Abstract
The estrogen receptor β (ERβ) is emerging as an important player in the physiology of the endocrine pancreas. We evaluated the role and antidiabetic actions of the ERβ selective agonist WAY200070 as an insulinotropic molecule. We demonstrate that WAY200070 enhances glucose-stimulated insulin secretion both in mouse and human islets. In vivo experiments showed that a single administration of WAY200070 leads to an increase in plasma insulin levels with a concomitant improved response to a glucose load. Two-week treatment administration increased glucose-induced insulin release and pancreatic β-cell mass and improved glucose and insulin sensitivity. In addition, streptozotocin-nicotinamide–induced diabetic mice treated with WAY200070 exhibited a significant improvement in plasma insulin levels and glucose tolerance as well as a regeneration of pancreatic β-cell mass. Studies performed in db/db mice demonstrated that this compound restored first-phase insulin secretion and enhanced pancreatic β-cell mass. We conclude that ERβ agonists should be considered as new targets for the treatment of diabetes.
Diabetes has become one of the most challenging health problems on a global scale, with an estimated 285 million people affected by this disease in 2010 (1,2). The most common form of diabetes is type 2 (T2D), which results from the interaction of a subject’s genetic background with the environment. Both insulin resistance and pancreatic β-cell dysfunction contribute importantly to the pathogenesis of this disease; however, T2D develops only when insulin secretion cannot meet the insulin demand (3–5). Therefore, the most effective therapy for T2D should control not only β-cell failure, but also the loss of β-cell mass. Today, although there is an extensive range of oral antidiabetic drugs that differ in their modes of action, none seem to be completely effective (6–9).
Estrogen receptors are emerging as important molecules involved in modulating pancreatic β-cell function. 17β-estradiol (E2) modulates insulin content in an estrogen receptor α (ERα)-dependent manner (10). In addition, the activation of the estrogen receptor β (ERβ) triggers the closure of ATP-sensitive K+ (KATP) channels, enhancing glucose-induced [Ca2+] oscillations and insulin release cooperatively with glucose (11). Selective ERβ agonists, such as diarylpropionitrile (DPN), elicit this rapid phenomenon (1–7 min). The KATP channel–dependent pathway in the pancreatic β-cell is the major trigger for glucose-stimulated insulin secretion (GSIS). Accordingly, the fact that ERβ selective ligands can activate this mechanism raises the possibility that these compounds may behave as rapid insulinotropic agents and, thus, lead to new antidiabetic drugs.
Since the discovery of ERβ in the mid-1990s, intense research efforts continue to focus on the biology of this receptor and on developing and evaluating the use of ERβ-specific agonists in animal models of human disease. Remarkably, some of the ERβ agonists are already under evaluation in clinical studies (12–14). At present, ERβ is a promising novel drug target for the treatment of cancer and multiple sclerosis because of distinct functional characteristics of this estrogen receptor subtype.
Here, we evaluate the action of a selective ERβ agonist (WAY200070) on glucose homeostasis in different animal models of diabetes. We analyze the capacity of this compound to normalize fasting glucose levels, to enhance endogenous insulin secretion, and to regulate β-cell mass. We hypothesize that the use of selective ERβ agonists offers great hope in the treatment of T2D.
RESEARCH DESIGN AND METHODS
Animals.
Adult male C57BL/6 mice aged 3–4 months were used. C57BL/6 (a globally standardized model) and db/db mice were obtained from Harlan Laboratories (Barcelona, Spain). ERβ knockout (BERKO) mice were generated as described in Krege et al. (15) and supplied by Dr. Gustafsson’s laboratory. Streptozotocin-nicotinamide (STZ-NA) diabetic mice were used, which is a model of moderate hyperglycemia combined with the loss of early phase insulin secretion (16,17). WAY200070 (Tocris Cookson Ltd, Bristol, U.K.) was injected intraperitoneally in a volume of 100 μL saline solution.
Islet and islet cell isolation.
Pancreatic islets of Langerhans were isolated by collagenase (Sigma, Madrid, Spain) digestion as previously described (18). Freshly isolated islets were used for calcium and insulin secretion measurements after a 2-h recovery. For experiments using isolated β-cells, islets were dispersed into single cells with trypsin as previously described (19).
Recording intracellular calcium concentration.
Freshly isolated pancreatic islets of Langerhans were loaded with 5 μmol/L Fura-2 acetoxymethyl ester (Molecular Probes, Invitrogen, Barcelona, Spain) for at least 1 h at room temperature. Calcium recordings were obtained as previously described (20).
Insulin secretion measurements.
Groups of five mouse islets were transferred to 400 μL of a buffer solution containing 140 mmol/L NaCl, 4.5 mmol/L KCl, 2.5 mmol/L CaCl2, 1 mmol/L MgCl2, 20 mmol/L HEPES, and the corresponding glucose concentration with final pH of 7.4. Afterward, 100 μL corresponding buffer solution with 5% BSA was added and cooled down for 15 min on ice. The medium was then collected, and insulin was measured in duplicate samples by radioimmunoassay with a Coat-A-Count kit (Siemens, Los Angeles, CA). Protein concentration was measured by the Bradford dye method (21).
Isolated human pancreatic islets from a nondiabetic male were provided by the Nordic Network for Clinical Islet Transplantation (Prof. Olle Korsgren, Uppsala University, Uppsala, Sweden). All procedures were approved by the ethical committees at Uppsala and Lund Universities. The islets were cultured as previously described (22). The secreted insulin was measured with a radioimmunoassay kit (Millipore).
Patch clamp recordings.
KATP channel activity was recorded using standard patch clamp recording procedures from isolated pancreatic β-cells as previously described (22).
Glucose and insulin tolerance test.
For intraperitoneal glucose tolerance tests (IGTTs), animals were fasted overnight for 12 h, and blood samples were taken from the tail vein. Animals were then injected intraperitoneally with 2 g/kg glucose, and blood samples were taken at the indicated intervals. In STZ-NA and db/db mice, the glucose load was 1.5 g/kg.
For intraperitoneal insulin tolerance tests, fed animals were used. Animals were injected intraperitoneally with 0.75 IU/kg soluble insulin. The db/db mice were fasted for 6 h and injected with 1.25 IU/kg soluble insulin. Blood glucose was measured in each sample using an Accu-Chek compact glucometer (Roche, Madrid, Spain).
Immunohistochemistry and β-cell mass.
