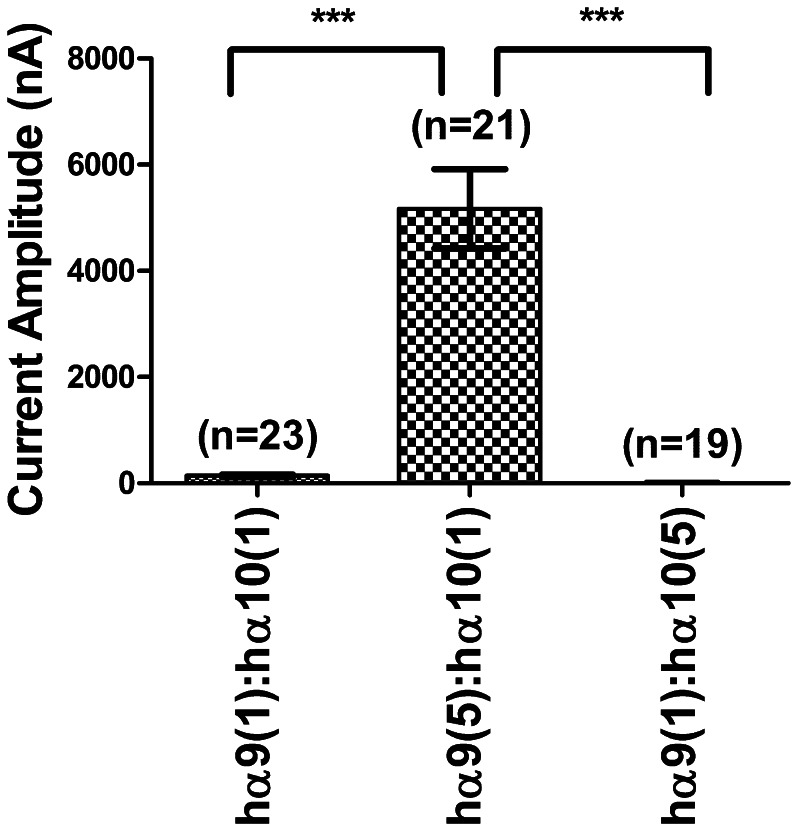
When cRNAs encoding human α9 and human α10 subunits were co-injected at a 1∶1 ratio, the IACh rarely reached 1 µA with the average response equal to 142±23 nA. Oocytes injected with a 5∶1 ratio had currents averaging 5171±748 nA. Injections at a 1∶5 ratio produced oocytes with low average IACh amplitude equal to 6.5±3.9 nA (Fig. 3 and Table 2). Thus, more abundant cRNA for the α9 subunit leads to substantially enhanced functional expression of α9α10 nAChRs. This increased functional expression suggests that translation of the human α9 subunit is likely a limiting factor in the assembly of α9α10 receptors.
Figure 3. Comparison of functional receptor expression following injection of different ratios of receptor subunit cRNA.
Differing subunit ratios of cRNA were injected into oocytes and the resulting levels of expression of functional receptors were compared. Recordings were performed 2 days after injection. The data from oocytes of four different batches were combined to determine the mean current amplitudes. Values are given in Table 2. A one-way ANOVA test with Tukey's post-hoc comparison indicated a significant difference between hα9(1):hα10(1) vs. hα9(5):hα10(1), p<0.001, and between hα9(5):hα10(1) vs. hα9(1):hα10(5), p<0.001. There was no significant difference between hα9(1):hα10(1) and hα9(1):hα10(5), p>0.05.
Table 2. Comparison of the functional expression of receptors upon co-injection of different ratios of cRNA for specific subunit.a .
| Receptor | Mean current amplitude (nA) | SEM | n |
| hα9(1):hα10(1) | 142 | 23 | 23 |
| hα9(5):hα10(1) | 5171 | 748 | 21 |
| hα9(1):hα10(5) | 6.5 | 3.9 | 19 |
Graphical representations of these results are provided in Fig. 3.
AMV insertion and expression of human α9-containing nAChRs
Previous investigators have shown that incorporation of 5′UTR of the Xenopus laevis β-globin gene facilitates the in vitro translation of different proteins in oocytes and other expression systems [50], [51], [54]. In pGEMHE and pSGEM vectors the 5′ leader sequence of the receptor subunit includes the 5′UTR of X. laevis β-globin, restriction sites of the vector's multiple cloning site, and the native 5′UTR of the subunit.
Plant viruses use host translational machinery for replication. RNAs of many plant viruses possess efficient translation enhancers [55] that can be used in order to improve the translation of recombinant proteins or expression of receptors in heterologous systems. Among such enhancers are untranslated regions from different viral RNAs. The 5′UTR from alfalfa mosaic virus RNA4, the 3′UTR of brome mosaic virus and the 5′leader of tobacco mosaic virus were shown to be able to enhance the mRNA translation of foreign proteins [56], [57], [58], [59], [60].
