Abstract
The Banting Medal for Scientific Achievement Award is the American Diabetes Association's highest scientific award and honors an individual who has made significant, long-term contributions to the understanding of diabetes, its treatment, and/or prevention.The award is named after Nobel Prize winner Sir Frederick Banting, who codiscovered insulin treatment for diabetes.
Bruce M. Spiegelman, PhD, of Harvard Medical School and the Dana-Farber Cancer Institute in Boston, received the American Diabetes Association's Banting Medal for Scientific Achievement at the Association's 72nd Scientific Sessions, 8–12 June 2012, Philadelphia, Pennsylvania. He presented the Banting Lecture, “Transcriptional Control of Adipogenesis—Toward a New Generation of Therapeutics for Metabolic Disease,” on Sunday, 10 June 2012. In his lecture, Dr. Spiegelman described the discovery of several transcriptional components that control adipose cell development: PPAR-γ, PGC1-α, and PRDM16. He also described the cloning and characterization of beige fat cells, the thermogenic “brown-like” cells that can develop in white fat depots. Lastly, Dr. Spiegelman discussed irisin, a newly discovered regulatory hormone that converts white fat into the more thermogenic beige fat. Dr. Spiegelman’s research has found that irisin, which is induced by exercise, appears to activate some of the same health benefits as exercise, including improvement of glycemic control. Understanding the regulation of adipose tissue, white, brown, and beige, can potentially lead to the development of a new generation of therapeutics for diabetes prevention and treatment.
We are all familiar with data indicating that the United States has led the way in the epidemic of obesity and type 2 diabetes. More recently, it has become very clear that this is not just a domestic problem, but a worldwide concern. Mexico and Brazil are two very populous countries that are projected to soon surpass the United States in the frequency of type 2 diabetes, and most of Europe is also experiencing this epidemic. Hence, diabetes and obesity are worldwide challenges.
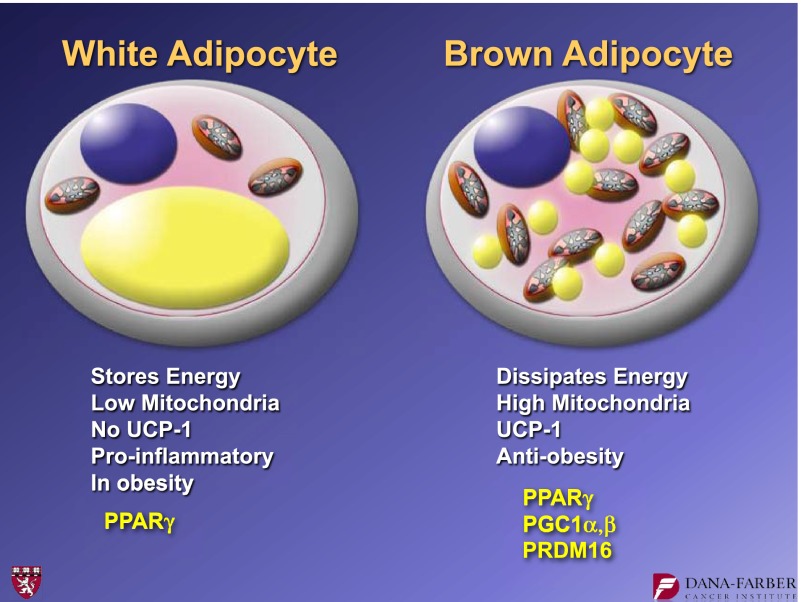
Our laboratory's research interest is related to the control of fat cell biology. When we began our studies in this area more than 25 years ago, we were particularly interested in two issues: 1) the biology of white and brown fat cells and 2) the regulatory molecules that might play even broader roles in the physiology and homeostasis of animals, including humans. The white adipose cell (Fig. 1) stores energy in a single lipid droplet. It has relatively low mitochondrial content and no uncoupling protein 1 (UCP1). It has been known for almost 20 years that white fat cells can be proinflammatory in the context of obesity (1). In the early and mid-1990s, we identified peroxisome proliferator–activated receptor (PPAR)-γ, the dominant regulator of fat cell development (2).
