Abstract
The actin cytoskeleton–regulating GTPase Rac1 is required for insulin-stimulated GLUT4 translocation in cultured muscle cells. However, involvement of Rac1 and its downstream signaling in glucose transport in insulin-sensitive and insulin-resistant mature skeletal muscle has not previously been investigated. We hypothesized that Rac1 and its downstream target, p21-activated kinase (PAK), are regulators of insulin-stimulated glucose uptake in mouse and human skeletal muscle and are dysregulated in insulin-resistant states. Muscle-specific inducible Rac1 knockout (KO) mice and pharmacological inhibition of Rac1 were used to determine whether Rac1 regulates insulin-stimulated glucose transport in mature skeletal muscle. Furthermore, Rac1 and PAK1 expression and signaling were investigated in muscle of insulin-resistant mice and humans. Inhibition and KO of Rac1 decreased insulin-stimulated glucose transport in mouse soleus and extensor digitorum longus muscles ex vivo. Rac1 KO mice showed decreased insulin and glucose tolerance and trended toward higher plasma insulin concentrations after intraperitoneal glucose injection. Rac1 protein expression and insulin-stimulated PAKThr423 phosphorylation were decreased in muscles of high fat–fed mice. In humans, insulin-stimulated PAK activation was decreased in both acute insulin-resistant (intralipid infusion) and chronic insulin-resistant states (obesity and diabetes). These findings show that Rac1 is a regulator of insulin-stimulated glucose uptake and a novel candidate involved in skeletal muscle insulin resistance.
Insulin increases glucose uptake in skeletal muscle by stimulating translocation of GLUT4 from intracellular compartments to the plasma membrane and transverse tubuli (1–4). Skeletal muscle accounts for up to 75% of postprandial glucose disposal in humans (5), and normal insulin action in skeletal muscle is therefore crucial for maintaining glucose homeostasis.
The Rho family GTPase Rac1 has been shown to regulate insulin-stimulated GLUT4 translocation and glucose transport in cultured muscle cells (6–8). Insulin activates Rac1, which leads to reorganization of the cortical actin cytoskeleton. Downregulation of Rac1 by small interfering RNA prevents this process (7,9) and also abolishes insulin-stimulated glucose uptake and GLUT4 translocation in L6 myoblasts (6,7). In addition, expression of a constitutively active Rac1 increases GLUT4 translocation to the same level seen after maximal insulin stimulation in this cell line (6).
Even though cultured muscle cell lines are powerful tools to understand intracellular mechanisms, they differ from mature skeletal muscle in the expression and reliance of various proteins in the regulation of insulin-stimulated glucose uptake (10). Cultured muscle myoblasts, although able to fuse into myotubes, do not reach the same end-stage differentiation (e.g., do not have cross striations and do not develop transverse tubules) as muscles in vivo and therefore do not fully mature into a system that mimics fully developed skeletal muscles (11,12). Furthermore, the location, expression, and insulin-stimulated GLUT4 translocation are very different in cultured cells compared with mature muscle and may not require the same trafficking steps (2,3,13,14). As a consequence, it is imperative to investigate the role of Rac1 in insulin-stimulated glucose uptake in fully matured skeletal muscle in order to understand its role in glucose metabolism. Furthermore, the importance of skeletal muscle Rac1 on whole-body glucose homeostasis has not been determined.
Rac1 activates p21-activated kinase (PAK) by facilitating autophosphorylation of PAK on threonine 423 (p-PAKThr423), and this pathway induces actin remodeling of the actin cytoskeleton (15). Accordingly, disruption of the actin cytoskeleton by actin-depolymerizing agents, such as latrunculin B, inhibits insulin-stimulated GLUT4 translocation in L6 myotubes (16,17). Dynamic rearrangement of the actin cytoskeleton is thus necessary for insulin to induce GLUT4 translocation in these cells (18).
These findings also apply to mature skeletal muscle, since latrunculin B inhibits insulin-stimulated glucose uptake in rat epitrochlearis muscle (19). Furthermore, Ueda et al. (20) recently showed that Rac1 is activated by insulin in mouse skeletal muscle and that insulin-stimulated GLUT4 translocation is decreased in muscle-specific Rac1 knockout (KO) mice. PAK1 was also recently shown to be implicated in the regulation of insulin-stimulated GLUT4 translocation in mouse skeletal muscle (21). However, GLUT4 translocation does not always mimic glucose uptake, and numerous studies have reported experimental conditions where GLUT4 translocation and transport can be clearly dissociated (22–27), suggesting that GLUT4 translocation is not always an adequate measure of the functional end point, glucose uptake. Thus, the involvement of Rac1 and its downstream signaling in insulin-stimulated glucose uptake in mature skeletal muscle has not yet been investigated, and Rac1-dependent signaling has not been characterized in animal or human models of insulin resistance.
A decreased ability to rearrange the cortical actin cytoskeleton in response to insulin has been proposed as a central defect in insulin-resistant muscle cells (28–30). Although exposure to insulin resistance–inducing agents decreased Rac1 activation and GLUT4 translocation (7), only small reductions in Akt signaling were observed in L6 myotubes (8). It is therefore possible that Rac1 is a major regulator of glucose uptake in mature skeletal muscle, and its dysregulation might contribute to the phenotype of muscular insulin resistance and type 2 diabetes (T2D). In the current study, we hypothesized that activation of Rac1 and its downstream target, PAK, is crucial for insulin-induced glucose uptake in mature skeletal muscle and for maintaining whole-body glucose homeostasis. We further hypothesized that Rac1-dependent signaling is downregulated in insulin-resistant states.
RESEARCH DESIGN AND METHODS
Animals.
Female C57BL/6 mice (Taconic, Silkeborg, Denmark) 12–16 weeks of age were used for all inhibitor incubation experiments.
Tetracycline-inducible muscle-specific Rac1 KO mice.
Whole-body Rac1-floxed mice (31) were crossed with mice containing a tetracycline-controlled transactivator coupled to the human skeletal actin promoter, which drives the muscle-specific expression of the Cre recombinase (32). Mice were back-crossed until the fifth generation (96.9% congenial) on a C57BL/6 background. Transgenic mice (14–18 weeks of age) were littermates from breeding of heterozygous Cre- and Rac1-floxed transgenic mice. Rac1 KO was obtained by adding doxycycline (1 g/L; Sigma-Aldrich) to the drinking water of homozygous Rac1-floxed and heterozygous Cre mice for 21 days, after which it was switched to normal tap water for 4–6 weeks before experiments (doxycycline treatment was initiated at 5–7 weeks of age). Homozygous Rac1-floxed and Cre hemizygous mice treated only with tap water served as wild type (WT) controls.
All animals were maintained on a 10:14-h light-dark cycle and received standard rodent chow diet (Altromin no. 1324; Chr. Pedersen, Ringsted, Denmark) and water ad libitum. All experiments were approved by the Danish Animal Experimental Inspectorate and complied with the European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes.
Glucose and insulin tolerance tests.