Pancreas samples were removed and fixed overnight in 4% paraformaldehyde. Subsequently, pancreatic tissue was embedded in paraffin, and sections were prepared. After dehydration, the sections were heated to 100°C in the presence of 10 mmol/L citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase was blocked by incubation for 30 min with a solution of 3% hydrogen peroxidase in 50% methanol. To block nonspecific binding, the sections were incubated in 3% BSA in PBS for 1 h at room temperature. Tissue sections were then stained for β-cells with a rabbit anti-human insulin antibody (1:100) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. The sections were incubated with the secondary antibody biotinylated anti-rabbit IgG (H+L) (Vector Laboratories, Burlingame, CA). The Vectastain ABC kit (Vector Laboratories) was used for the avidin-biotin complex method. Peroxidase activity was visualized with 3,3′-diaminobenzidine (Dako, Barcelona, Spain). The sections were lightly counterstained with hematoxylin. The islet cross-sectional area and the total pancreatic area were measured using MetaMorph Software. At least two sections, separated by 200 μm, were measured per animal.
β-Cell replication.
Mice were injected intraperitoneally with 100 µg/g BrdU 6 h before being killed. Pancreatic tissue was collected, fixed, and processed as just described. Samples were then incubated with antibodies for insulin (1:100 rabbit polyclonal; Santa Cruz Biotechnology, Madrid, Spain) and BrdU (1:100 monoclonal; Dako) overnight at 4°C. After incubation with secondary antibodies, sections were mounted using ProLong Gold Antifade Reagent (Invitrogen).
Measurement of the β-cell proliferation rate in vitro.
Single islets cells were cultured for 48 h in the presence of 10 μmol/L BrdU (Sigma) and the vehicle or WAY200070. They were then fixed for 5 min with 4% paraformaldehyde, washed with PBS, and made permeable with 1% Triton X-100. Nonspecific interactions were blocked with PBS plus 5% normal goat serum for 1 h. Cells were then incubated with the primary antibody rabbit anti-human insulin antibody (1:100) (Santa Cruz Biotechnology, Inc.) overnight at 4°C. As a secondary antibody, goat anti-rabbit Alexa Fluor 546 was used at 1/500 for 1 h at room temperature. After this, cells were fixed again and washed with PBS. The cells were then treated with 2 mol/L HCl for 20 min at 37°C and washed with 0.1 mol/L Na2B4O7 (pH 8.5). To detect BrdU, the mouse anti-BrdU antibody (M0744; Dako) at 1/100 was used overnight at 4°C along with goat anti-mouse Alexa Fluor 488. The nuclei were stained with 1 μmol/L DAPI for 15 min at room temperature.
Plasma analysis.
Insulin was measured by ELISA (Mercodia, Uppsala, Sweden, and Crystal Chem, Downers Grove, IL) and leptin was measured by ELISA (Crystal Chem, Downers Grove, IL). Triglyceride and glycerol levels were measured with the GTO-Trinder Triglycerides assay (Sigma).
Statistical analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using Student t test or one-way ANOVA, as indicated (SigmaStat 3.1 software; Systat Software, Inc., Chicago, IL). P < 0.05 was considered significant.
Study approval.
All animal experiments were performed in accordance with national and institutional guidelines for animal care. The ethical committee of Miguel Hernandez University of Elche (Comisión de Ética en la Investigación Experimental) reviewed and approved the method used (Approval ID Number IB-ARL-002-12).
RESULTS
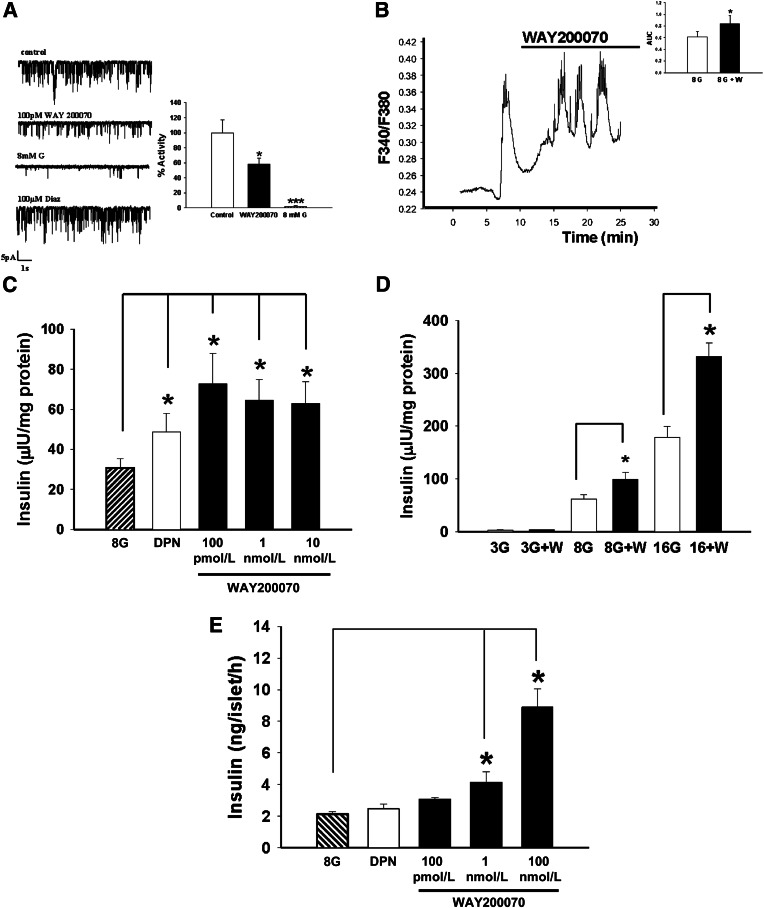
In vitro experiments performed with isolated pancreatic β-cells from mice showed that WAY200070 (100 pmol/L) decreased KATP channel activity (Fig. 1A). In whole mouse islets of Langerhans, the agonist promoted both a greater global intracellular Ca2+ entry (Fig. 1B) and an increase of insulin secretion in response to glucose stimulation (8 mmol/L glucose) (Fig. 1C). WAY200070 at 100 pmol/L enhanced GSIS in the presence of 8 and 16 mmol/L glucose but, of interest, it did not have any effect when applied in the presence of low glucose concentrations (3 mmol/L) (Fig. 1D). The effect of WAY200070 on GSIS of human islets was also analyzed. Insulin release was enhanced in a dose-dependent manner, with a maximal effect at a dose of 100 nmol/L (Fig. 1E).
