Abstract
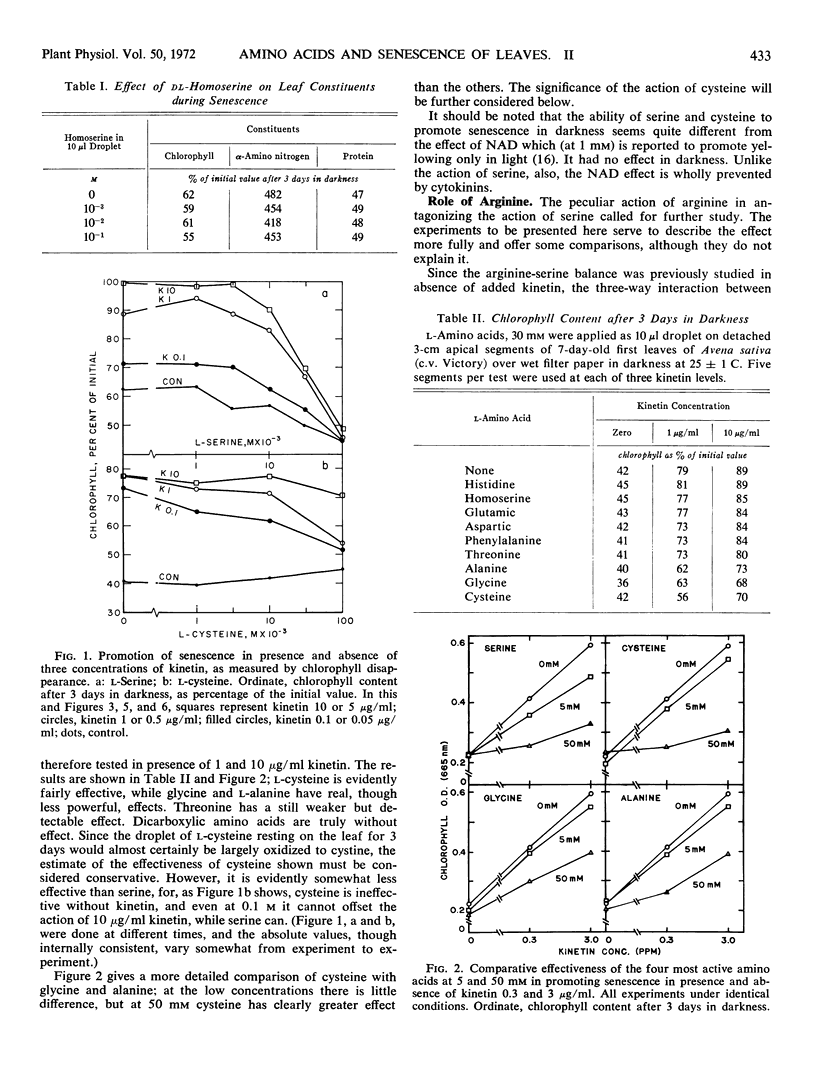
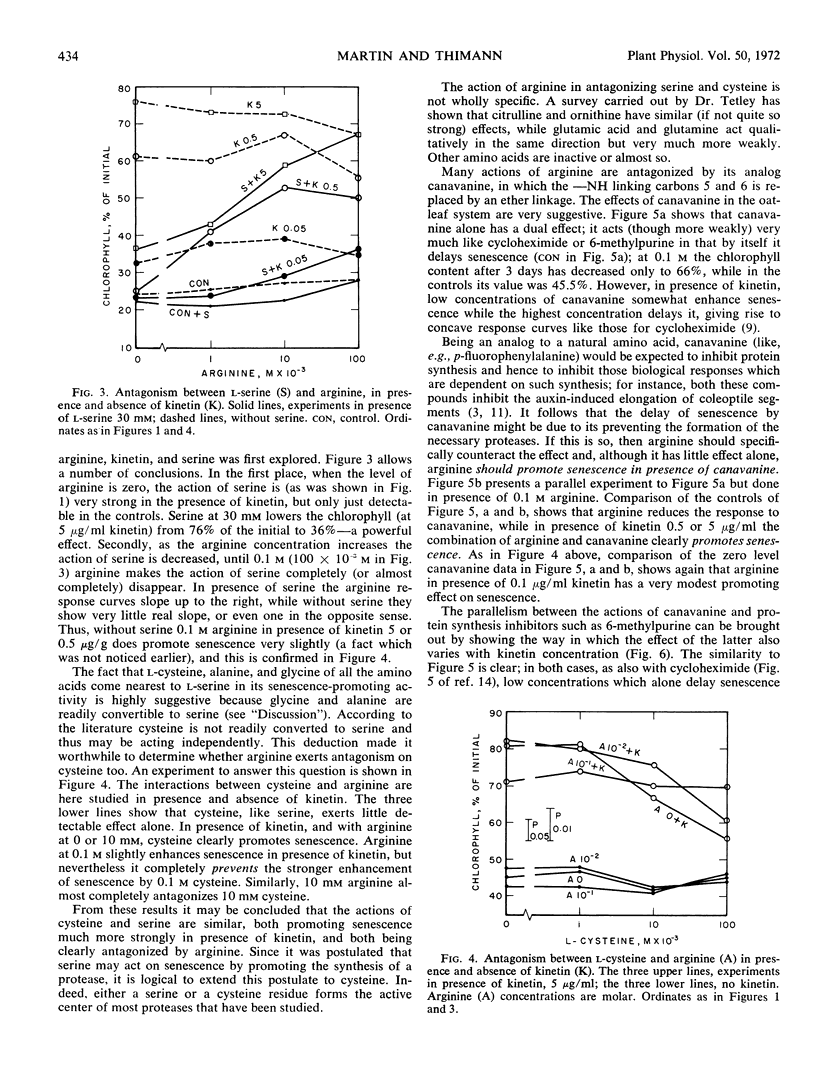
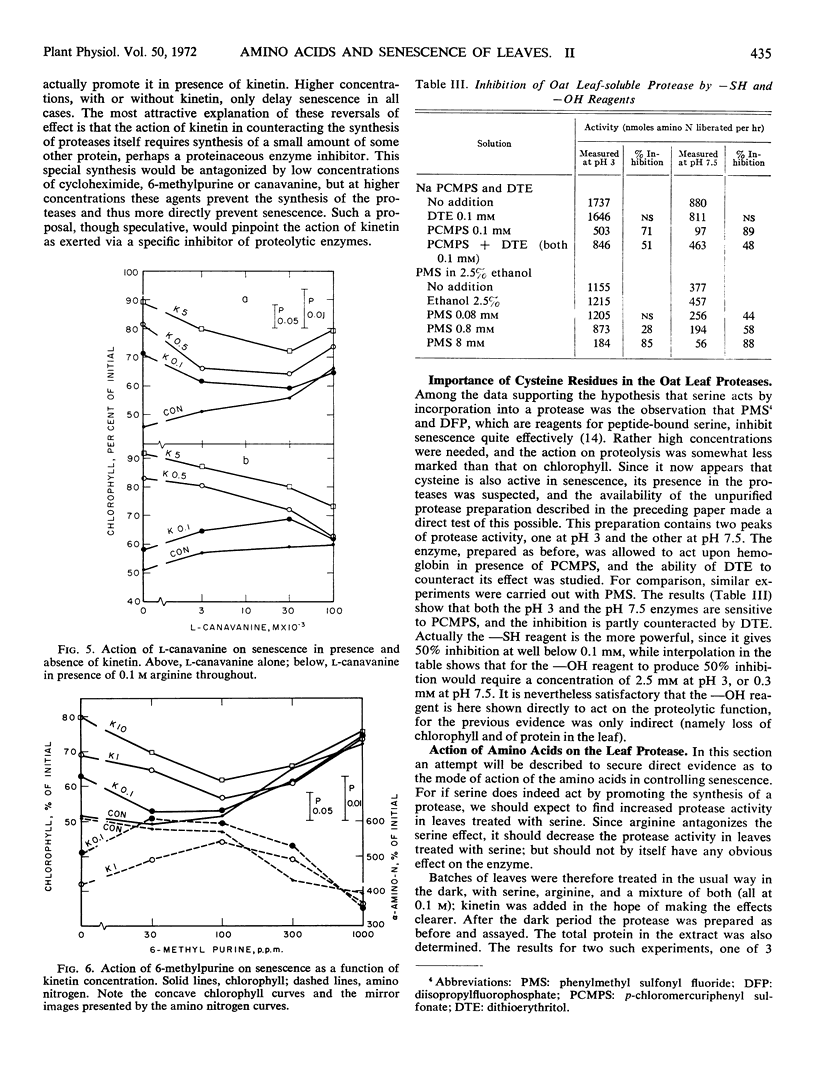
When the first leaf of the oat (Avena sativa) seedling is detached and placed in the dark, yellowing and proteolysis take place rapidly. The earlier finding that d-serine promotes this process has led to a further study of the controlling roles of several amino acids. Since the action of serine was found to be more powerful in presence of kinetin than alone, the effects of other amino acids have been restudied in presence of kinetin. Cysteine emerges as a moderately strong promotor of senescence, with glycine and alanine having definite but weaker effects. The serine effect is antagonized by arginine, especially in presence of kinetin, and so is the cysteine effect. This is considered to indicate that these two amino acids act in the same way. The antagonism exerted by arginine is in turn antagonized by canavanine. The protease activities at two pH regions which increase in the oat leaf during senescence react to both p-chlorimercuri-phenylsulfonate and to phenylmethyl-sulfonyl fluoride, and thus may