Mice were fasted from 07:00 a.m. for 6 and 2 h before intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT), respectively. Glucose (2 g) or 0.5 units insulin per kg body weight was administered for GTT and ITT, respectively. During GTT and ITT, blood was collected from the tail vein at time points 0, 20, 40, 60, 90, and 120 min, and blood glucose was determined in duplicate. For measurements of plasma insulin, blood was sampled from female wild-type (WT) and Rac1 KO mice at time points 0 and 20 min after intraperitoneal glucose injection and analyzed in triplicate (80-INSRTU-E10; ALPCO Diagnostics). No sex differences were observed (n = 11–13, compared by Student t test); therefore, results for male and female mice were pooled for GTT and ITT as stated.
Metabolic measurements.
Indirect calorimetry was performed in a 16-chamber indirect calorimetry system (PhenoMaster; TSE Systems, Frankfurt, Germany). Male mice were individually housed and placed in the chambers for 8 days, of which the last days were recorded and data collected for analysis. Oxygen consumption rate (VO2; mL/h/kg), respiratory exchange ratio (RER), total activity (beam breaks), and food intake were simultaneously measured for each mouse.
Muscle incubations.
Soleus and extensor digitorum longus (EDL) muscles were dissected from 4 h–fasted anesthetized female mice (6 mg pentobarbital sodium/100 g body weight) and suspended at resting tension (4–5 mN) in incubation chambers (Multi Myograph System; Danish Myo Technology, Aarhus, Denmark) in Krebs-Ringer-Henseleit (KRH) buffer with 2 mmol/L pyruvate and 8 mmol/L mannitol at 30°C, as described previously (33). For inhibitor experiments, the muscles were preincubated for 40 min in KRH buffer with Rac1 Inhibitor II (10 μmol/L; Calbiochem), or latrunculin B (1 and 10 μmol/L; Sigma-Aldrich), or a corresponding amount of DMSO as vehicle control. After the preincubation period, the muscles were stimulated with insulin (60 nmol/L) for 30 min.
2-Deoxyglucose uptake.
2-Deoxyglucose (2DG) uptake was measured with 1 mmol/L 2DG for 10 min during the last 10 min of the insulin stimulation period using [3H]2DG and [14C]mannitol tracers as described previously (33).
Immunohistochemistry on single fibers.
Basal and insulin-stimulated EDL or WT and Rac1 KO tibialis anterior muscles were immersed in ice-cold KRH buffer containing procaine hydrochloride (35 mg/10 mL) for 5 min. Right after, muscles were fixed by immersion in 2% formaldehyde and 0.15% picric acid for 30 min at room temperature, followed by 3.5 h at 4°C. After isolation of a minimum of 30 single muscle fibers per muscle, immunostaining against Rac1 was performed as previously described (34). In brief, isolated muscle fibers were incubated overnight with an anti-Rac1 antibody (Novus Biologicals) in immunobuffer containing 2% saponin, and after three washes with immunobuffer, single muscle fibers were incubated with a secondary antibody conjugated with Alexa Fluor488 or 568 (Invitrogen, Paisley, U.K.). Negative controls were performed by staining without primary antibody. Muscle fibers were mounted in Vectashield mounting medium. Confocal images were collected with a Zeiss LSM710 microscope, through a 63×/1.40 oil DIC Plan-Apochromat objective at 20°C. Images were analyzed using Zeiss Zen (2010) software.
Intralipid study.
Eight healthy men (28 ± 2 years of age, BMI = 22.3 ± 0.5 kg/m2) were included in the study. Samples of the vastus lateralis (VL) muscle were analyzed from subjects in which clinical characteristics and insulin sensitivity were described in a recently published study (35) approved by the Copenhagen Ethics Committee (KF01261127) and carried out in accordance with the Declaration of Helsinki II (1996). All subjects underwent two experimental trials only differing by infusion of either saline or intralipid for 7 h. After infusion of saline or intralipid, subjects underwent a 120-min hyperinsulinemic-euglycemic clamp (plasma insulin concentration of 100 µU/mL). Biopsies were obtained from VL muscles 30 min before the clamp and 30 and 120 min into the clamp. Intralipid infusion decreased glucose clearance by 30% compared with saline infusion (see reference 35 for further details).
Study on T2D patients.
Eight to ten normal glucose-tolerant (NGT) (BMI = 24 ± 1 kg/m2), obese NGT (BMI = 33 ± 1 kg/m2), and obese T2D (BMI = 33 ± 1 kg/m2) subjects underwent euglycemic-hyperinsulinemic clamp (plasma insulin concentration of 58 µU/mL) combined with muscle biopsies, which has been published elsewhere (36).
PAK1 expression.
For determination of PAK1 protein expression in skeletal muscle with different muscle fiber type composition, eight healthy, young, normal-weight (26 ± 2 years of age, 85 ± 2 kg, 184 ± 2 cm) males volunteered to participate. The study was approved by the Copenhagen Ethics Committee (HKF277313) and was carried out in accordance with the Declaration of Helsinki II (1996). After an overnight fast, muscle biopsies were taken under local anesthesia from the soleus, VL, and gastrocnemius muscles. Muscle fiber type composition and activation of several kinases in muscle during exercise in these subjects have been reported elsewhere (37).
All subjects were given oral and printed information on the study designs and risks and gave written, informed consent.
Muscle analyses.
Immediately after insulin stimulation, mouse and human muscle tissue was quickly frozen in liquid nitrogen and stored at –80°C. For Western blotting, tissue was homogenized two times for 30 s at 30 Hz using a TissueLyser II (Qiagen, Manheim, Germany) in either ice-cold Rac1 buffer (commercial Rac1 buffer from Cytoskeleton Inc.) or 50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 20 mmol/L sodium pyrophosphate, 20 mmol/L β-glycerophosphate, 10 mmol/L NaF, 2 mmol/L sodium orthovanadate, 2 mmol/L EDTA, 1% NP-40, 10% glycerol, 2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 10 µg/mL leupeptin, 10 µg/mL aprotinin, and 3 mmol/L benzamidine. After end-over-end rotation for 30 min, lysate supernatants were collected by centrifugation (12,000g) for 20 min at 4°C.
Immunoblotting.
Lysate protein concentrations were measured using the bicinchoninic acid (BCA) method using BSA standards (Pierce) and BCA assay reagents (Pierce). Total protein and phosphorylation levels of relevant proteins were determined by standard immunoblotting techniques loading equal amounts of protein. The primary antibodies used were p-AktSer473/Thr308, GLUT4, phospho-Akt substrate (PAS), actin, PAK1, p-PAKThr423 (Cell Signaling Technology), GLUT1 (Abcam), and Rac1 (Cytoskeleton). Polyvinylidene difluoride membranes (Immobilon Transfer Membrane; Millipore) were blocked in TBS-Tween 20 containing 2% skim milk or 5% BSA protein for 30 min at room temperature. Membranes were incubated with primary antibodies overnight at 4°C, followed by incubation with horseradish peroxidase–conjugated secondary antibody for 1 h at room temperature. Bands were visualized using an Eastman Kodak Image Station 2000MM or Bio-Rad ChemiDocTM MP Imaging System and enhanced chemiluminescence (ECL+; Amersham Biosciences).