FIG. 1.
In vitro exposure to an ERβ agonist (WAY200070) induced reduction of KATP channel activity and enhanced glucose-induced intracellular calcium concentration signals and insulin secretion. A: WAY200070 100 pmol/L decreased KATP channel activity in isolated pancreatic β-cells from mice (n = 6). B: This was accompanied by a greater increase in global intracellular Ca2+ entry after glucose stimulation (n = 6), as indicated by a greater AUC (inset). C: Insulin release in response to 8 mmol/L glucose, 8 mmol/L glucose and DPN (1 nmol/L), and 8 mmol/L glucose and WAY200070 (100 pmol/L, 1 nmol/L, and 10 nmol/L) was measured in intact mouse islets of Langerhans. Insulin levels were significantly higher in the presence of WAY200070 (n = 8). D: WAY200070-induced insulin secretion from mouse islets exposed to 3, 8, and 16 mmol/L glucose for 1 h (n = 8). WAY200070 was applied at 100 pmol/L. Note that WAY200070 action was significant only when stimulatory glucose concentrations were used. E: Insulin release in response to 8 mmol/L glucose, 8 mmol/L glucose and DPN (1 nmol/L), and 8 mmol/L glucose and WAY200070 (100 pmol/L, 1 nmol/L, and 100 nmol/L) was measured in intact human islets of Langerhans. Data are mean ± SE. *P < 0.05, ***P < 0.001 versus control (Student t test). Statistical analyses between groups in C, D, and E were evaluated by one-way ANOVA, with P < 0.05 considered significant. G, glucose; W, WAY200070.
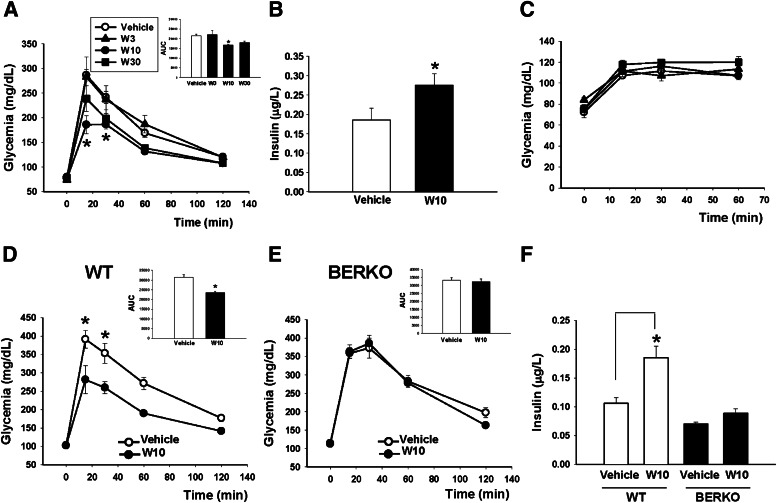
We next studied the in vivo effect of WAY200070. For this purpose, we used male C57BL/6 mice aged 3 months. Fasted animals were injected intraperitoneally with 2 g/kg glucose followed by the intraperitoneal administration of a vehicle or WAY200070 at different concentrations (3, 10, or 30 mg/kg). As shown in Figure 2A, glucose sensitivity was improved in those animals that received the agonist WAY200070, especially at a concentration of 10 mg/kg (W10) and 30 mg/kg (W30), although the effects were not statistically significant at the latter dose. When plasma insulin levels were measured at 30 min, a higher glucose-stimulated insulin release was observed at W10 compared with the vehicle-treated group (Fig. 2B). Under fasting conditions, no changes of glycemia were observed in animals treated with the ERβ agonist at any concentration assayed (Fig. 2C).
FIG. 2.
Single in vivo administration of the ERβ agonist (WAY200070) improved glucose tolerance. A: Fasted C57BL/6 male mice were injected intraperitoneally with a single dose of WAY200070 3 mg/kg (W3), 10 mg/kg (W10), or 30 mg/kg (W30). In parallel, they were administered a glucose challenge (2 g/kg). Through an IGTT we observed an improvement in glucose response in animals treated with the ERβ agonist at W10. Thus, the AUC (inset) was significantly reduced in this group (n = 5). B: In addition, we measured plasma insulin levels 30 min after the administration of the glucose challenge and the ERβ agonist and detected that glucose-stimulated insulin release was significantly higher in W10 mice than in controls (8–10 mice/group). C: In the fasted state, a single injection of WAY200070 did not have an effect on glucose sensitivity (5 mice/group). We confirmed that this finding was an ERβ-mediated effect by using WT and BERKO mice. D: Fasted WT mice were injected intraperitoneally with a single dose of W10 or vehicle in parallel with a glucose challenge of 2 g/kg. A better response to the glucose load was observed in the animals that received the agonist, with a decreased AUC (inset) (5–7 mice/group). E: No changes in glucose tolerance or the AUC were observed in BERKO mice in response to the agonist W10-treated mice compared with the vehicle-treated mice. F: Glucose-stimulated insulin release was clearly enhanced in W10 WT mice but not in W10 BERKO mice (5–7 mice/group). Data are mean ± SE. *P < 0.05 versus vehicle (Student t test). In A and C, statistical analysis between groups was evaluated by one-way ANOVA, with P < 0.05 considered significant. BERKO, ERβ knockout.
To unequivocally demonstrate that the in vivo insulinotropic effect of WAY200070 is an ERβ-mediated effect, we compared its action in wild type (WT) and ERβ knockout (BERKO) mice. A single administration of W10 in WT mice resulted in a lower rise of glycemia levels in response to a glucose challenge, indicating that these mice tolerated glucose better than WT vehicle-treated mice (Fig. 2D). However, when the same experiment was performed in BERKO mice, no differences were observed between vehicle and ERβ agonist-treated animals (Fig. 2E). When we measured plasma insulin levels 30 min after a glucose load, we observed a higher glucose-stimulated insulin release in the WT mice that had received a single dose of W10 (Fig. 2F) but no changes in the BERKO mice (Fig. 2F).