contain both SH and OH groups. The amounts of both these enzyme activities formed in the leaf during 3 days in the dark are increased over 50% by pretreatment with serine, and this increase is very largely prevented by arginine. The amounts of soluble proteins left in the leaf vary as expected in the opposite sense. It is deduced that control of the new formation of proteases plays an important part in senescence. A suggestion is made as to the mechanism of control of senescence in leaves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAKLEY R. L. The interconversion of serine and glycine: participation of pyridoxal phosphate. Biochem J. 1955 Oct;61(2):315–323. doi: 10.1042/bj0610315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham V. L., Bryan J. K. Synergistic effects of metabolically related amino acids on the growth of a multicellular plant. Plant Physiol. 1969 Nov;44(11):1601–1608. doi: 10.1104/pp.44.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest J. C., Wightman F. Metabolism of amino acids in plants. I. Changes in the soluble amino acid fractions of bushbean seedlings (Phaseolus vulgaris L.) and the development of transaminase activity. Can J Biochem. 1971 Jun;49(6):709–720. doi: 10.1139/o71-101. [DOI] [PubMed] [Google Scholar]
- Kende H. KINETINLIKE FACTORS IN THE ROOT EXUDATE OF SUNFLOWERS. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1302–1307. doi: 10.1073/pnas.53.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka H., Thimann K. V. Antagonisms between Kinetin and Amino Acids: Experiments on the Mode of Action of Cytokinins. Plant Physiol. 1970 Aug;46(2):212–220. doi: 10.1104/pp.46.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. C., Torrey J. G. Cytokinins in seedling roots of pea. Plant Physiol. 1972 Feb;49(2):155–160. doi: 10.1104/pp.49.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG D., BURRIS R. H. CARBON METABOLISM OF C-14-LABELED AMINO ACIDS IN WHEAT LEAVES. 3. FURTHER STUDIES ON THE ROLE OF SERINE IN GLYCINE METABOLISM. Plant Physiol. 1965 May;40:415–418. doi: 10.1104/pp.40.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Vaadia Y. Kinetin-like activity in root apices of sunflower plants. Life Sci. 1965 Jul;4(13):1323–1326. doi: 10.1016/0024-3205(65)90084-6. [DOI] [PubMed] [Google Scholar]