FIG. 1.
The general features of white and brown adipocytes. Key transcriptional regulators that are discussed in this article are shown in yellow.
Brown adipose cells (Fig. 1) are of great interest because they dissipate chemical energy in the form of heat. These cells play natural antiobesity, antidiabetes roles, and they protect against hypothermia in most, if not all, mammals. They do this because they have a relatively high mitochondrial content that contains a specialized mitochondrial protein called UCP1. It is UCP1 that dissipates chemical energy within a mitochondria. Essentially, UCP1 causes a proton leak across the inner membrane of the mitochondria, thereby dissipating chemical energy in the form of heat.
Our laboratory has studied these brown fat cells in considerable detail over the last 15 years. During this time we have identified some, although certainly not all, key transcriptional regulators. Similar to white fat, brown fat development also requires PPAR-γ, but brown fat requires additional molecular components for its development. Examples of these components include the peroxisome proliferator–activated receptor γ coactivator 1 (PGC1) coactivators (3), PGC1-α and PGC1-β, and PRDM16, a molecule that appears to control the fate of brown fat development (4).
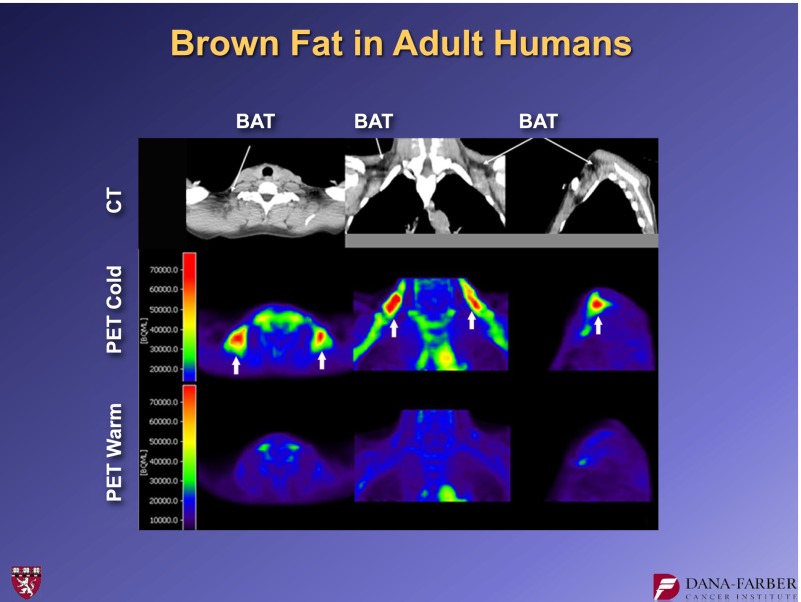
Brown fat in humans—especially in adult humans—was “rediscovered” several years ago through positron emission tomography (PET) imaging, which was being used in the oncology field. Oncologists noticed that when they used PET imaging with radioactive glucose to look for metastases, humans often had a ring of “hot spots” denoting glucose uptake. These hot spots were symmetrical in the supraclavicular area and were almost certainly not tumor metastases. Figure 2 shows the same individual at ambient temperature and then after a couple of hours in cold exposure. PET imaging lights up these regions (5–7).
FIG. 2.
PET images of an individual human at three different angles at ambient temperature and after brief cold exposure (6). CT, computed tomography; BAT, brown adipose tissue.
Analysis of open biopsies confirmed that most adult humans have significant deposits of brown adipose tissue. A key issue now is understanding the contribution that this tissue makes to overall energy balance in adult humans. In particular, it is reasonable to ask if there are things that we can do to increase the amount and/or function of brown adipose tissue.