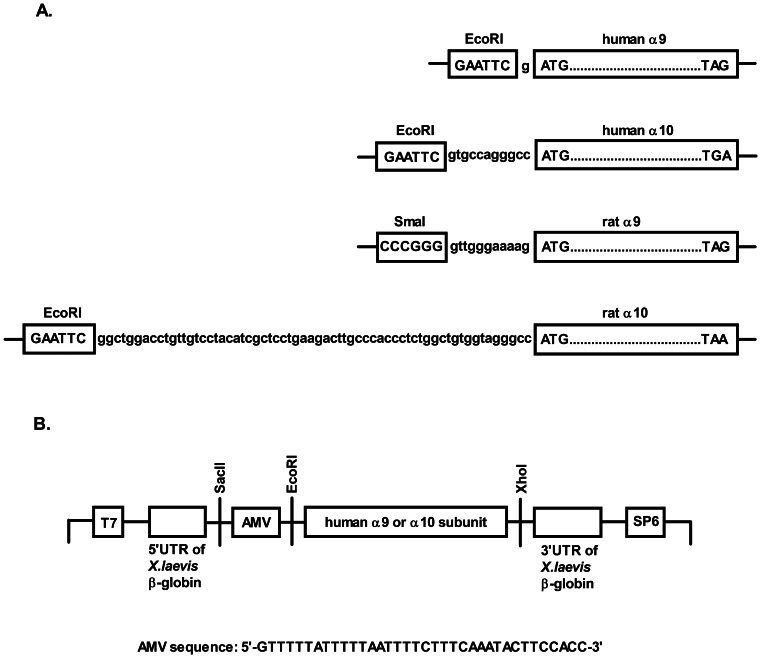
In an attempt to improve the translation of human α9/α10 we modified the 5′leader sequence of human α9 and human α10 subunits by introducing the 5′UTR of RNA4 of alfalfa mosaic virus (AMV) into the multiple cloning site of the pSGEM vector (Fig. 4B) between SacII and EcoRI sites, after the 5′UTR of β-globin and in front of the nAChR subunit.
Figure 4. Comparison of the 5′ untranslated regions in human α9, human α10, rat α9, and rat α10 subunits.
(A) Native 5′UTRs of subunits are between the restriction site and the start codon. (B) The modifications made to the 5′ untranslated region of human α9 and α10 subunits are shown. The 5′UTR of RNA4 of the alfalfa mosaic virus coat protein was inserted into the multiple cloning site of the pSGEM vector between SacII and EcoRI sites. The subunit-encoding sequence is between the EcoRI and XhoI sites.
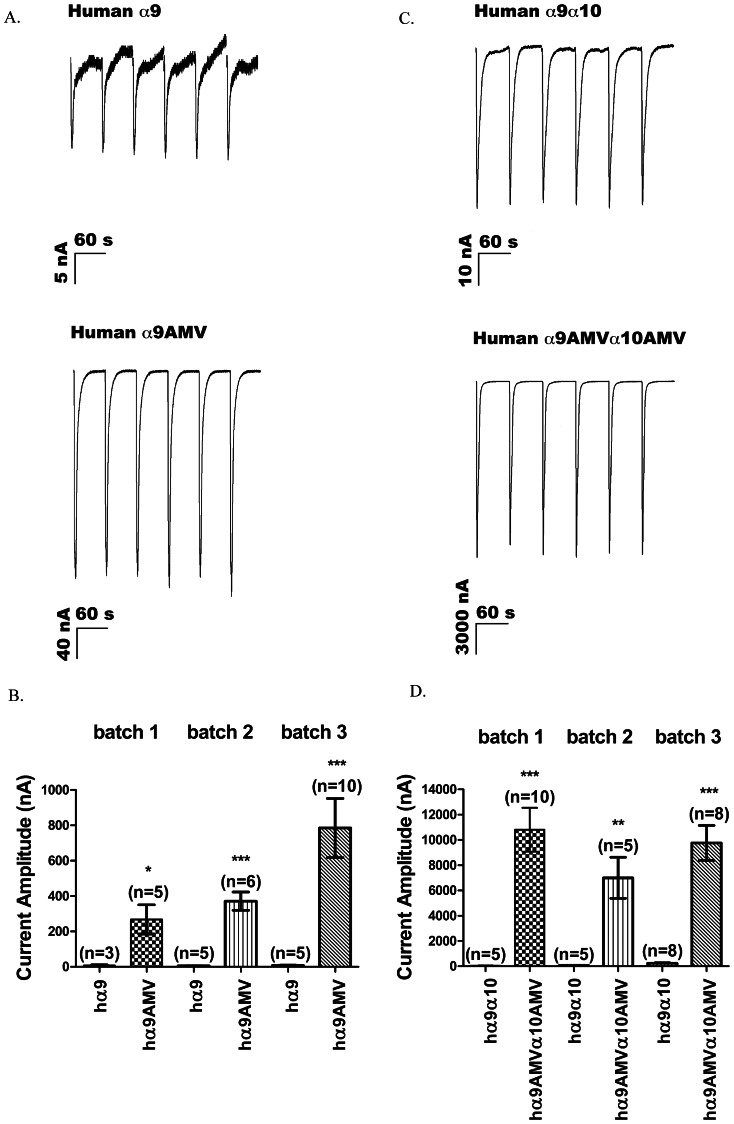
The AMV incorporation improved the functional expression of human α9 homomeric receptors by 37- to 101-fold, and the human α9α10 heteromeric receptors by 41- to 250-fold, depending on the batch of oocytes used (Fig. 5 and Tables 3 and 4). Despite the variability in the expression levels of human α9 and α9α10 receptors, which is also commonly observed for other nAChRs, the large improvement in expression was highly reproducible.