Rac1 activity assay in muscle samples.
Frozen solei, EDL, and VL muscles were pulverized and homogenized in Rac1 homogenization buffer at 30 Hz for 30 s using a TissueLyser II. Lysates were generated by centrifuging the homogenate for 2 min at 10,000g. Rac1-GTP loading was measured in the supernatant using a commercially available Rac1 activation assay kit (BK 126; Cytoskeleton Inc.). In short, lysates (100–200 µg protein) were immediately loaded onto wells coated with the Rac1/cdc42 binding domain (RBD) domain of PAK and incubated on a shaker at 4°C for 30 min. Bound Rac1 was detected colorimetrically using specific antibodies toward Rac1, as described by the manufacturer. To test the activation profile of the GTPase-linked immunosorbent assay (GLISA), Rac1-GTP binding was analyzed in lysates from L6 myoblasts (grown and treated as previously described) (15) incubated with either GDP (inactivates Rac1) or GTP-ϒ S (maximally activates Rac1). Basal and insulin-stimulated L6 myoblasts were also analyzed on the same plate to compare the Rac1-GTP binding of inactive versus maximally activated Rac1 to a physiological response known to activate Rac1 (Supplementary Fig. 1). These data show that a substantial pool of Rac1 is not activated by maximal insulin stimulation in L6 myoblasts and that the assay is sensitive enough to detect levels of activated Rac1 four times higher than observed in response to insulin.
Statistical analyses.
Results are shown as means ± SEM. Statistical testing was performed using paired Student t tests or one- or two-way ANOVA (repeated or nonrepeated measurements) as appropriate. Tukey post hoc test was performed when ANOVA revealed significant interaction. Statistical evaluation was performed using Sigmaplot 11.0. The significance level was set at P < 0.05.
RESULTS
Rac1 and PAK are activated in response to insulin in mouse and human skeletal muscle.
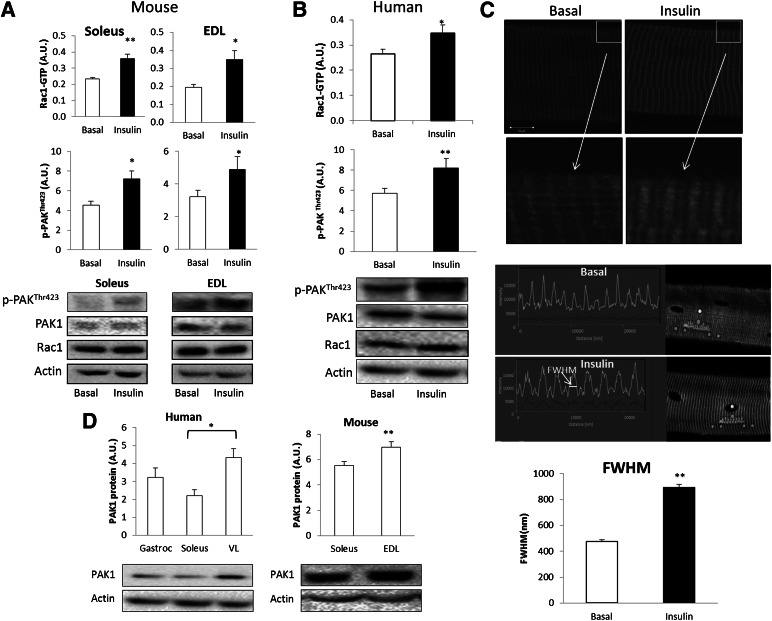
In mice, insulin stimulation increased Rac1-GTP binding by 20 and 25% in incubated soleus and EDL muscles, respectively (Fig. 1A). In addition, phosphorylation of Rac1’s downstream target, PAK (p-PAKThr423), increased by 20% in both muscles compared with basal. Likewise, Rac1-GTP binding as well as p-PAKThr423 increased by 20 and 30%, respectively, after a hyperinsulinemic-euglycemic clamp, in human VL muscle (Fig. 1B). Furthermore, we show insulin-induced redistribution of Rac1 in the myofilaments (Fig. 1C). In isolated mouse EDL single muscle fibers, Rac1 presents a striated pattern of localization. In response to insulin, the thickness of the Rac1-positive striations, measured by determining the full width at half maximum, increased by 90%. Skeletal muscle Rac1 expression has been described previously (38), but expression of PAK1 in muscles of various fiber type compositions has not previously been investigated. PAK1 protein abundance was 15% higher in EDL compared with soleus in mice (Fig. 1D). In humans, PAK1 protein expression was 45% higher in VL compared with soleus (Fig. 1D).
FIG. 1.
A: Rac1-GTP binding and p-PAK1Thr423 in isolated incubated mouse soleus and EDL muscle (n = 8). B: Rac1-GTP loading and p-PAK1Thr423 in human VL muscle prior to and after a 2-h hyperinsulinemic-euglycemic clamp (n = 8). C: Representative images of Rac1 localization in mouse EDL single fibers ± 10 min 60 nmol/L ex vivo insulin stimulation and bar graph showing mean ± SEM. Arrows indicate magnification of the highlighted area in the top panel. Full width at half maximum (FWHM) of the striated Rac1 staining (n = 4). D: Bar graphs showing protein expression of PAK1 in human (n = 8) and mouse (n = 24) skeletal muscle of different fiber type composition. Statistical significance between basal and insulin or between muscles: *P < 0.05; **P < 0.01. Values are mean ± SEM. A.U., arbitrary units.
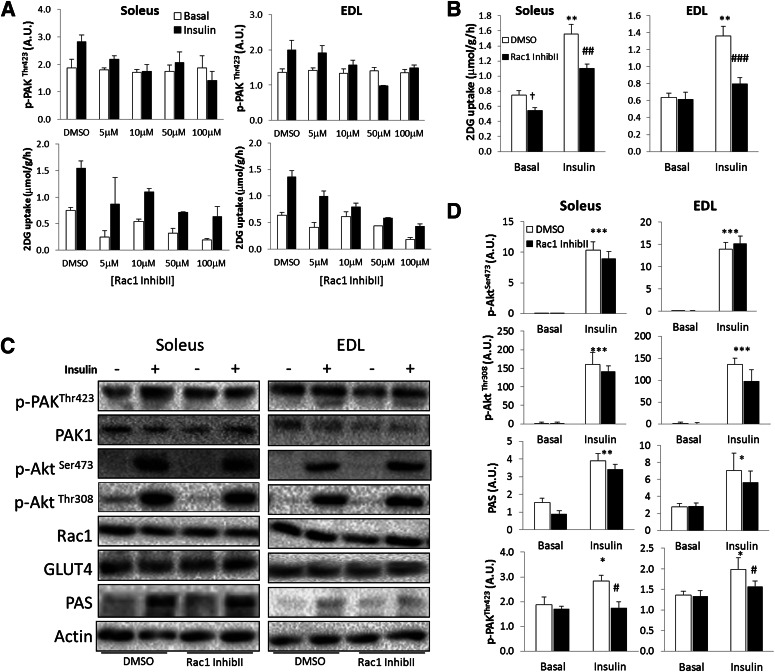
Insulin-stimulated glucose uptake is decreased by pharmacological inhibition of Rac1.