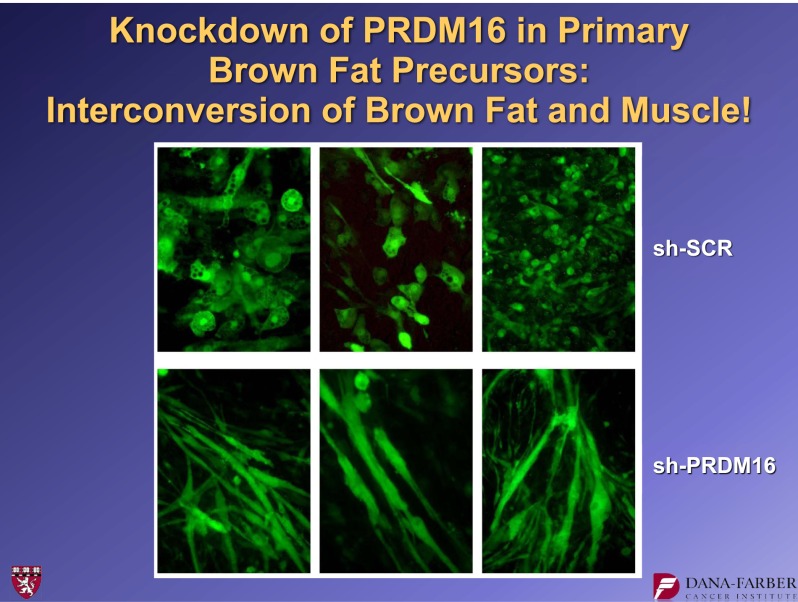
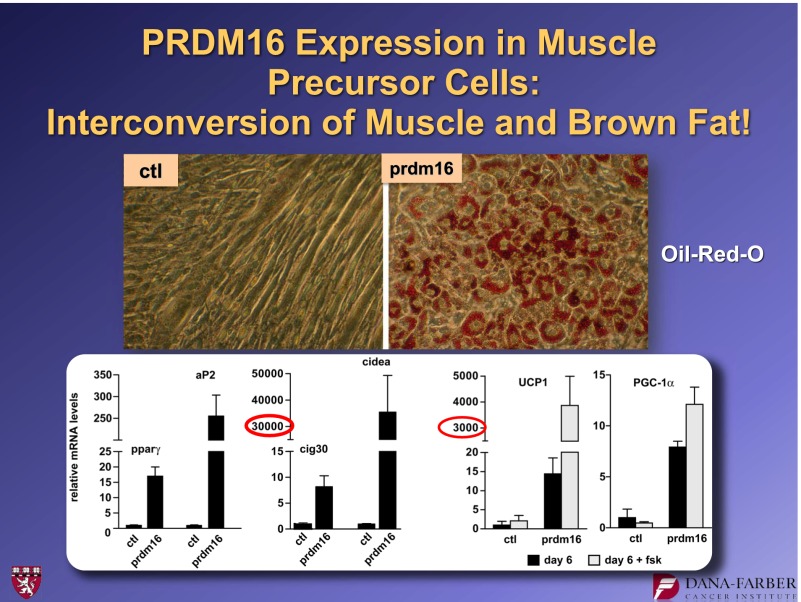
In 2008, a very striking observation was made about the role of PRDM16 in brown fat development (8). PRDM16 was suppressed in cultures of brown fat precursor cells using a shRNA, a short hairpin RNA. This biochemical method suppresses the function of a specific gene. These experiments showed that cells treated with a control shRNA started with brown fat precursors and ended with brown fat. However, cultures receiving the shRNA suppressor of PRDM16 started with brown fat precursors, but the cells on the dish fused to form large tubes that—if you watched under the microscope—began to twitch (Fig. 3). By staining these cells for muscle proteins, we showed that suppressing this one molecule, PRDM16, had essentially switched the fate of the brown fat cells into skeletal muscle cells. We also showed that the converse was true: PRDM16 expression converted myoblasts into brown fat cells (Fig. 4). These findings led us to do lineage-tracing studies (8) that indicated that brown fat is derived from the same lineage as skeletal muscle. In contrast, white fat is derived from a different lineage.