Figure 5. AMV improves the level of functional expression of α9-containing nAChRs.
(A) Representative traces and (B) comparisons of the levels of functional expression of homomeric human α9 receptors encoded by cRNA without (A, top) and with (A, bottom) AMV. The results are from three different batches of oocytes, each isolated from a different frog and recorded on 3rd day after injection, are presented. (C and D) Comparison of the level of expression of heteromeric receptors. Recordings were conducted on the second day after injection. Values for mean current amplitude are shown in Tables 3 and 4. *p<0.05; **p<0.01. Error bars indicate SEM.
Table 3. Insertion of AMV improves the expression of human α9 homomeric receptors.a .
| Oocyte Batch # | Receptor | Mean current amplitude (nA) | SEM | n |
| 1 | α9 | 7 | 4 | 3 |
| 1 | α9AMV | 268 | 83 | 5 |
| 2 | α9 | 5 | 1 | 5 |
| 2 | α9AMV | 372 | 52 | 6 |
| 3 | α9 | 8 | 2 | 5 |
| 3 | α9AMV | 785 | 167 | 10 |
Graphical representations of these results are provided in Fig. 5.
Table 4. AMV improves the expression of human α9α10 heteromeric receptors.a .
| Oocyte Batch # | Receptor | Mean current amplitude (nA) | SEM | n |
| 1 | hα9α10 | 43 | 6 | 5 |
| 1 | hα9AMVα10AMV | 10813 | 1739 | 10 |
| 2 | hα9α10 | 60 | 11 | 5 |
| 2 | hα9AMVα10AMV | 6999 | 1627 | 5 |
| 3 | hα9α10 | 237 | 41 | 8 |
| 3 | hα9AMVα10AMV | 9763 | 1379 | 8 |
Graphical representations of these results are provided in Fig. 5.
Discussion
In this study, we determined that the functional expression of human α9 subunits of nAChRs in X. laevis oocytes depended on the composition of its 5′untranslated region. By introducing the 5′ leader sequence of alfalfa mosaic virus RNA4 into the multiple cloning site of the pSGEM vector just preceding the coding region of human α9 or α10 subunits, we created a vector that gave ∼70-fold higher expression levels of α9 homomeric receptors and ∼80-fold higher expression levels of α9α10 heteromeric receptors compared to those achieved with unmodified vectors.
Since the early demonstration that mRNA encoding nicotinic receptors from Torpedo californicus electric organ could produce functional receptors when it is injected into oocytes of X. laevis [61], [62], oocytes have frequently been used as an exogenous expression system to study the pharmacology of nAChRs. In most cases, the receptor subunits assemble into functional receptors [36], [63], [64]. However, sometimes the cRNA injected into oocytes fails to yield functional receptors. For example, when cRNA encoding the α6 subunit is co-injected with cRNA encoding the β2 or β4 subunit, there is little or no detectable ACh-gated current [65]. In our laboratory, the unmodified cRNA of human α9 nAChRs failed to produce functional receptors. Other authors also reported difficulties in expressing human α9-containing nAChRs [38], [39]. The ability of cRNAs of rat α9 and human α10 subunits, but not those of human α9 and rat α10, to form receptors with high levels of functional expression suggests that human α9 is a limiting factor in the assembly of functional receptors.
There are several possible factors that can influence the level of functional expression of nAChRs in the X. laevis oocyte system. First, the cRNA composition might prevent or interfere with efficient translation. For example, formation of secondary structures may take place that prevent efficient binding of cap-binding proteins and initiation of translation [44]. The nucleotide sequence just preceding the start codon is important for translation initiation. In eukaryotes, the optimal sequence surrounding the start codon is GCCA/GCCaugG [66]. If the purine at the −3 position is changed to a pyrimidine, the efficiency of translation initiation might be reduced. Second, a high G+C content of mRNA can halt efficient transcription and translation by formation of secondary structures. For example, the gene encoding human acetylcholinesterase (AChE) is highly G+C rich (65%) which results in the formation of a secondary structure in the 5′region [67] that serves as an attenuator of transcription. In addition, two highly homologous and highly G+C-rich genes encoding Bungarus and rat acetylcholinesterases have strikingly different rates of transcription with approximately equal translation in oocyte functional tests [68]. The difference in the transcription rate is believed to be determined by the differences in the coding sequences.
The G+C content of human α9 mRNA is 49 % for the gene-coding sequence compared to 51% for rat mRNA. α10 subunits are richer in G+C content with a 65% in the human and 59% in the rat subunit. Thus, the G+C content of human α9 is only slightly lower than its rat counterpart. Based on the relatively equal G+C composition of human and rat α9 mRNAs and high homology in nucleotide sequences of gene-coding regions it is unlikely that G+C content contributes to the low level of functional expression observed from unmodified human α9 subunit in our study.