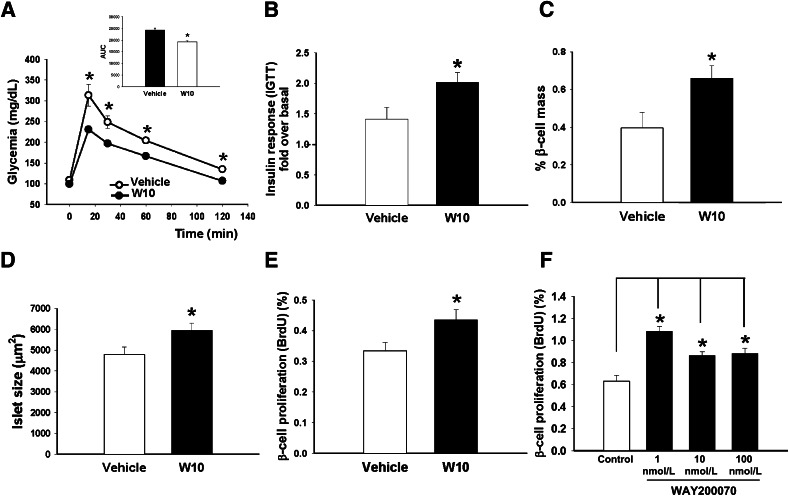
We studied the extent to which an ERβ agonist can be a positive modulator of energy balance and glucose homeostasis under healthy conditions. For this purpose, we analyzed glucose and insulin sensitivity in C57BL/6 male mice treated with W10 for 14 days. The treatment with the agonist was found to improve glucose tolerance (Fig. 3A) and increase the glucose-stimulated in vivo insulin release 30 min after the glucose load (1.41 ± 0.19-fold increase over basal insulin levels in vehicle-treated mice versus 2.01 ± 0.16-fold increase in W10-treated mice) (Fig. 3B), suggesting that the pancreatic β-cells were working more vigorously. A slightly better insulin sensitivity was also observed in these animals compared with the controls (Supplementary Fig. 1A). In addition to this, β-cell mass and islet size were higher in W10-treated mice compared with the vehicle-treated mice (Fig. 3C and D). We studied whether the augmented β-cell mass was a result of an increased β-cell proliferation. We performed coimmunostaining for insulin and BrdU and found that animals treated with the ERβ agonist exhibited a larger number of proliferating β-cells (Fig. 3E). Dispersed islet cells were treated with a range of doses of this compound for 2 days, and β-cell proliferation was quantified by using BrdU incorporation over the same period. We observed a significant increase in pancreatic β-cell proliferation in the ERβ agonist–treated cultures, which suggests that the activation of ERβ signaling directly affects pancreatic β-cell replication (Fig. 3F).
FIG. 3.
In vivo administration of the ERβ agonist (WAY200070) in C57BL/6 mice improved glucose homeostasis. A: IGTT performed in C57BL/6 male mice treated with W10 or vehicle (7 mice/group) during a 14-day period. The treatment resulted in better glucose sensitivity and in the reduction of the AUC (inset). B: Insulin levels during an IGTT performed in the same group of animals. Data are provided as the fold increase in plasma insulin (over basal) at 30 min after the glucose bolus injection. Note that there is a marked increased in in vivo insulin release in W10 mice. C: Measurement of β-cell area (area occupied by insulin-positive cells expressed as a percentage of the total area). W10 showed a clear increase of pancreatic β-cell mass compared with vehicle-treated mice. D: Islet size was also increased in W10 mice. E: β-Cell replication measured as the percentage of BrdU-positive cells (7 mice/group). F: β-Cell proliferation rate in cultures of dispersed primary mouse islet cells. Cells were treated with vehicle or WAY200070 (1, 10, and 100 nmol/L) for 2 days. BrdU was added over the same period. Data are mean ± SE. *P < 0.05 versus vehicle (Student t test). In F, the statistical analysis between groups was evaluated by one-way ANOVA.
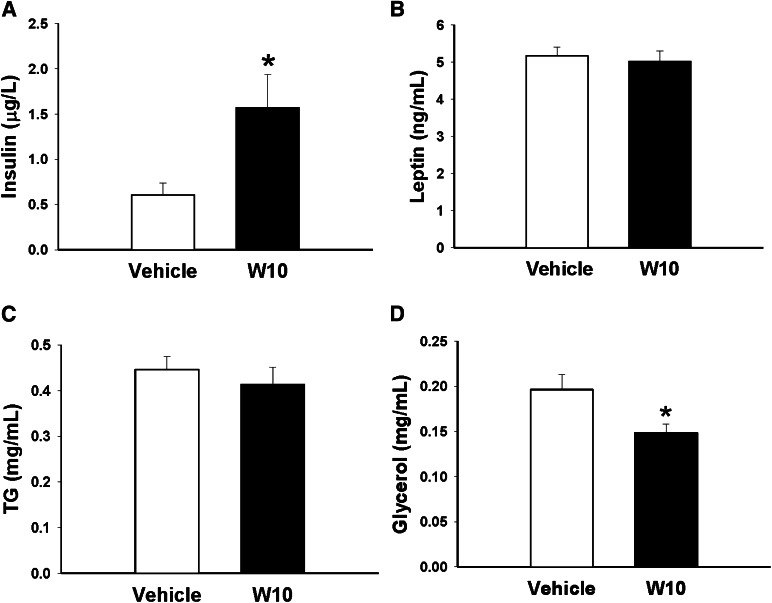
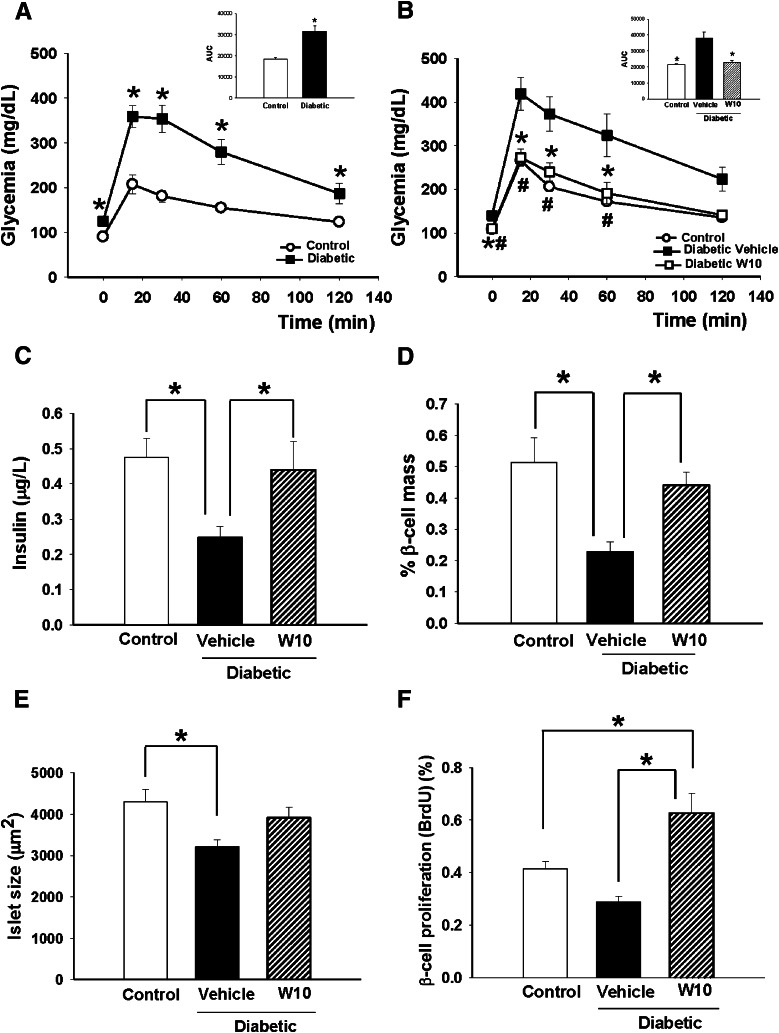
We also observed clear hyperinsulinemia in the fed state (Fig. 4A) but no differences in leptin (Fig. 4B) or triglyceride (Fig. 4C) levels, although glycerol levels were decreased in the ERβ agonist-treated group (Fig. 4D). Mild diabetes was induced by administering a single dose of STZ 150 mg/kg and NA 1,000 mg/kg. As shown in Figure 5A, 10 days after the STZ-NA treatment, these animals exhibited moderate fasting hyperglycemia (vehicle-treated control mice 90 ± 4.31 mg/dL, STZ-NA diabetic mice 126 ± 5.3 mg/dL, P < 0.05). An IGTT showed that STZ-NA mice presented consistent glucose intolerance as indicated by the bigger area