FIG. 3.
Suppression of PRDM16 expression with a specific shRNA causes a phenotypic switch to myotubes in brown fat cell cultures. For further details, see ref. 8. shSCR, scrambled shRNA.
FIG. 4.
Forced expression of PRDM16 causes brown adipogenesis in C2C12 myoblasts. For further details, see ref. 8. ctl, control.
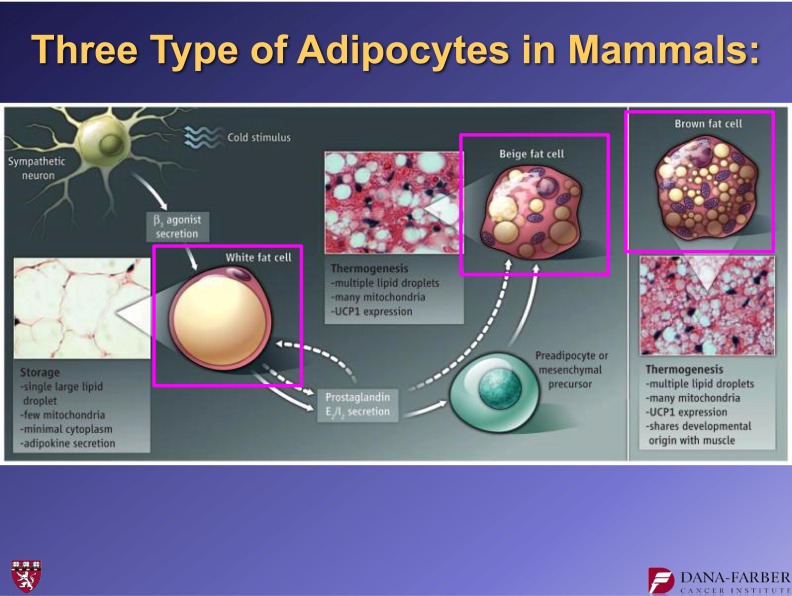
In this same article (8), we showed that patches of brown fat cells, which can emerge in white adipose tissue under conditions of extreme cold or extreme β-adrenergic signaling, do not come from the same muscle-like lineage as the classical brown fat. These observations provided the first evidence that there were two different cell types that had been called brown fat cells: 1) the classical brown fat, which is found in the interscapular and perirenal depots, and 2) the UCP1-positive cells emerging in white fat depots. We named the latter “beige” adipose cells (Fig. 5), although others call them “brite” cells (9). It is intriguing to speculate that there may be additional subtypes of adipose cells that have yet to be discovered.
FIG. 5.
Illustration of three distinct types of adipose cells (17).