The UTR is another factor influencing translational efficiency. It was shown to be important for the translation of different proteins in different expression systems. Mutations in the UTR affect the translation of aspartyl protease BACE1 protein and HT3A receptor [69], [70], [71]. When the 5′UTR of BACE1 is present, the protein, but not mRNA, level in transfected HEK293, COS7 and H4 cells is reduced as much as 90%. The inhibitory effect of the 5′UTR is due to the upstream open reading frame (uORF) [69], [71]. Due to their importance, the UTR regions are frequently modified to improve translation. For example, it became a common practice to include 5′- and 3′- UTRs of Xenopus β-globin into expression vectors to flank the gene-coding region [51]. UTRs of viruses have also been used to replace native UTRs, which results in improved yields of translated proteins or improved functional expression of receptors. For example, the 5′UTR of tobacco mosaic virus enhances the translation of chloramphenicol acetyltransferase and β-glucuronidase in tobacco mesophyll protoplasts, E. coli, and Xenopus oocytes [56], [58], [59], [72]. The facilitatory effect of the 5′leader is due to recruitment of eukaryotic initiation factor 4G indirectly via heat shock protein 101 [73].
The alfalfa mosaic virus is an RNA virus consisting of three genomic RNAs and one subgenomic RNA (RNA4). RNA1 and RNA2 encode the replicase proteins P1 and P2, whereas RNA3 encodes viral movement protein (MP). RNA4 is 881-nucleotides long, with a 661-nucleotide long coding sequence that encodes a coat protein required for infectivity and replication of the virus [74]. The 5′UTR of RNA4 is 39-nucleotides long, uracil rich and was shown to be able to improve the translation of foreign proteins. Computer-based structure prediction as well as nuclease-sensitivity analysis indicate the unstructured character of the 5′leader sequence, which can facilitate cap-independent translation initiation [75]. This fact might be relevant if cap-dependent translation initiation of unmodified human α9 subunit is disrupted. The substitution of the native 5′UTR with a 37-base-pair AMV RNA4 leader was shown to improve the translation of several proteins [58], [72]. For example, in vitro translation of human interleukin 1β and barley α-amylase improved as much as 35-fold [59]. Also, the introduction of AMV into the 5′leader of GABAA receptors improved the expression of those receptors in X. laevis oocytes [72].
The 5′UTRs of human nicotinic receptors may be an important factor for receptor function, considering evidence from other systems suggesting that this region could have the regulatory elements important for translation initiation [69], [76], [77]. Many human nAChR subunits have upstream uATG repeats (uATGs) and upstream open reading frames (uORFs). For example, human α9 has an uORF with a length of 36 codons. uORFs are involved in translational regulation of oncogenes by suppressing the level of translation [78], [79]. It is believed that the uORF causes the small ribosomal subunit to stall and therefore halt translation initiation [80]. How the uORF affects the translation of the α9 subunit is an open question. When cRNAs encoding nicotinic receptor subunits are injected into oocytes at a 1∶1 molar ratio, it is assumed that the two subunits will be translated with equal efficiencies so the amount of protein of the two subunits will also be produced in a 1∶1 ratio. However, different receptor subunits might be translated with different efficiencies.
mRNA stability might be a contributing factor to the observed different levels of expression between unmodified human α9-containing receptors and rat α9-containing receptors. One of the factors that determines the stability of mRNA is located within 3′-end of mRNA. In particular, the poly(A) tail is required to ensure high functional stability of the mRNA as was shown for rabbit globin protein [81], [82]. The 3′UTR of the human α9 subunit had a short (6 nucleotides) native 3′UTR, followed by the 3′ UTR of Xenopus β-globin, followed by a poly(A) tail. In contrast, the modified construct incorporated the 5′ UTR of the alfalfa mosaic virus RNA4. This addition may slow degradation of the mRNA.
Another factor that may contributes to the fast turnover of mRNA is an AU-rich region at 3′-untranslated region. Many RNA-binding proteins such as ELAV-like proteins (HuD, Hel-N1, HuC, HuR) bind to AU-rich regions at the 3′-untranslated region of RNA and prevent degradation of mRNA [83]. Human α9 as well as rat α9 subunit 3′UTRs have six non-overlapping AUUUA motifs separated by non-AU nucleotides in a U-poor region. In addition, they have one AAAAUUUAAAA motif.
A second possibility for low expression level of receptors in oocytes is the lack of postrtranslational modifications in the oocyte expression system. The possible posttranslational modifications of nAChRs include proteolytic cleavage, disulfide bond formation, glycosylation, palmitoylation, fatty acid acylation, phosphorylation, amidation, hydroxyprolination, proline isomerization, etc. [84], [85], [86], [87]. The lack of functional expression of α6-containing receptors is likely due to posttranslational mechanisms, insofar as functionality is achieved when the C-terminus of the α6 subunit is replaced with the C-terminus of an α3 subunit implying that important regulatory elements for efficient receptor function are located outside of ligand-binding domain [65].
A third possibility is the lack of appropriate chaperones in oocytes. There are several chaperones described for nicotinic receptors such as BiP, calnexin, Erp57, and RIC3 [88], [89], which facilitate proper folding and improve functional expression of receptors. Nicotine exposure causes an upregulation of nicotinic AChRs in brain as well as in vitro, and a possible explanation of this effect is through the chaperoning by nicotine [90], [91], [92]. The RIC-3 is a chaperone that upregulates the expression of α7 nAChRs in oocytes [93], [94], [95], [96]. Interestingly, RIC-3 has no effect on the expression of α9 receptors [40], [97].