To test whether Rac1 is a regulator of insulin-induced glucose uptake, mouse soleus and EDL muscles were incubated in the presence or absence of insulin and Rac1 Inhibitor II. The concentration used was the lowest necessary to inhibit insulin-stimulated Rac1 activation and glucose uptake as found in pilot experiments (Fig. 2A). The Rac1 Inhibitor II decreased insulin-stimulated glucose uptake in soleus and EDL by 50 and 90%, respectively (Fig. 2B). While p-PAKThr423 was prevented, the Rac1 Inhibitor II did not affect insulin signaling of Akt or PAS (at 150–160 kDa) (Fig. 2C and D). These data suggest that Rac1 is a regulator of insulin-stimulated glucose uptake in mature skeletal muscle independently of Akt signaling.
FIG. 2.
A: Bar graphs showing dose response of Rac1 Inhibitor II on insulin-stimulated p-PAKThr423 and 2DG uptake in soleus and EDL muscles. B: Insulin-stimulated (60 nmol/L, 30 min) 2DG transport in isolated incubated soleus and EDL muscles ± 10 μmol/L Rac1 Inhibitor II (InhibII) or a corresponding amount of DMSO, 40 min preincubation (n = 5–6). C: Bar graphs showing quantifications of p-PAKThr423, p-Akt Ser473, p-AktThr308, and PAS in soleus and EDL ± 10 μmol/L Rac1 Inhibitor II (n = 5–6). D: Representative blots showing insulin-stimulated signaling in soleus and EDL muscle ± 10 μmol/L Rac1 Inhibitor II. Statistically significant effects of the inhibitor on insulin-stimulated 2DG transport and signaling: #P < 0.05; ##P < 0.01; ###P < 0.001. Statistical significance between basal and insulin: *P < 0.05; **P < 0.01; ***P < 0.001. Main effect of inhibitor, †P < 0.05. Values are mean ± SEM.
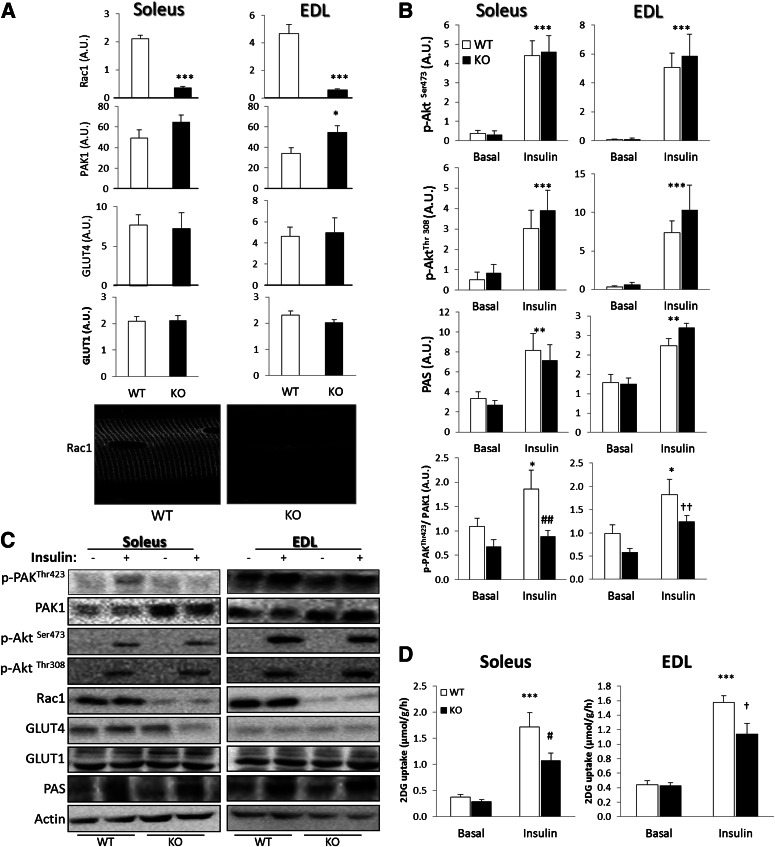
Insulin-stimulated glucose uptake is decreased in muscles of Rac1 KO mice.
After doxycycline treatment of inducible muscle-specific Rac1 KO mice, Rac1 protein content was decreased by 85 and 90% in soleus and EDL, respectively, compared with control (untreated WT) mice (Fig. 3A). Whereas GLUT4, GLUT1, and actin protein content was not affected, PAK1 protein expression was increased 20% in soleus and 40% in EDL from Rac1 KO mice compared with floxed mice (Fig. 3A). Insulin-stimulated AktSer473/Thr308 phosphorylation and PAS signaling were similar between genotypes (Fig. 3B and C). In contrast, insulin-stimulated p-PAKThr423 was prevented in soleus and decreased by 70% in EDL in the KO mice. To investigate the functional significance of the Rac1 signaling pathway, insulin-induced glucose uptake was measured in isolated soleus and EDL muscles from Rac1 KO and WT control mice. Insulin-stimulated 2DG uptake was decreased by 50% in soleus and 40% in EDL muscle (Fig. 3D), implicating Rac1-dependent signaling in the regulation of insulin-stimulated glucose transport in skeletal muscle.
FIG. 3.
A: Rac1, PAK1, GLUT4, and GLUT1 protein expression in soleus and EDL muscles of Rac1 KO mice after doxycycline treatment. Bottom: Single fiber image of WT and Rac1 KO tibialis anterior muscle. B: Bar graphs showing SEM of insulin-induced p-AktSer473, p-AktThr308, p-PAKThr423, and p-PAS in KO and WT in soleus and EDL muscles. C: Representative blots of insulin-induced p-AktSer473, p-AktThr308, p-PAKThr423, and p-PAS and total PAK1, Rac1, GLUT4, GLUT1, and actin in KO and WT in soleus and EDL muscles. D: Insulin-stimulated (60 nmol/L, 30 min) 2DG transport in isolated incubated soleus and EDL muscles from WT and Rac1 KO mice (n = 8). Statistically significant differences between basal and insulin (panels B and D), or WT and Rac1 KO (panel A). *P < 0.05; **P < 0.01; ***P < 0.001. Effect of genotype on insulin-stimulated 2DG transport: #P < 0.05; ##P < 0.01. Main effect of genotype: †P < 0.05; ††P < 0.01. Values are mean ± SEM. A.U., arbitrary units.