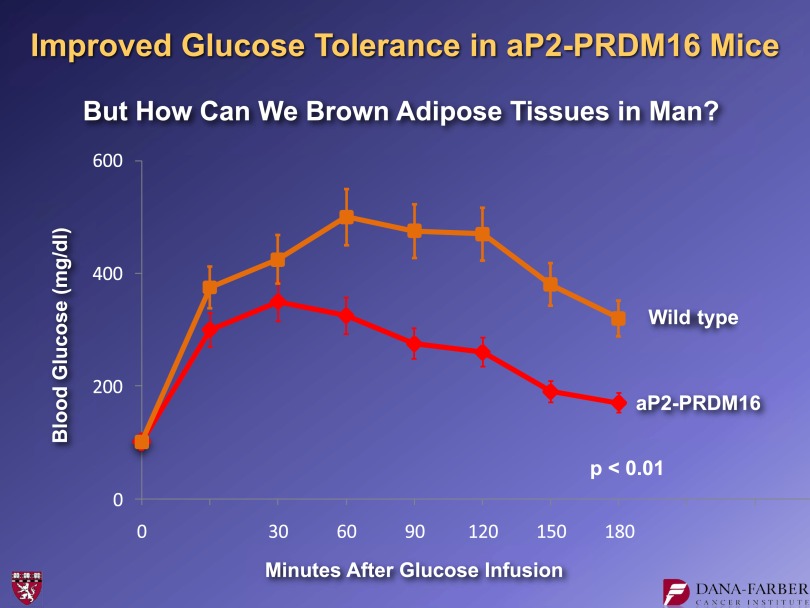
Figure 6 shows data from experiments in which we put PRDM16 under the control of a fat-selective genetic element from the adipose fatty acid binding protein (aP2) promoter and made transgenic strains of mice. These mice did not show alterations in visceral fat or in their classical brown fat. However, these mice developed profound browning of subcutaneous white fat (10). We analyzed these animals metabolically using glucose tolerance tests in high fat–fed animals. These experiments showed that, relative to controls, the transgenic mice with enhanced “beigeing” of their adipose tissues had a smaller area under the curve, indicating improved glucose tolerance (Fig. 6). These results show that more beige fat can improve animals' metabolic homeostasis.
FIG. 6.
Glucose tolerance test performed in high fat–fed control mice and mice with adipose-specific expression of PRDM16 (10).
These findings lead directly to the question of how we might use this knowledge to develop practical methods to increase the brown or beige adipose tissues in humans. One answer that we and others have recently found is surprising: Exercise can induce browning of white adipose tissues. One of the molecules that we identified in the late 1990s in our studies of brown adipose cells is a powerful transcriptional coactivator called PGC1-α. PGC1-α functions as a master regulator of mitochondrial biogenesis in mammals. Its expression is higher in brown fat than in white fat, and if this molecule is incorporated into white fat, white fat expresses more mitochondria and some, but not all, elements of browning. Although we were mainly studying brown fat at the time, we found that PGC1-α is expressed at higher levels in red, oxidative muscle compared with white, glycolytic muscle. All mammals have different types of muscle fibers. Type 1 and type 2A fibers are classified as red fibers. Importantly, John Holloszy’s laboratory showed that PGC1-α expression is increased by exercise in mice, in rats, and in human beings, including running and swimming (11). These and other observations raised the question as to whether this one molecule, PGC1-α, can regulate many of the benefits that exercise provides to humans and other mammals. Indeed, over the last 10 years, we and many others have shown that this is true. When PGC1-α is expressed in muscle, it causes mitochondrial biogenesis. But it also stimulates glucose uptake, neuromuscular junction formation, angiogenesis, fiber-type switching, and fatty acid oxidation (12–14). While we know that exercise is very important, it is complicated to study. There are so many variables at play when a human or an animal exercises that it is hard to identify the key components. In contrast, muscle cells in a dish or a transgenic animal that has just one change (e.g., elevated PGC1-α) provide an experimental system that is accessible and potentially informative.