There are few reports of successful expression of α9 receptors in mammalian cells [98]. GH4C1 cell line derived from pituitary gland was successfully transfected with rat α9α10 receptors [41]. Here, the average ACh-evoked currents ranged between 16 pA to 300 pA. Also, an α9/HT3a chimera, where the N-terminus of rat α9 was fused to the C-terminus of mouse HT3a receptor, produced functional receptors [99]. Mouse α9α10 receptors were successfully transfected into HEK293 cells [98]. The problem of the lack of expression of human α9 receptor in mammalian cell lines was addressed in several reports [40], [98], [99], [100]. It was shown that co-transfection of human α9 and α10 subunit with AChR-associated proteins rapsyn and chaperone RIC-3 in CL4 cells increased the cytosolic calcium level after application of 100 µACh but no measurements of ionic current from α9-containing receptors were reported [100]. It is still an open question as to whether the lack of functionality in mammalian cells is due to inefficient transcription, translation, improper folding, and lack of chaperoning or posttranslational modifications or a combination of these.
In our current study, we observed the effect of the 5′UTR of the human α9 subunit on the expression of functional receptor. We conclude that the inefficient expression of human α9-containing receptors can be improved by modifying 5′UTR of the cRNA encoding the subunit. It is possible that the initiation codon of the original unmodified subunit is in unfavorable form such that the small ribosomal subunit fails to associate with the RNA. By including the 5′UTR of RNA4 of alfalfa mosaic virus, we were able to construct an RNA, which when expressed in X. laevis oocytes, can be used to screen new ligands which bind to the α9* receptor (* denotes possibility of other subunits). The reasons for the poor ability of α9 receptors (both rat and human) to be expressed in the mammalian cells still remain to be explored.
Transcriptional and translational mechanisms are likely involved in regulation of human and rat α9 subunit expression in native tissues. In the rat adrenal medulla expression levels of α9, α3, and α7 subunits were determined by quantitative PCR [19] and the level was lowest for the α9 subunit. However, the same study showed that transcription of α9, but not α3 and α7 subunits, is upregulated in response to stress. Regulation of transcription and translation of nAChRs may also be relevant in the context of smoking. The concentration of nicotine in active smoker plasma can be 100 nM to 1 µM. Chronic exposure to nicotine leads to activation and desensitization of nAChR subtypes including α4β2 and α7. As a result, the level of expression of α4β2 nAChRs is increased in the brain [101]. Smoking is also associated with carcinogenesis, and nicotine-derived metabolites NNK and NNN are considered carcinogenic in lung, breast, and bladder cancers. α9 receptors mediate cell proliferation of breast cancer cells, and increased α9 nAChR subunit mRNA levels were observed in breast tumor tissues [102]. Moreover, α9-nAChR mRNA expression was higher in advanced-stage tumors. It was also shown that nicotine upregulates the mRNA as well as protein level for α9 receptors in breast tumor tissue [102]. The mechanism by which nicotine treatment leads to this upregulation remains elusive. α9 subunit expression seems to be important for cell proliferation, therefore, the mechanisms, whether transcriptional or translational, that control subunit expression might open exciting new avenues for control of tumorigenesis.
Our findings suggest the involvement of 5′-untranslated region in the efficient expression of human α9-containing receptors in oocytes. It remains to be investigated whether 5′untransated region contributes to the regulation of translation of α9 subunit in vivo.
Acknowledgments
We thank A. B. Elgoyhen (University of Buenos Aires, Argentina) and L. Lustig (University of California San Francisco, San Francisco, CA) for generously providing clones for rat and human receptors, respectively.