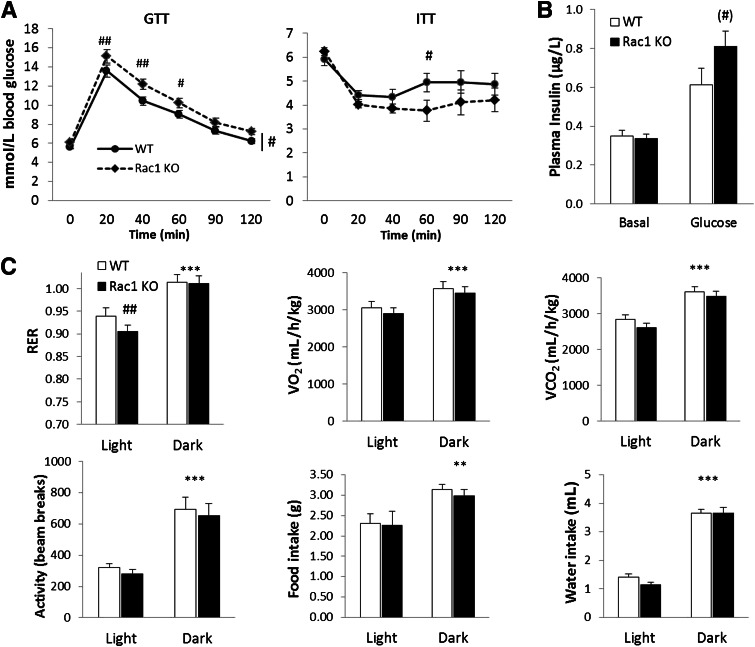
Muscle-specific Rac1 KO mice have altered whole-body glucose metabolism.
Since we observed a decreased insulin-stimulated glucose uptake in the isolated muscles of the Rac1 KO mice, we investigated whether muscle-specific KO of Rac1 affected whole-body glucose and insulin tolerance. Glucose tolerance evaluated during an intraperitoneal GTT was significantly decreased in the Rac1 KO mice compared with controls (Fig. 4A). In addition, glucose-stimulated plasma insulin concentration trended higher, although not statistically significantly (P = 0.07), in the Rac1 KO mice compared with WT at the 20-min time point (Fig. 4B). Blood glucose concentrations decreased more in the WT mice during an intraperitoneal ITT, suggesting that the Rac1 KO mice also have decreased response to insulin (Fig. 4A). We also observed a decreased RER during light hours in Rac1 KO mice compared with WT, suggesting a lower glucose oxidation (Fig. 4C). Between the genotypes, no differences in VCO2, VO2, food and water intake, or physical activity were observed.
FIG. 4.
A: Intraperitoneal GTT (male and female mice, n = 28–31) and ITT (male and female mice, n = 15–16) on Rac1 KO mice compared with WT. B: Plasma insulin concentrations before and after 20-min intraperitoneal glucose injection (female mice, n = 8). C: RER, VO2, VCO2, food and water intake, and activity level during dark and light hours (male and female mice, n = 14). Statistical significance between day and night: **P < 0.01; ***P < 0.001. Effect of genotype: (#)P < 0.1; #P < 0.05; ##P < 0.01. Values are mean ± SEM.
Insulin-induced glucose transport in both soleus and EDL muscle relies on an intact actin cytoskeleton.
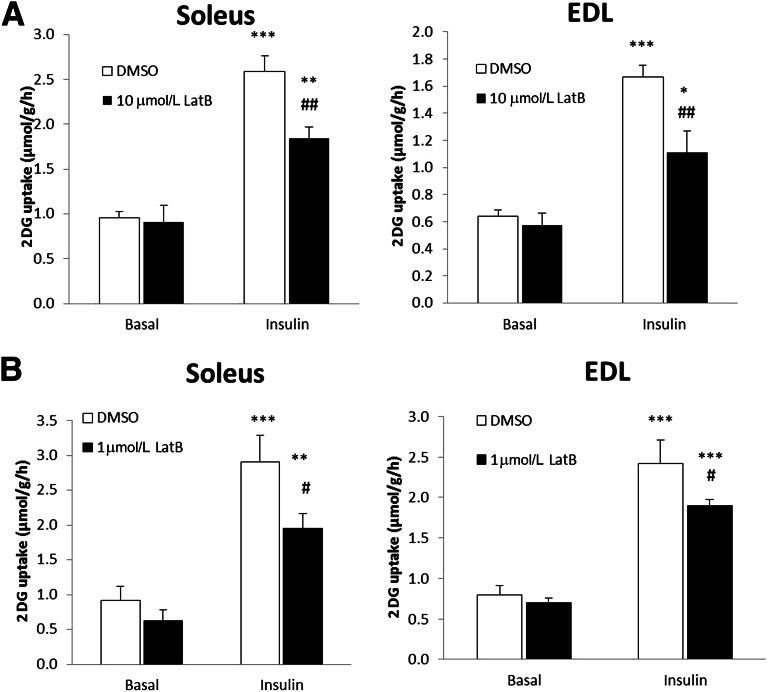
Rac1 is a major regulator of actin remodeling, and dynamic actin rearrangement is required for GLUT4 translocation in response to insulin in muscle cell culture (7,18). Since we found that inhibition of Rac1 decreased insulin-stimulated glucose uptake, we hypothesized that this might be due to impaired Rac1-dependent regulation of the actin cytoskeleton. At concentrations that have previously been found to inhibit insulin-stimulated glucose uptake in rat skeletal muscle (19), the actin-depolymerizing agent latrunculin B decreased insulin-induced 2DG uptake by 40 and 30% in soleus and EDL, respectively (Fig. 5A). However, in other studies, we have found that this concentration inhibited force production during electrical stimulation of muscle by ∼50% (38). High concentrations of latrunculin B could therefore affect sarcomere organization, which could lead to indirect effects on glucose transport. Therefore, soleus and EDL muscles were also incubated with one-tenth of the previously applied concentration. This concentration did not inhibit force production (and hence is deemed unlikely to affect contractile actin filaments) (38), yet inhibited insulin-stimulated glucose uptake in soleus by 30% and EDL by 20% (Fig. 5B). These results suggest that an intact actin cytoskeleton is necessary for both slow- and fast-twitch fibers to increase glucose transport in response to insulin.
FIG. 5.
A: Insulin-stimulated (60 nmol/L, 30 min) 2DG transport in isolated incubated soleus and EDL muscles ± 10 μmol/L latrunculin B (LatB) or a corresponding amount of DMSO, 40 min preincubation. B: Insulin-stimulated (60 nmol/L, 30 min) 2DG transport in soleus and EDL ± 1 μmol/L LatB, 40 min preincubation. Statistically significant effects of the inhibitor on insulin-stimulated 2DG transport: #P < 0.05; ##P < 0.001. Statistical significance between basal and insulin: **P < 0.01; ***P < 0.001 (n = 8). Values are mean ± SEM.
High-fat feeding induces insulin resistance and decreases Rac1 expression and insulin-induced PAK signaling.