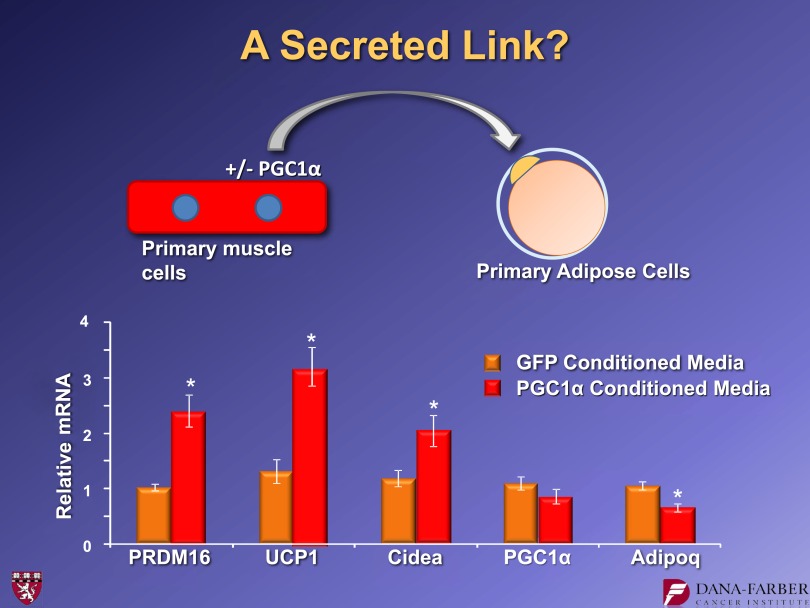
We observed that mice with transgenic expression of PGC1-α specifically in the muscle had more browning of white subcutaneous adipose tissues. Patches of UCP1 positively stained cells are visible in the inguinal fat of mice that have elevated PGC1-α in the muscle (15). This is accompanied by elevated UCP1 mRNA in the fat. We asked whether there was a secreted molecule coming out of muscle cells under the control of PGC1-α that could link muscle function to the adipose tissues. We took primary muscle cells and introduced either PGC1-α or green fluorescent protein (GFP) as a control. The supernatants from those cells were collected and put on primary adipose cultures (Fig. 7). These induced UCP1 when they came from cells receiving PGC1-α but not GFP. This indicated that at least some of the activity that generates these observations is a soluble molecule. We took many different approaches and eventually settled on informatics to help us identify the key molecule. We took gene expression arrays from PGC1-α−expressing muscle in vivo and combined these data with several algorithms that could predict protein secretion. We then validated the data in cell culture. Eventually, our studies centered on a molecule called FNDC5.
FIG. 7.
Medium conditioned by cells expressing PGC1-α have a “browning effect” on primary inguinal adipose cells with the induction of UCP1. For further details, see ref. 15. *P < 0.05.
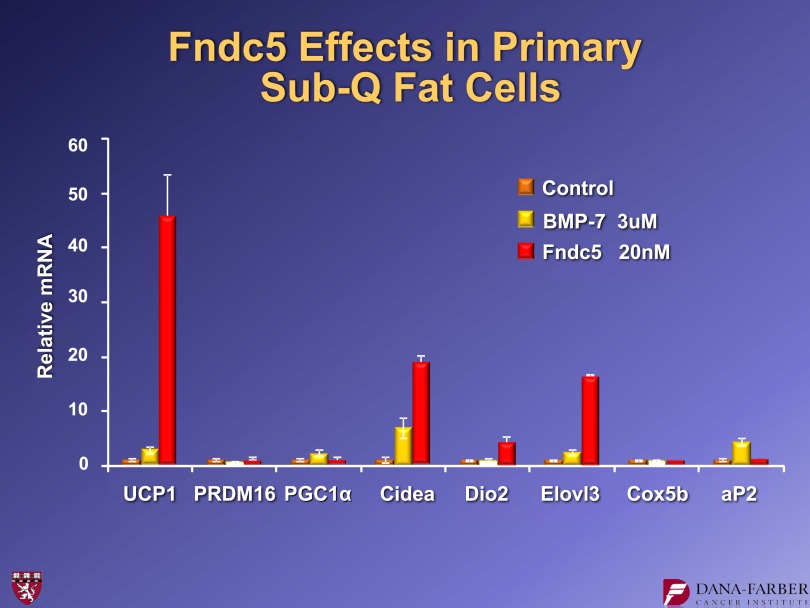
The FNDC5 protein, while not well studied, was commercially available. Figure 8 shows data from experiments in which we put either BMP7 or commercial FNDC5 on inguinal adipose cultures. There was a robust induction of UCP1 and several other genes in these cells as a result of applying 20 nmol/L of FNDC5. Importantly, we do not see this response in classical brown fat. These results are consistent with the idea that activation of classical brown fat and the browning of white fat is not the same thing. Thus, it was not surprising that FNDC5 worked on one fat type and not the other.