Funding Statement
The work was supported by National Institute of Health Grants GM48677 and GM103801. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Skok VI (2002) Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci 97: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Wang N, Orr-Urtreger A, Korczyn AD (2002) The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice. Prog Neurobiol 68: 341–360. [DOI] [PubMed] [Google Scholar]
- 3. Kuffler SW, Yoshikami D (1975) The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol 251: 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuffler SW, Yoshikami D (1975) The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol 244: 703–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marchi M, Grilli M (2010) Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Prog Neurobiol 92: 105–111. [DOI] [PubMed] [Google Scholar]
- 6. Robinson L, Platt B, Riedel G (2011) Involvement of the cholinergic system in conditioning and perceptual memory. Behav Brain Res 221: 443–465. [DOI] [PubMed] [Google Scholar]
- 7. Levin ED (2012) alpha7-Nicotinic receptors and cognition. Curr Drug Targets 13: 602–606. [DOI] [PubMed] [Google Scholar]
- 8. Quik M, McIntosh JM (2006) Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson's disease therapy. J Pharmacol Exp Ther 316: 481–489. [DOI] [PubMed] [Google Scholar]
- 9. Parri HR, Hernandez CM, Dineley KT (2011) Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer's disease. Biochem Pharmacol 82: 931–942. [DOI] [PubMed] [Google Scholar]
- 10. Dani JA, Jenson D, Broussard JI, De Biasi M (2011) Neurophysiology of Nicotine Addiction. J Addict Res Ther S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Biasi M, Dani JA (2011) Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34: 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, et al. (2002) Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 59: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 13. Leonard S, Freedman R (2006) Genetics of chromosome 15q13-q14 in schizophrenia. Biol Psychiatry 60: 115–122. [DOI] [PubMed] [Google Scholar]
- 14. Millar NS, Gotti C (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56: 237–246. [DOI] [PubMed] [Google Scholar]
- 15. Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S (1994) Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79: 705–715. [DOI] [PubMed] [Google Scholar]
- 16. Lips KS, Pfeil U, Kummer W (2002) Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience 115: 1–5. [DOI] [PubMed] [Google Scholar]
- 17. Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, et al. (2005) Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26: 735–746. [DOI] [PubMed] [Google Scholar]
- 18. Kummer W, Lips KS, Pfeil U (2008) The epithelial cholinergic system of the airways. Histochem Cell Biol 130: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colomer C, Olivos-Ore LA, Vincent A, McIntosh JM, Artalejo AR, et al. (2010) Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity. J Neurosci 30: 6732–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB (2000) Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology 39: 2515–2524. [DOI] [PubMed] [Google Scholar]
- 21. Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, et al. (2002) A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol 61: 150–159. [DOI] [PubMed] [Google Scholar]
- 22. Vincler M (2005) Neuronal nicotinic receptors as targets for novel analgesics. Expert Opin Investig Drugs 14: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 23. Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, et al. (2006) Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A 103: 17880–17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincler M, McIntosh JM (2007) Targeting the alpha9alpha10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin Ther Targets 11: 891–897. [DOI] [PubMed] [Google Scholar]
- 25. McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M (2009) Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem Pharmacol 78: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holtman JR, Dwoskin LP, Dowell C, Wala EP, Zhang Z, et al. (2011) The novel small molecule alpha9alpha10 nicotinic acetylcholine receptor antagonist ZZ-204G is analgesic. Eur J Pharmacol 670: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wala EP, Crooks PA, McIntosh JM, Holtman JR Jr (2012) Novel Small Molecule alpha9alpha10 Nicotinic Receptor Antagonist Prevents and Reverses Chemotherapy-Evoked Neuropathic Pain in Rats. Anesth Analg 115: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen VT, Ndoye A, Grando SA (2000) Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by Pemphigus vulgaris autoimmunity. Am J Pathol 157: 1377–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, et al. (1999) Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93–103. [DOI] [PubMed] [Google Scholar]
- 30. Maison SF, Luebke AE, Liberman MC, Zuo J (2002) Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci 22: 10838–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, et al. (2001) alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 98: 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA (2001) Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10). Genomics 73: 272–283. [DOI] [PubMed] [Google Scholar]
- 33. Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-Kaplan M, et al. (1996) Stable expression, pharmacologic properties and regulation of the human neuronal nicotinic acetylcholine alpha 4 beta 2 receptor. J Pharmacol Exp Ther 276: 289–297. [PubMed] [Google Scholar]
- 34. Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, et al. (2003) Characterization of human alpha 4 beta 2-nicotinic acetylcholine receptors stably and heterologously expressed in native nicotinic receptor-null SH-EP1 human epithelial cells. Mol Pharmacol 64: 1283–1294. [DOI] [PubMed] [Google Scholar]
- 35. Deneris ES, Connolly J, Boulter J, Wada E, Wada K, et al. (1988) Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron 1: 45–54. [DOI] [PubMed] [Google Scholar]
- 36. Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, et al. (1990) A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron 5: 847–856. [DOI] [PubMed] [Google Scholar]