under the curve (AUC) (Fig. 5A). At this point, we divided STZ-NA mice into two groups: One received an intraperitoneal injection of W10 for 7 days, and the other received a vehicle injection for the same period. The 1-week treatment provoked a decrease in fasting glycemia levels in the diabetic mice treated with the ERβ agonist (control mice 108 ± 2.36 mg/dL, diabetic mice treated with WAY200070 109 ± 4.03 mg/dL, diabetic mice 140 ± 9.16 mg/dL, P < 0.05). We also observed from the IGTT that glucose tolerance was significantly improved in the diabetic group treated with WAY200070, with similar levels of glycemia as the control group (Fig. 5B). This improvement in glucose sensitivity was accompanied by a restoration of plasma insulin levels (Fig. 5C). Remarkably, we found that pancreatic β-cell mass in diabetic mice exhibited a clear decrease compared with that of controls, whereas diabetic mice treated with the agonist presented not only similar levels to the control mice (Fig. 5D), but also similar islet size (Fig. 5E). The restoration of β-cell mass is related to an increase of β-cell replication (Fig. 5F). No differences in the plasma leptin or triglyceride levels were found between groups (Supplementary Fig. 1B and C). A marked tendency for glycerol levels to decrease was observed in the diabetic group treated with the ERβ agonist, although it was not statistically significant (Supplementary Fig. 1D).
FIG. 4.
Plasma hormones and lipid metabolites in C57BL/6 mice after administration of WAY200070. Plasma was collected from C57BL/6 mice treated with the ERβ agonist W10 or vehicle (6–7 mice/group) during a 14-day period, and insulin (A), leptin (B), TG (C), and glycerol (D) levels were measured. Data are mean ± SE. *P < 0.05 versus vehicle (Student t test). TG, triglyceride.
FIG. 5.
In vivo administration of the ERβ agonist (WAY200070) improved glucose tolerance in mildly diabetic mice and restored plasma insulin levels and pancreatic β-cell mass. A: Mild diabetes was induced by the administration of a single dose of STZ 150 mg/kg and NA 1,000 mg/kg. Ten days after the treatment, these animals exhibited moderate hyperglycemia and impaired glucose tolerance compared with controls (8–15 mice/group). The AUC in these mice was significantly increased (inset). *P < 0.05 versus control (Student t test). B: At this point, 1-week treatment with the ERβ agonist resulted in a decrease of fasting glycemia as well as a better sensitivity to a glucose challenge in the W10 diabetic group compared with the vehicle-treated diabetic group (7–8 mice/group). *P < 0.05 diabetic vehicle vs. diabetic W10; #P < 0.05 diabetic vehicle vs. control. C: Plasma insulin levels in diabetic mice treated with WAY200070 during 1 week compared with control and nontreated diabetic mice. We observed that levels of plasma insulin in treated diabetic mice were similar to controls; meanwhile, levels in diabetic mice were significantly reduced (8–10 mice/group). D, E, and F: Measurement of pancreatic β-cell mass and islet size in treated diabetic mice (5 mice/group) and β-cell replication measured as the percentage of BrdU-positive cells (5 mice/group). Data are mean ± SE. Statistical analysis between groups was evaluated by one-way ANOVA, with *P < 0.05 considered significant.
At ∼14 weeks of age, db/db mice presented hyperglycemia, glucose intolerance, and decreased in vivo insulin secretion in response to a glucose load (Supplementary Fig. 2A and B). At this moment, the animals were treated with W10 for 14 days or with the vehicle.