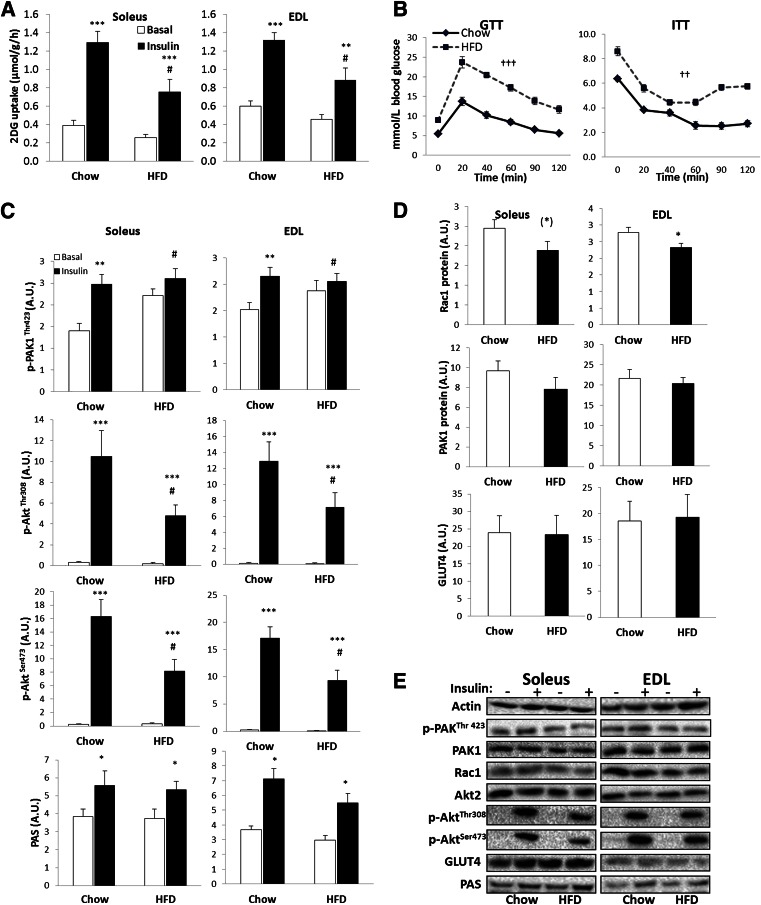
To investigate if Rac1 signaling is dysregulated in insulin-resistant states, we investigated the effect of 14 weeks of high-fat feeding (65% fat) on Rac1 signaling in mice. High-fat diet (HFD) induced a significant decrease in glucose transport in both soleus and EDL (Fig. 6A) and significantly impaired glucose and insulin tolerance (Fig. 6B) compared with mice fed chow. Insulin-stimulated Akt phosphorylation was decreased, and p-PAKThr423 in response to insulin was completely prevented, in both soleus and EDL muscles of mice on an HFD compared with chow due to an increased basal p-PAKThr423 (Fig. 6C). In addition, Rac1 protein expression tended to be decreased by 20% in soleus and was significantly reduced by 15% in EDL after HFD. GLUT4, Akt, and PAK1 expression was unaffected by diet intervention (Fig. 6D and E).
FIG. 6.
A: Insulin-stimulated (60 nmol/L, 30 min) 2DG transport in isolated incubated mouse soleus and EDL after 14 weeks of chow or 65% HFD, 40 min preincubation. B: Intraperitoneal GTT and ITT on chow- vs. HFD-fed mice. C: Bar graph showing mean ± SEM of p-PAKThr423, p-AktSer473, p-AktThr308, and PAS. D: Bar graph showing mean ± SEM of total PAK1, Rac1, and GLUT4 protein expression in soleus and EDL muscles from chow- or HFD-fed mice. E: Representative Western blots of phosphorylated and total proteins (n = 7–9). Statistically significant effects of HFD on insulin-stimulated 2DG transport or signaling: #P < 0.05. Statistically significant effect of HFD on blood glucose during GTT or ITT: ††P < 0.01; †††P < 0.001. Statistical significance between basal and insulin conditions: (*)P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001. Values are mean ± SEM. A.U., arbitrary units.
PAK signaling is decreased by intralipid infusion, obesity, and T2D in human skeletal muscle.
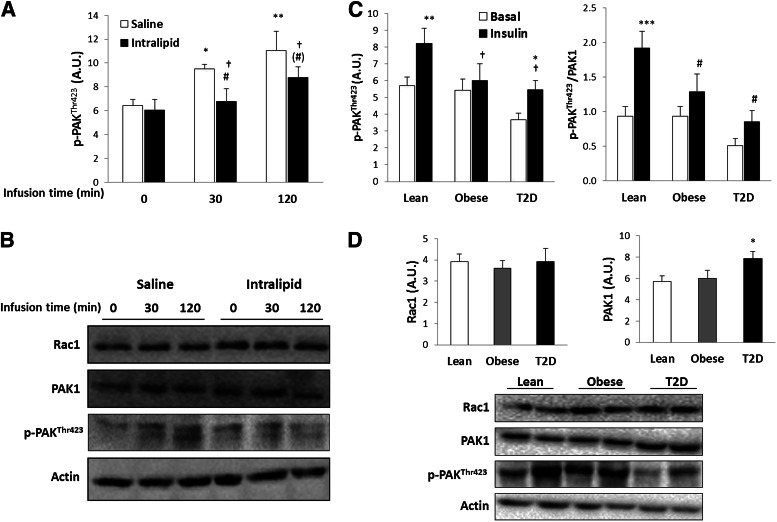
In humans, intralipid infusion induces skeletal muscle insulin resistance during a hyperinsulinemic-euglycemic clamp (35). Insulin increased p-PAKThr423 by 30% after 30 min and by 40% after 120-min clamp (Fig. 7A). Intralipid infusion completely abolished insulin-stimulated p-PAKThr423 after 30 min of the clamp and decreased it by 50% after 120 min (Fig. 7A). No change in protein expression of Rac1 or PAK1 was observed (Fig. 7B). This identifies PAK as a novel protein dysregulated in human muscle in insulin-resistant states.
FIG. 7.
A: p-PAKThr423 in VL before and after a 2-h hyperinsulinemic-euglycemic clamp after infusion of either saline or intralipid for 7 h (n = 8). B: Representative Western blots of p-PAKThr423, total Rac1, PAK1, and actin protein content (n = 8). C: p-PAKThr423 before and after a 2-h hyperinsulinemic-euglycemic clamp in VL of lean NGT healthy (Lean) and obese NGT (Obese) subjects and patients with T2D (n = 9–10). Left: p-PAKThr423 related to total amount of loaded protein. Right: p-PAKThr423 related to total amount of PAK1. D: Rac1 and PAK1 expression in VL of lean NGT healthy and obese NGT subjects and patients with T2D and representative Western blots of p-PAKThr423, total Rac1, PAK1, and actin protein content (n = 9–10). Statistically significant effects of intralipid infusion on insulin-stimulated signaling: (#)P = 0.06; #P < 0.05. Statistical significance between basal and insulin conditions: *P < 0.05; **P < 0.01; ***P < 0.001. Main effect of intralipid (A) and obesity/T2D (C) is indicated by †. Values are mean ± SEM. A.U., arbitrary units.
To investigate whether this dysregulation also applied to more chronic states of insulin resistance, such as in human T2D subjects, we analyzed p-PAKThr423 and total Rac1 and PAK1 expression in three groups: 1) lean NGT, 2) obese NGT, and 3) T2D subjects (36). Obese and diabetic subjects had a lower insulin-stimulated p-PAKThr423 compared with control subjects after a hyperinsulinemic-euglycemic clamp (Fig. 7C). Basal p-PAKThr423 was decreased by 48% in the diabetic subjects compared with control and obese subjects. Insulin-stimulated p-PAKThr423 was reduced by 33% in the obese group and by 66% in the T2D group, compared with the control subjects, suggesting a severe dysfunction in Rac1-dependent signaling toward PAK. Whereas Rac1 protein expression was not altered, we observed that PAK1 protein expression was 27% higher in T2D compared with lean and obese individuals (Fig. 7D). Because of this, PAK phosphorylation is shown both related to total protein and related to total PAK1 in the figure (Fig. 7C). These findings show that Rac1-dependent signaling is impaired in insulin-resistant human skeletal muscle.