- 37. Gotti C, Hanke W, Maury K, Moretti M, Ballivet M, et al. (1994) Pharmacology and biophysical properties of alpha 7 and alpha 7-alpha 8 alpha-bungarotoxin receptor subtypes immunopurified from the chick optic lobe. Eur J Neurosci 6: 1281–1291. [DOI] [PubMed] [Google Scholar]
- 38. van Kleef RG, Vijverberg HP, Westerink RH (2008) Selective inhibition of human heteromeric alpha9alpha10 nicotinic acetylcholine receptors at a low agonist concentration by low concentrations of ototoxic organic solvents. Toxicol In Vitro 22: 1568–1572. [DOI] [PubMed] [Google Scholar]
- 39. Halai R, Clark RJ, Nevin ST, Jensen JE, Adams DJ, et al. (2009) Scanning mutagenesis of alpha-conotoxin Vc1.1 reveals residues crucial for activity at the alpha9alpha10 nicotinic acetylcholine receptor. J Biol Chem 284: 20275–20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, et al. (2005) RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol 68: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 41. Fucile S, Sucapane A, Eusebi F (2006) Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium 39: 349–355. [DOI] [PubMed] [Google Scholar]
- 42. Gray NK, Hentze MW (1994) Regulation of protein synthesis by mRNA structure. Mol Biol Rep 19: 195–200. [DOI] [PubMed] [Google Scholar]
- 43. Jacobs E, Mills JD, Janitz M (2012) The role of RNA structure in posttranscriptional regulation of gene expression. J Genet Genomics 39: 535–543. [DOI] [PubMed] [Google Scholar]
- 44. Kozak M (2005) Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 361: 13–37. [DOI] [PubMed] [Google Scholar]
- 45. Nomura M, Ohsuye K, Mizuno A, Sakuragawa Y, Tanaka S (1984) Influence of messenger RNA secondary structure on translation efficiency. Nucleic Acids Symp Ser 173–176. [PubMed] [Google Scholar]
- 46. Kozak M (1990) Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc Natl Acad Sci U S A 87: 8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pesole G, Grillo G, Larizza A, Liuni S (2000) The untranslated regions of eukaryotic mRNAs: structure, function, evolution and bioinformatic tools for their analysis. Brief Bioinform 1: 236–249. [DOI] [PubMed] [Google Scholar]
- 48. Araujo PR, Yoon K, Ko D, Smith AD, Qiao M, et al. (2012) Before It Gets Started: Regulating Translation at the 5′ UTR. Comp Funct Genomics 2012: 475731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilkie GS, Dickson KS, Gray NK (2003) Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem Sci 28: 182–188. [DOI] [PubMed] [Google Scholar]
- 50. Falcone D, Andrews DW (1991) Both the 5′ untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro. Mol Cell Biol 11: 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liman ER, Tytgat J, Hess P (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861–871. [DOI] [PubMed] [Google Scholar]
- 52. Kruys V, Wathelet M, Poupart P, Contreras R, Fiers W, et al. (1987) The 3′ untranslated region of the human interferon-beta mRNA has an inhibitory effect on translation. Proc Natl Acad Sci U S A 84: 6030–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, et al. (1996) A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem 271: 7522–7528. [DOI] [PubMed] [Google Scholar]
- 54. Kariko K, Kuo A, Barnathan E (1999) Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene Ther 6: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 55. Fan Q, Treder K, Miller WA (2012) Untranslated regions of diverse plant viral RNAs vary greatly in translation enhancement efficiency. BMC Biotechnol 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TM (1987) The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res 15: 3257–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gallie DR, Walbot V (1992) Identification of the motifs within the tobacco mosaic virus 5′-leader responsible for enhancing translation. Nucleic Acids Res 20: 4631–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TM (1987) A comparison of eukaryotic viral 5′-leader sequences as enhancers of mRNA expression in vivo. Nucleic Acids Res 15: 8693–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jobling SA, Gehrke L (1987) Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature 325: 622–625. [DOI] [PubMed] [Google Scholar]
- 60. Sleat DE, Gallie DR, Jefferson RA, Bevan MW, Turner PC, et al. (1987) Characterisation of the 5′-leader sequence of tobacco mosaic virus RNA as a general enhancer of translation in vitro. Gene 60: 217–225. [DOI] [PubMed] [Google Scholar]
- 61. Sumikawa K, Houghton M, Emtage JS, Richards BM, Barnard EA (1981) Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature 292: 862–864. [DOI] [PubMed] [Google Scholar]
- 62. Barnard EA, Miledi R, Sumikawa K (1982) Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci 215: 241–246. [DOI] [PubMed] [Google Scholar]
- 63. Papke RL, Boulter J, Patrick J, Heinemann S (1989) Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron 3: 589–596. [DOI] [PubMed] [Google Scholar]
- 64. Duvoisin RM, Deneris ES, Patrick J, Heinemann S (1989) The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron 3: 487–496. [DOI] [PubMed] [Google Scholar]
- 65. Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J (2000) Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 39: 2570–2590. [DOI] [PubMed] [Google Scholar]
- 66. Kozak M (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44: 283–292. [DOI] [PubMed] [Google Scholar]
- 67. Soreq H, Ben-Aziz R, Prody CA, Seidman S, Gnatt A, et al. (1990) Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A 87: 9688–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morel N, Massoulie J (2000) Comparative expression of homologous proteins. A novel mode of transcriptional regulation by the coding sequence folding compatibility of chimeras. J Biol Chem 275: 7304–7312. [DOI] [PubMed] [Google Scholar]
- 69. Lammich S, Schobel S, Zimmer AK, Lichtenthaler SF, Haass C (2004) Expression of the Alzheimer protease BACE1 is suppressed via its 5′-untranslated region. EMBO Rep 5: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Niesler B, Flohr T, Nothen MM, Fischer C, Rietschel M, et al. (2001) Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 11: 471–475. [DOI] [PubMed] [Google Scholar]