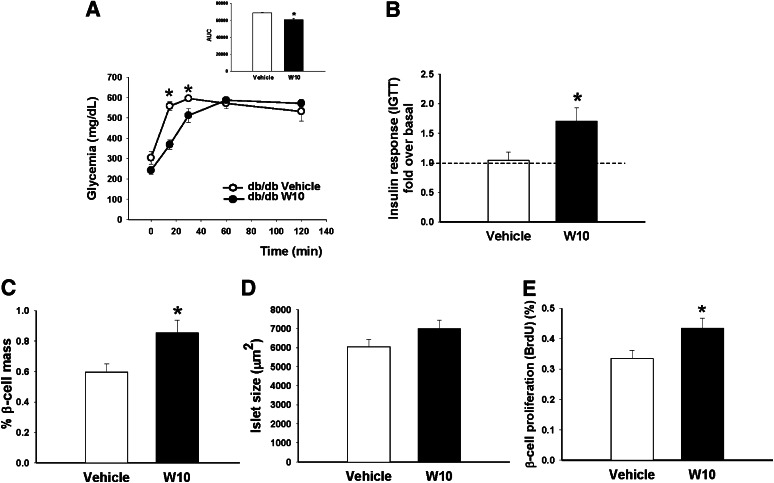
Glucose levels after overnight fasting were slightly reduced in W10 db/db mice (241 ± 18.5 mg/dL) compared with the vehicle db/db mice (303 ± 31.5 mg/dL), although differences were not significant (P = 0.1). To explore differences in glucose homeostasis, we performed an IGTT. At 15 and 30 min, blood glucose levels were lower in treated diabetic mice than in untreated mice. We also observed a smaller AUC, suggesting a better glucose tolerance at least after short periods (Fig. 6A). We measured the plasma insulin of mice in a fasted state and 30 min after a glucose load (Fig. 6B). Of interest, we found that in vivo GSIS was practically absent in vehicle db/db mice, showing a 1.04 ± 0.14-fold increase over basal insulin levels, whereas in db/db animals treated with the ERβ agonist, insulin secretion showed a 1.70 ± 0.22-fold increase over basal insulin. No differences were observed in the significance of insulin sensitivity between groups (Supplementary Fig. 2C).
FIG. 6.
In vivo administration of the ERβ agonist (WAY200070) in db/db mice enhanced first-phase insulin secretion. A: IGTT performed in db/db mice treated with W10 or vehicle (6–7 mice/group) during a 14-day period. B: Insulin levels during an IGTT performed in the same group of animals. Data are provided as the fold increase in plasma insulin (over basal) at 30 min after the glucose bolus injection. C and D: Measurement of β-cell area (area occupied by insulin-positive cells expressed as a percentage of the total area) and islet size. W10-treated db/db mice showed an increase of pancreatic β-cell mass compared with vehicle-treated db/db mice. E: β-Cell replication measured as the percentage of BrdU-positive cells (6–7 mice/group). Data are mean ± SE. *P < 0.05 versus vehicle (Student t test).
In addition to the ERβ-agonist effect on pancreatic β-cell function, ERβ activation also increased pancreatic β-cell mass (Fig. 6C). We observed an increase in the size of the islets, although differences between groups were not significant (P = 0.1) (Fig. 6D). We found a higher rate of β-cell proliferation in the treated db/db mice (Fig. 6E).
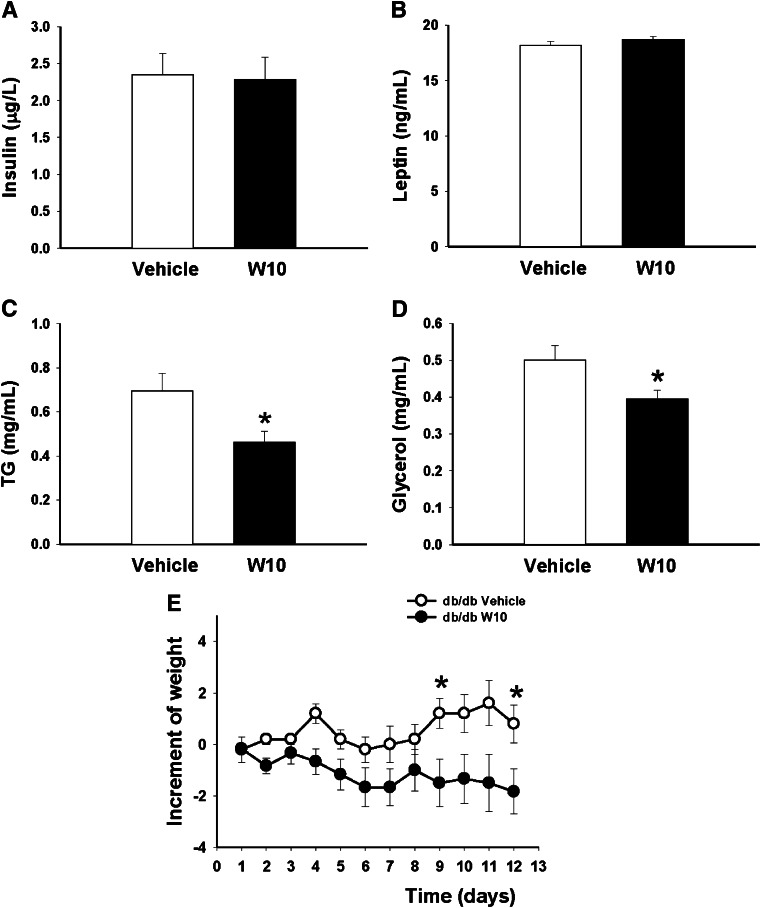
In the fed state, no differences in plasma insulin or leptin levels were found (Fig. 7A and B); however, a clear decrease of triglyceride and glycerol plasma levels was detected in the W10 db/db mice (Fig. 7C and D), which was in consonance with the weight loss observed in these animals throughout the treatment from mainly day 8 to the end (Fig. 7E). No changes in food intake were observed (Supplementary Fig. 2D).
FIG. 7.
Plasma hormones, lipid metabolites, and increment of weight in db/db mice. Plasma was collected from W10 db/db or vehicle-treated db/db mice after 14 days of treatment, and insulin (A), leptin (B), TG (C), and glycerol (D) levels were measured. E: Increment of weight in db/db mice during the treatment with W10 or vehicle (6–7 mice/group). Data are mean ± SE. *P < 0.05 versus vehicle (Student t test). TG, triglyceride.
DISCUSSION
In the current study, we demonstrate the potential use of the ERβ ligand WAY200070 as an effective modulator of insulin release and pancreatic β-cell mass, with a significant improvement in glucose homeostasis. It is clear now that both ERα and ERβ play a pivotal role in the regulation of lipid and glucose metabolism and that a correct balance of the ERα/ERβ ratio is crucial for that (23,24). However, most studies have focused on the role of both receptors in the central nervous system, adipose tissue, liver, and skeletal muscle. It has been described that the anorectic effect of E2 seems to be mediated mainly by ERα (25,26), although the involvement of ERβ cannot be disregarded (27). In the liver, ERα is the main regulator of hepatic insulin sensitivity (28,29). In skeletal muscle, ERβ is suggested to play a repressive role on GLUT4 expression because the ERβ agonist DPN severely downregulates its expression in ArKO (aromatase-deficient) mice (30). Finally, in adipose tissue, as is described later, ERβ could have antiobesity effects (31).