FIG. 8.
FNDC5 protein stimulates UCP1 expression in inguinal adipose cell cultures. BMP7 and FNDC5 were used at the doses shown for 4 days. For further details, see ref. 15. Sub-Q, subcutaneous.
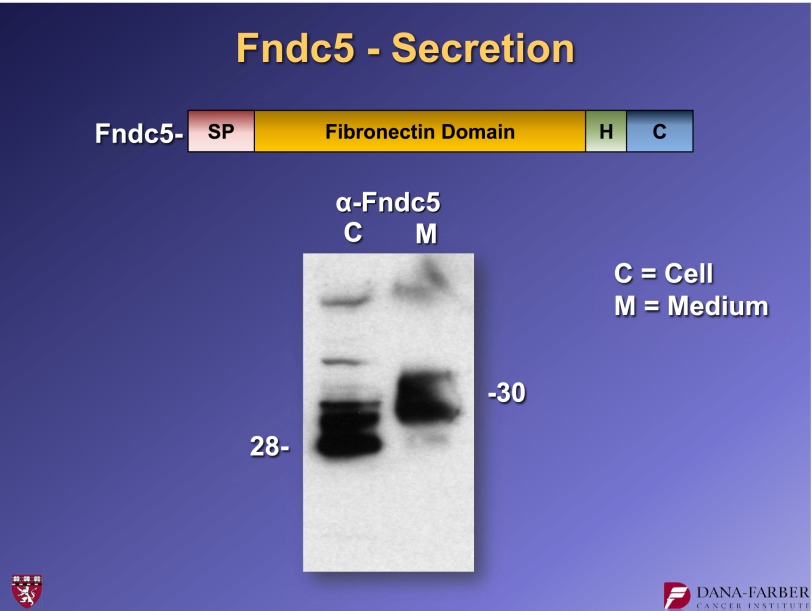
So what is FNDC5? This molecule was cloned by two groups in 2002 and is annotated as an intracellular protein. It has a signal peptide, a fibronectin type 3 domain, and a short hydrophobic stretch that resembles a transmembrane domain (Fig. 9). Essentially, this could be a type 1 membrane protein that sticks through the membrane and has an extracellular domain that could possibly be shed. It is enriched in skeletal muscle, heart, and the central nervous system. We transfected the gene expressing this protein into cells, collected the cellular material and the secreted medium, and did a Western blot for FNDC5. These experiments showed that FNDC5 is in fact secreted, and the material that is secreted has a slightly slower electrophoretic mobility than the material inside the cells (Fig. 9). This change in electrophoretic mobility was because of glycosylation.
FIG. 9.
A fragment of FNDC5 protein is secreted when the cDNA encoding FNDC5 is expressed in 293 cells. FNDC5-derived protein was detected by Western blotting. For further details, see ref. 15. H, hydrophobic domain. SP, signal peptide.
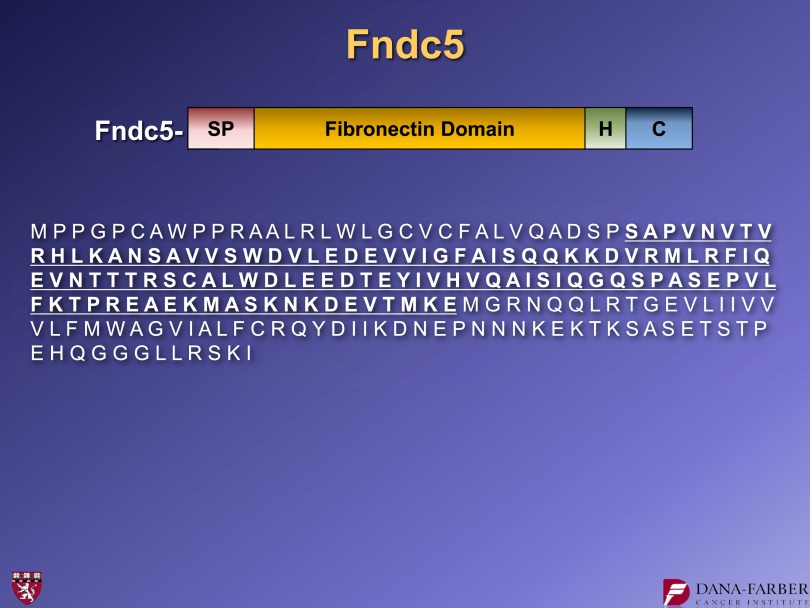
Collaborating with the laboratory of Steve Gygi, we identified the secreted fragment of FNDC5. And indeed, the N-terminal clip is just at the end of the signal peptide, as we predicted. There is a second cleavage just to the N-terminal side of the hydrophobic domain. Irisin represents amino acids 30–143 of FNDC5 (Fig. 10).