- 71. Mihailovich M, Thermann R, Grohovaz F, Hentze MW, Zacchetti D (2007) Complex translational regulation of BACE1 involves upstream AUGs and stimulatory elements within the 5′ untranslated region. Nucleic Acids Res 35: 2975–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Venkatachalan SP, Bushman JD, Mercado JL, Sancar F, Christopherson KR, et al. (2007) Optimized expression vector for ion channel studies in Xenopus oocytes and mammalian cells using alfalfa mosaic virus. Pflugers Arch 454: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gallie DR (2002) The 5′-leader of tobacco mosaic virus promotes translation through enhanced recruitment of eIF4F. Nucleic Acids Res 30: 3401–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brederode FT, Koper-Zwarthoff EC, Bol JF (1980) Complete nucleotide sequence of alfalfa mosaic virus RNA 4. Nucleic Acids Res 8: 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gehrke L, Auron PE, Quigley GJ, Rich A, Sonenberg N (1983) 5′-Conformation of capped alfalfa mosaic virus ribonucleic acid 4 may reflect its independence of the cap structure or of cap-binding protein for efficient translation. Biochemistry 22: 5157–5164. [DOI] [PubMed] [Google Scholar]
- 76. Chatterjee S, Pal JK (2009) Role of 5′- and 3′-untranslated regions of mRNAs in human diseases. Biol Cell 101: 251–262. [DOI] [PubMed] [Google Scholar]
- 77. Hudder A, Werner R (2000) Analysis of a Charcot-Marie-Tooth disease mutation reveals an essential internal ribosome entry site element in the connexin-32 gene. J Biol Chem 275: 34586–34591. [DOI] [PubMed] [Google Scholar]
- 78. Brown CY, Mize GJ, Pineda M, George DL, Morris DR (1999) Role of two upstream open reading frames in the translational control of oncogene mdm2. Oncogene 18: 5631–5637. [DOI] [PubMed] [Google Scholar]
- 79. Jin X, Turcott E, Englehardt S, Mize GJ, Morris DR (2003) The two upstream open reading frames of oncogene mdm2 have different translational regulatory properties. J Biol Chem 278: 25716–25721. [DOI] [PubMed] [Google Scholar]
- 80. Morris DR, Geballe AP (2000) Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol 20: 8635–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Soreq H, Nudel U, Salomon R, Revel M, Littauer UZ (1974) In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol 88: 233–245. [DOI] [PubMed] [Google Scholar]
- 82. Huez G, Marbaix G, Hubert E, Leclercq M, Nudel U, et al. (1974) Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A 71: 3143–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pascale A, Govoni S (2012) The complex world of post-transcriptional mechanisms: is their deregulation a common link for diseases? Focus on ELAV-like RNA-binding proteins. Cell Mol Life Sci 69: 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alexander JK, Govind AP, Drisdel RC, Blanton MP, Vallejo Y, et al. (2010) Palmitoylation of nicotinic acetylcholine receptors. J Mol Neurosci 40: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Swope SL, Moss SJ, Raymond LA, Huganir RL (1999) Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res 33: 49–78. [DOI] [PubMed] [Google Scholar]
- 86. daCosta CJ, Kaiser DE, Baenziger JE (2005) Role of glycosylation and membrane environment in nicotinic acetylcholine receptor stability. Biophys J 88: 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nishizaki T (2003) N-glycosylation sites on the nicotinic ACh receptor subunits regulate receptor channel desensitization and conductance. Brain Res Mol Brain Res 114: 172–176. [DOI] [PubMed] [Google Scholar]
- 88. Chang W, Gelman MS, Prives JM (1997) Calnexin-dependent enhancement of nicotinic acetylcholine receptor assembly and surface expression. J Biol Chem 272: 28925–28932. [DOI] [PubMed] [Google Scholar]
- 89. Wanamaker CP, Green WN (2007) Endoplasmic reticulum chaperones stabilize nicotinic receptor subunits and regulate receptor assembly. J Biol Chem 282: 31113–31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, et al. (2011) Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137: 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, et al. (2009) Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kuryatov A, Luo J, Cooper J, Lindstrom J (2005) Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 68: 1839–1851. [DOI] [PubMed] [Google Scholar]
- 93. Valles AS, Barrantes FJ (2012) Chaperoning alpha7 neuronal nicotinic acetylcholine receptors. Biochim Biophys Acta 1818: 718–729. [DOI] [PubMed] [Google Scholar]
- 94. Valles AS, Roccamo AM, Barrantes FJ (2009) Ric-3 chaperone-mediated stable cell-surface expression of the neuronal alpha7 nicotinic acetylcholine receptor in mammalian cells. Acta Pharmacol Sin 30: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, et al. (2005) Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J Biol Chem 280: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 96. Roncarati R, Seredenina T, Jow B, Jow F, Papini S, et al. (2008) Functional properties of alpha7 nicotinic acetylcholine receptors co-expressed with RIC-3 in a stable recombinant CHO-K1 cell line. Assay Drug Dev Technol 6: 181–193. [DOI] [PubMed] [Google Scholar]
- 97. Cheng A, McDonald NA, Connolly CN (2005) Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J Biol Chem 280: 22502–22507. [DOI] [PubMed] [Google Scholar]
- 98. Nie L, Song H, Chen MF, Chiamvimonvat N, Beisel KW, et al. (2004) Cloning and expression of a small-conductance Ca(2+)-activated K+ channel from the mouse cochlea: coexpression with alpha9/alpha10 acetylcholine receptors. J Neurophysiol 91: 1536–1544. [DOI] [PubMed] [Google Scholar]
- 99. Baker ER, Zwart R, Sher E, Millar NS (2004) Pharmacological properties of alpha 9 alpha 10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol Pharmacol 65: 453–460. [DOI] [PubMed] [Google Scholar]
- 100. Osman AA, Schrader AD, Hawkes AJ, Akil O, Bergeron A, et al. (2008) Muscle-like nicotinic receptor accessory molecules in sensory hair cells of the inner ear. Mol Cell Neurosci 38: 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ (1992) A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41: 31–37. [PubMed] [Google Scholar]
- 102. Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, et al. (2010) Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst 102: 1322–1335. [DOI] [PubMed] [Google Scholar]