DISCUSSION
We show that Rac1 and its downstream target, PAK, are activated by insulin in human and murine skeletal muscle and are important regulators of insulin-stimulated glucose uptake in skeletal muscle and thereby whole-body glucose homeostasis. In addition, we demonstrate that Rac1 and PAK signaling are impaired in insulin-resistant muscles in mice and humans.
In the current study, pharmacological inhibition of Rac1 decreased insulin-stimulated 2DG transport in mouse soleus and EDL muscles, without effects on Akt or AS160 signaling, suggesting that Rac1 is not upstream of Akt or AS160. The impaired glucose transport was therefore likely due to specific inhibition of Rac1 and PAK signaling. To further validate our results, we used an inducible muscle-specific Rac1 KO mouse. Even though ∼15% of Rac1 was still present in the KO muscles, insulin-stimulated 2DG uptake in both soleus and EDL was significantly decreased compared with WT. The Rac1 Inhibitor II decreased insulin-stimulated glucose transport more potently in EDL than in soleus, whereas Rac1 KO had the largest effect in soleus. This discrepancy could be due to the higher PAK1 expression observed in the EDL of the Rac1 KO mice, which may have compensated for the decreased Rac1 signaling. Consequently, phosphorylation of PAKThr423 was completely blocked by Rac1 Inhibitor II in both muscles, whereas PAKThr423 phosphorylation was only completely prevented in the soleus of the Rac1 KO mice. Our results are in agreement with data obtained from muscle cell cultures. In L6 myoblasts, downregulation of Rac1 by small interfering RNA decreases insulin-stimulated GLUT4 translocation and glucose uptake (7,15). Although insulin-stimulated GLUT4 translocation has recently been reported to be dependent upon Rac1 in mouse muscle, actual glucose transport was not reported (20). Thus, the involvement of Rac1 in regulation of glucose uptake in murine and human skeletal muscle has not previously been demonstrated, and we here provide novel evidence to show that Rac1 is a regulator of insulin-stimulated glucose uptake in mature skeletal muscle. Interestingly, we have recently shown that Rac1 is also an important regulator of contraction-induced glucose uptake in muscle (38). Thus, Rac1 is a convergence point between the contraction- and the insulin-stimulated molecular pathway leading to increased glucose uptake.
Interestingly, we observed that high-fat feeding of mice (which induced severe insulin resistance) decreased Rac1 protein expression and completely abolished the increase in insulin-stimulated p-PAKThr423 in soleus and EDL muscles. We further showed that intralipid infusion, which induced whole-body and muscular insulin resistance (35), significantly impaired p-PAKThr423 during a hyperinsulinemic-euglycemic clamp in humans. Importantly, obese and T2D humans also displayed impaired PAK signaling during a hyperinsulinemic-euglycemic clamp. Decreased Rac1-dependent signaling thus seems common to many models of skeletal muscle insulin resistance. This is in agreement with a previous study in L6 myotubes where insulin resistance–inducing agents decreased insulin-stimulated Rac1 activity (7). Insulin-resistant states have been associated with decreased filamentous actin (e.g., the actin cytoskeleton) (29), which, when replenished, restored insulin sensitivity (30). These findings suggest that Rac1 and downstream signaling to the actin cytoskeleton constitute an important dysfunctional pathway in insulin-resistant states.
In agreement with the observed reductions in ex vivo insulin-stimulated glucose uptake, the Rac1 KO mice exhibited altered glucose and insulin tolerance in vivo. In addition, we observed a trend toward higher insulin secretion in response to intraperitoneal glucose injection. It is therefore likely that the Rac1 KO mice compensate for the decreased muscle insulin sensitivity by increasing insulin secretion. Decreased skeletal muscle glucose metabolism in the Rac1 KO mice is supported by a lower RER compared with WT, suggesting that Rac1 KO mice rely more on fat oxidation. Higher fat utilization could potentially lead to increased production of reactive oxygen species, mitochondrial dysfunction, and/or ceramide accumulation, which could further exacerbate muscle insulin resistance (39,40). The effect of Rac1 KO on these parameters should be further investigated.
Our finding that insulin activates Rac1 is in agreement with previous studies in muscle cells showing that Rac1-GTP binding increases in response to insulin (6,7,15,20). Interestingly, we also observed in single muscle fibers that Rac1 relocalizes in response to insulin. We speculate that the observed movement of Rac1 reflects the well-described activation-dependent release of Rac1 from its cytosolic RhoGDI-associated pool to allow membrane association (41). We observed increased PAK1 expression in the Rac1 KO mice as well as in the T2D subjects, both models in which we found decreased Rac1 signaling to PAK. Rac1 might thus be a negative regulator of PAK1 protein expression. Taken together, these data strongly implicate Rac1 and downstream signaling as important regulators of skeletal muscle glucose uptake.
Rac1-induced activation of PAK and downstream signaling are believed to be necessary for actin cytoskeleton reorganization and regulate GLUT4 translocation in response to insulin in cultured muscle cells (42). A requirement for an intact actin cytoskeleton to regulate glucose transport has previously been shown in rat epitrochlearis muscle (19). However, since the expression patterns of Rac1 and PAK1 protein are different in red and white muscle fibers, we investigated if soleus (90% oxidative fibers) and EDL (66% glycolytic fibers) (43) equally rely on the actin cytoskeleton to take up glucose. This was indeed the case since latrunculin B–induced actin cytoskeleton depolymerization decreased insulin-stimulated glucose uptake in both muscles. Even though we here do not provide a direct link between insulin-stimulated activation of Rac1 and rearrangement of the actin cytoskeleton, it is likely that KO or inhibition of Rac1 affects actin cytoskeleton dynamics, which in turn inhibits insulin-stimulated glucose uptake. This needs further investigation.
In conclusion, our data show that Rac1 and downstream signaling are activated in skeletal muscle by insulin in humans as well as mice. We also provide evidence to suggest that Rac1, possibly via its effects on the actin cytoskeleton, is an important regulator of insulin-stimulated glucose uptake in mouse muscle. Furthermore, our findings show that Rac1-dependent signaling is decreased in insulin-resistant states in mice and humans. Dysregulation of Rac1 and the actin cytoskeleton in skeletal muscle might be novel molecular candidates contributing to the phenotype of insulin resistance and T2D.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Danish Diabetes Association, the Danish Medical Research Council, the Novo Nordisk Foundation, The Lundbeck Foundation, and the UNIK–Food Fitness and Pharma. K.H. was supported by grants from the Danish Medical Research Council and the Novo Nordisk Foundation (Excellence Project 2009).