Glucose homeostasis requires a tight communication between peripheral tissues and the pancreas, but ultimately, hyperglycemia only develops when pancreatic β-cell function fails. In previous studies, we demonstrated that ERβ regulates stimulus-secretion coupling in pancreatic β-cells, as does E2 (11). In the present study, we confirm these results, showing that the selective ERβ agonist WAY200070 rapidly decreased KATP channel activity and increased glucose-stimulated calcium signaling and GSIS. Remarkably, in human islets, WAY200070 also enhanced insulin release, with a maximal effect at a dose of 100 nmol/L. In mice, we observed that a single injection of WAY200070 in parallel with a glucose load rapidly enhances insulin secretion. Under fasting conditions, the selective ERβ agonist does not have an effect on glycemia, suggesting that the action of this compound depends on the actual glucose concentration and provides the possibility of glucose normalization without the risk of hypoglycemia.
This phenomenon is mediated at least partially by ERβ, as judged from experiments performed in WT and BERKO mice, in a process that mimics the effect of low physiological concentrations of E2 (100 pmol/L–1 nmol/L), a natural ligand of ERβ. However, we cannot rule out the possibility that other estrogen receptors can provoke similar effects. A recent report described that the activation of G protein-coupled receptor 30 enhanced GSIS and decreased glucagon and somatostatin release in female human and mouse islets, although this effect is only visible when E2 is applied at supraphysiological concentrations of ≥ 100 nmol/L (32–34). ERα has been proposed to play a significant role in the regulation of insulin synthesis by E2 but with no effects on pancreatic β-cell mass (10). Its role in cytoprotection has also been suggested (35).
Potential clinical implications of the insulinotropic ERβ-mediated effect.
Insulin deficiency is the common denominator for both type I diabetes and T2D. In the current study, we evaluated the insulinotropic effect of an ERβ agonist by using STZ-NA diabetic mice. This model has been reported to be useful for the assay of insulin secretagogues (17,36,37). We observed that the ERβ agonist modulates insulin secretion capacity and restores plasma insulin levels, but beyond this regulatory role of insulin secretion, we demonstrate that a selective ERβ agonist enhances β-cell proliferation in pancreatic islets, and thus, it could act as a regulator of β-cell growth. When pancreatic β-cell mass was quantified in STZ-NA diabetic mice, a clear decrease was observed compared with controls. However, in the diabetic animals that were treated with the agonist, there was a marked increase that resulted in the recovery of β-cell mass. Of interest, in vitro studies suggested that the ERβ agonist directly stimulates β-cell division, although the molecular basis mediating this phenomenon still needs further clarification. Mechanistically, E2 promotes islet regeneration in different animal models of diabetes through increased β-cell proliferation (38–41). Choi et al. (41) proposed that the E2 proliferative effect could be mediated by the activation of the cAMP-responsive element–binding protein, which in turn leads to an increased level of insulin receptor substrate 2 and pancreatic duodenal homeobox-1 levels and most probably leads to the activation of an IGF-I signaling cascade. In addition, genistein, another natural estrogenic compound, has been shown to improve islet β-cell proliferation, survival, and mass in diabetic mice in a process mediated by the activation of the protein kinase A and the subsequent phosphorylation of extracellular signal–related kinase 1/2 in β-cells (42). The authors speculated about the possibility that this signaling cascade is connected with an upregulation of cyclin D1 and, thus, with the regulation of the cell cycle machinery. Whether the ERβ agonist used in this study works through any of the presently proposed mechanisms remains unknown. In any case, we propose that ERβ selective agonists are considered as newcomers in the field of β-cell regeneration that potentially act alone or in synergy with other known factors, such as the hepatocyte growth factor, the glucose-dependent insulinotropic polypeptide, the transcription factors Pax 4, and the orphan nuclear liver receptor homolog-1 (43).
In addition to the STZ-NA model, we studied the possible benefits of ERβ-agonist treatment in a different model of diabetes, db/db mice. The administration of this drug led to an improvement of fasting glucose levels and glucose homeostasis, although the latter seemed to be a short-term effect. Importantly, while no insulin release in response to a rapid glucose stimulus was detected in the group of db/db vehicle-treated mice, the ERβ-agonist–treated group exhibited a clear release of insulin, indicating that this compound is able to restore the blunted first phase of insulin secretion. This phase is almost absent in individuals with T2D (44,45). Moreover, it has been shown that there is a delay of the first-phase insulin secretion in BERKO mice (46), reinforcing the importance of ERβ in the maintenance of postprandial glucose homeostasis.
When looking at the effects of WAY200070 in STZ-NA diabetic mice compared with db/db mice, we found that the recovery of pancreatic β-cell function and glucose homeostasis in the first model was much more effective than in the second model. We hypothesized that this recovery is a result of the animals exhibiting a less severe degree of diabetes in the first case than in the second case. Similarly, the hypoglycemic effect of E2 depends on the severity of the diabetic condition, with no positive effects when hyperglycemia exceeds 500 mg/dL and islet damage is too severe (38). For these reasons, we believe that treatment at an earlier stage of the disease would be more effective for the db/db mice.
We also observed that the ERβ-agonist treatment has other extrapancreatic effects. It significantly decreased triglyceride and glycerol plasma levels in db/db mice, which were accompanied by a reduction of body weight. However, more information in terms of energy expenditure would largely contribute to the understanding of the role of WAY200070 from an energetic point of view. One report indicated that the administration of selective ERβ ligands prevented body weight gain in animals fed with a high-fat diet as well as in ovariectomized mice because of the reduction in fat mass and the increase in lean mass (31). In contrast, a diabetogenic role for ERβ has been proposed because BERKO mice exposed to a high-fat diet had improved whole-body insulin sensitivity and glucose tolerance (47). In any case, the activation of ERβ demonstrates antilipogenic actions in pancreatic β-cells (40). Its role in cytoprotection has also been suggested (35).