No potential conflicts of interest relevant to this article were reported.
L.S. designed the study, conducted the experiments, performed the laboratory analysis, and wrote the manuscript. T.E.J. and E.A.R. designed the study and conducted the experiments. M.K., K.H., B.K., J.W., C.P., and P.S. conducted the experiments. All authors commented on and approved the final version of the manuscript. E.A.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the skilled technical assistance of Betina Bolmgren (University of Copenhagen). Rac1-floxed mice were a kind gift from Cord Brakebusch (University of Copenhagen). Tetracycline-activated Cre mice were a kind gift from Ashley Monks (University of Toronto Mississauga). The authors thank Dr. Klaus Levin (Odense University Hospital) for muscle biopsies in the study on patients with diabetes.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1148/-/DC1.
See accompanying commentary, p. 1831.
REFERENCES
- 1.Marette A, Burdett E, Douen A, Vranic M, Klip A. Insulin induces the translocation of GLUT4 from a unique intracellular organelle to transverse tubules in rat skeletal muscle. Diabetes 1992;41:1562–1569 [DOI] [PubMed] [Google Scholar]
- 2.Lauritzen HP, Ploug T, Prats C, Tavaré JM, Galbo H. Imaging of insulin signaling in skeletal muscle of living mice shows major role of T-tubules. Diabetes 2006;55:1300–1306 [DOI] [PubMed] [Google Scholar]
- 3.Lauritzen HP, Reynet C, Schjerling P, et al. Gene gun bombardment-mediated expression and translocation of EGFP-tagged GLUT4 in skeletal muscle fibres in vivo. Pflugers Arch 2002;444:710–721 [DOI] [PubMed] [Google Scholar]
- 4.James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 1988;333:183–185 [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 1985;76:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda S, Kataoka T, Satoh T. Activation of the small GTPase Rac1 by a specific guanine-nucleotide-exchange factor suffices to induce glucose uptake into skeletal-muscle cells. Biol Cell 2008;100:645–657 [DOI] [PubMed] [Google Scholar]
- 7.JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 2007;56:394–403 [DOI] [PubMed] [Google Scholar]
- 8.Nozaki S, Ueda S, Takenaka N, Kataoka T, Satoh T. Role of RalA downstream of Rac1 in insulin-dependent glucose uptake in muscle cells. Cell Signal 2012;24:2111–2117 [DOI] [PubMed] [Google Scholar]
- 9.Khayat ZA, Tong P, Yaworsky K, Bloch RJ, Klip A. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J Cell Sci 2000;113:279–290 [DOI] [PubMed] [Google Scholar]
- 10.Tondeleir D, Vandamme D, Vandekerckhove J, Ampe C, Lambrechts A. Actin isoform expression patterns during mammalian development and in pathology: insights from mouse models. Cell Motil Cytoskeleton 2009;66:798–815 [DOI] [PubMed] [Google Scholar]
- 11.Ravenscroft G, Nowak KJ, Jackaman C, et al. Dissociated flexor digitorum brevis myofiber culture system—a more mature muscle culture system. Cell Motil Cytoskeleton 2007;64:727–738 [DOI] [PubMed] [Google Scholar]
- 12.Lauritzen HP, Schertzer JD. Measuring GLUT4 translocation in mature muscle fibers. Am J Physiol Endocrinol Metab 2010;299:E169–E179 [DOI] [PubMed] [Google Scholar]
- 13.Lauritzen HP, Ploug T, Ai H, Donsmark M, Prats C, Galbo H. Denervation and high-fat diet reduce insulin signaling in T-tubules in skeletal muscle of living mice. Diabetes 2008;57:13–23 [DOI] [PubMed] [Google Scholar]
- 14.Lizunov VA, Stenkula KG, Lisinski I, et al. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. Am J Physiol Endocrinol Metab 2012;302:E950–E960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol 2004;18:359–372 [DOI] [PubMed] [Google Scholar]
- 16.Liu LZ, Zhao HL, Zuo J, et al. Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell 2006;17:2322–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsakiridis T, Vranic M, Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem 1994;269:29934–29942 [PubMed] [Google Scholar]
- 18.Török D, Patel N, Jebailey L, et al. Insulin but not PDGF relies on actin remodeling and on VAMP2 for GLUT4 translocation in myoblasts. J Cell Sci 2004;117:5447–5455 [DOI] [PubMed] [Google Scholar]
- 19.Brozinick JT, Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem 2004;279:40699–40706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda S, Kitazawa S, Ishida K, et al. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J 2010;24:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem 2011;286:41359–41367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somwar R, Kim DY, Sweeney G, et al. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J 2001;359:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somwar R, Niu W, Kim DY, et al. Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J Biol Chem 2001;276:46079–46087 [DOI] [PubMed] [Google Scholar]
- 24.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem 1999;274:10071–10078 [DOI] [PubMed] [Google Scholar]
- 25.Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab 2008;7:348–356 [DOI] [PubMed] [Google Scholar]
- 26.Funaki M, Benincasa K, Randhawa PK. Peptide rescues GLUT4 recruitment, but not GLUT4 activation, in insulin resistance. Biochem Biophys Res Commun 2007;360:891–896 [DOI] [PubMed] [Google Scholar]
- 27.Funaki M, Randhawa P, Janmey PA. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol Cell Biol 2004;24:7567–7577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest 2001;108:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath EM, Tackett L, McCarthy AM, Raman P, Brozinick JT, Elmendorf JS. Antidiabetogenic effects of chromium mitigate hyperinsulinemia-induced cellular insulin resistance via correction of plasma membrane cholesterol imbalance [retracted in: Mol Endocrinol 2010;24:1308]. Mol Endocrinol 2008;22:937–950 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Habegger KM, Penque BA, Sealls W, et al. Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle. Diabetologia 2012;55:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrostek A, Wu X, Quondamatteo F, et al. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol Cell Biol 2006;26:6957–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao P, Monks DA. A tetracycline-inducible and skeletal muscle-specific Cre recombinase transgenic mouse. Dev Neurobiol 2009;69:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen TE, Rose AJ, Jørgensen SB, et al. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab 2007;292:E1308–E1317 [DOI] [PubMed] [Google Scholar]
- 34.Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol 1998;142:1429–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pehmøller C, Brandt N, Birk JB, et al. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes 2012;61:2743–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Højlund K, Birk JB, Klein DK, et al. Dysregulation of glycogen synthase COOH- and NH2-terminal phosphorylation by insulin in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab 2009;94:4547–4556 [DOI] [PubMed] [Google Scholar]
- 37.Jensen TE, Leutert R, Rasmussen ST, et al. EMG-normalised kinase activation during exercise is higher in human gastrocnemius compared to soleus muscle. PLoS ONE 2012;7:e31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sylow L, Jensen TE, Kleinert M, et al. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes 2013;62:1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 2008;29:381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012;15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cell Signal 2011;23:1546–1554 [DOI] [PubMed] [Google Scholar]
- 42.Chiu TT, Patel N, Shaw AE, Bamburg JR, Klip A. Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol Biol Cell 2010;21:3529–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol 2001;280:C637–C645 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.