In summary, we propose that an ERβ agonist could act as a regulator of pancreatic β-cell growth and that it promotes an increase of β-cell mass under pathological conditions. In addition, by using different experimental animal models of diabetes, we demonstrate the capacity of this agonist to normalize fasting glucose levels, to enhance endogenous insulin secretion, and to improve glucose homeostasis. Therefore, ERβ agonists may be useful new drugs for the treatment of T2D.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Generalitat Valenciana grant PROMETEO/2011/080, Ministerio de Economia y Competitividad BFU2011-28358, and Ministerio de Economía y Competitividad BFU2010-21773 and BFU2008-1942; the Swedish Cancer Fund; the Emerging Technology Fund of Texas; and the Robert A. Welch Foundation (E-0004).
No potential conflicts of interest relevant to this article were reported.
P.A.-M. designed the study, researched data, and wrote the paper. A.B.R. contributed to discussion and researched data. M.G.-A., S.S., S.J.M., and A.S. researched data. I.Q. and J.A.-G. contributed to discussion and reviewed and edited the manuscript. A.N. helped with the study design, contributed to discussion, and reviewed and edited the manuscript. P.A.-M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank M. Luisa Navarro, Institute of Bioengineering and Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders, Miguel Hernandez University of Elche, Alicante, Spain, for her technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1562/-/DC1.
REFERENCES
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2011;8:228–236 [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):82–90 [DOI] [PubMed] [Google Scholar]
- 6.Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus: what is their place in therapy? Drugs 2008;68:2131–2162 [DOI] [PubMed] [Google Scholar]
- 7.Tharakan G, Tan T, Bloom S. Emerging therapies in the treatment of ‘diabesity’: beyond GLP-1. Trends Pharmacol Sci 2011;32:8–15 [DOI] [PubMed] [Google Scholar]
- 8.Gallwitz B, Häring HU. Future perspectives for insulinotropic agents in the treatment of type 2 diabetes-DPP-4 inhibitors and sulphonylureas. Diabetes Obes Metab 2010;12:1–11 [DOI] [PubMed] [Google Scholar]
- 9.Pearson ER. Pharmacogenetics and future strategies in treating hyperglycaemia in diabetes. Front Biosci 2009;14:4348–4362 [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008;3:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano S, Ropero AB, Alonso-Magdalena P, et al. Rapid regulation of K(ATP) channel activity by 17beta-estradiol in pancreatic beta-cells involves the estrogen receptor beta and the atrial natriuretic peptide receptor. Mol Endocrinol 2009;23:1973–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov 2011;10:778–792 [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama N, Barros RP, Warner M, Gustafsson JA. ERbeta: recent understanding of estrogen signaling. Trends Endocrinol Metab 2010;21:545–552 [DOI] [PubMed] [Google Scholar]
- 14.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer 2011;11:597–608 [DOI] [PubMed] [Google Scholar]
- 15.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A 1998;95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahara A, Matsuyama-Yokono A, Shibasaki M. Effects of antidiabetic drugs in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice. Eur J Pharmacol 2011;655:108–116 [DOI] [PubMed] [Google Scholar]
- 17.Matsuyama-Yokono A, Tahara A, Nakano R, et al. Antidiabetic effects of dipeptidyl peptidase-IV inhibitors and sulfonylureas in streptozotocin-nicotinamide-induced mildly diabetic mice. Metabolism 2009;58:379–386 [DOI] [PubMed] [Google Scholar]
- 18.Nadal A, Rovira JM, Laribi O, et al. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB J 1998;12:1341–1348 [DOI] [PubMed] [Google Scholar]
- 19.Valdeolmillos M, Nadal A, Contreras D, Soria B. The relationship between glucose-induced K+ATP channel closure and the rise in [Ca2+]i in single mouse pancreatic beta-cells. J Physiol 1992;455:173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juan-Picó P, Fuentes E, Bermúdez-Silva FJ, et al. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium 2006;39:155–162 [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 22.Soriano S, Alonso-Magdalena P, García-Arévalo M, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS ONE 2012;7:e31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab 2011;14:289–299 [DOI] [PubMed] [Google Scholar]
- 24.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009;304:63–68 [DOI] [PubMed] [Google Scholar]
- 25.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes 2012;61:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A 2007;104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang YQ, Akishita M, Kim S, et al. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 2002;26:1103–1109 [DOI] [PubMed] [Google Scholar]
- 28.Bryzgalova G, Gao H, Ahren B, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 2006;49:588–597 [DOI] [PubMed] [Google Scholar]
- 29.Lundholm L, Bryzgalova G, Gao H, et al. The estrogen receptor alpha-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol 2008;199:275–286 [DOI] [PubMed] [Google Scholar]
- 30.Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A 2006;103:1605–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yepuru M, Eswaraka J, Kearbey JD, et al. Estrogen receptor-beta-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 2010;285:31292–31303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology 2011;152:2568–2579 [DOI] [PubMed] [Google Scholar]
- 33.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol 2010;320:16–24 [DOI] [PubMed] [Google Scholar]
- 34.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology 2011;152:3030–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Le May C, Wong WP, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 2009;58:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A 1977;74:2485–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Shibasaki M. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin Pharmacol Toxicol 2008;103:560–568 [DOI] [PubMed] [Google Scholar]
- 38.Yamabe N, Kang KS, Zhu BT. Beneficial effect of 17β-estradiol on hyperglycemia and islet β-cell functions in a streptozotocin-induced diabetic rat model. Toxicol Appl Pharmacol 2010;249:76–85 [DOI] [PubMed] [Google Scholar]
- 39.Goodman MN, Hazelwood RL. Short-term effects of oestradiol benzoate in normal, hypophysectomized and alloxan-diabetic male rats. J Endocrinol 1974;62:439–449 [DOI] [PubMed] [Google Scholar]
- 40.Tiano JP, Delghingaro-Augusto V, Le May C, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest 2011;121:3331–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 2005;146:4786–4794 [DOI] [PubMed] [Google Scholar]
- 42.Fu Z, Zhang W, Zhen W, et al. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 2010;151:3026–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellado-Gil JM, Cobo-Vuilleumier N, Gauthier BR. Islet β-Cell mass preservation and regeneration in diabetes mellitus: four factors with potential therapeutic interest. J Transplant 2012;2012:230870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn SE, Montgomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2001;86:5824–5829 [DOI] [PubMed] [Google Scholar]
- 45.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 2002;51(Suppl. 1):S117–S121 [DOI] [PubMed] [Google Scholar]
- 46.Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 2009;297:E124–E133 [DOI] [PubMed] [Google Scholar]
- 47.Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 2008;4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.