FIG. 10.
The amino acid sequence of FNDC5 and irisin (underlined), the secreted fragment of FNDC5. For further details, see ref. 15. C, C-terminal region. H, hydrophobic domain. SP, signal peptide.
Irisin is named for Iris, the Greek goddess who took messages from Mount Olympus and brought them down to humans on Earth. We did not want to name it for any particular function, but to highlight its role as a messenger that comes from skeletal muscle to other parts of the body.
An intriguing aspect about irisin is that it is 100% identical among most mammalian species. Figure 11 shows the amino acid sequence of human and mouse irisin. They are 100% identical. This suggests a very high degree of both conservation and function.
FIG. 11.
Alignment of amino acid sequences for human and mouse irisin.
When we applied FNDC5 to cells, we observed a dramatic increase in oxygen consumption both in the basal state and (especially) in the uncoupled state. This is a 300% increase in uncoupled respiration. Again, this is consistent with UCP1 expression and browning reaction.
Importantly, irisin circulates in mice and in humans. Our assay is a rather cumbersome Western blot. We have to clean up the plasma by removing albumin and immunoglobulins and deglycosylate this plasma so that the irisin forms a sharp band on SDS gels. Exercise in mice and humans stimulates an increase in irisin at the mRNA in muscle and protein in the blood (15). Based on standard curves with Western blots (not the most accurate method), we estimate that irisin circulates at roughly 50 nmol/L in both species.
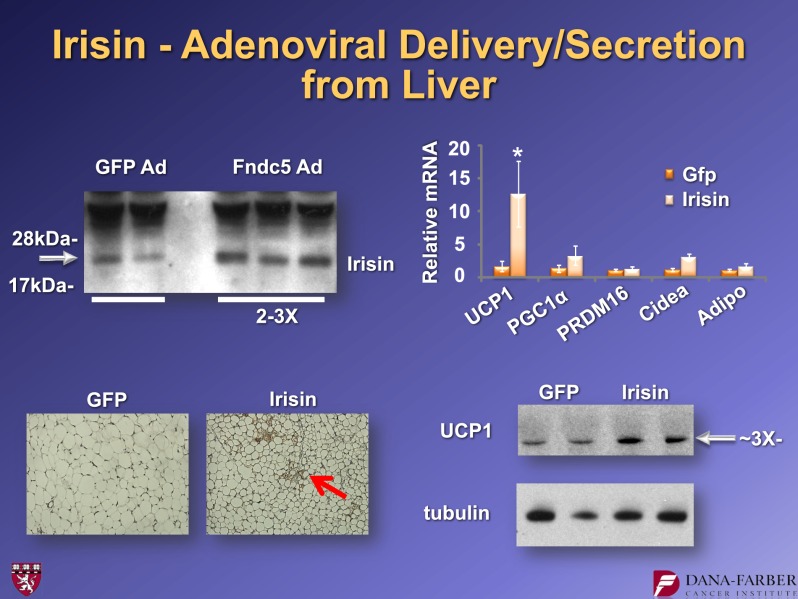
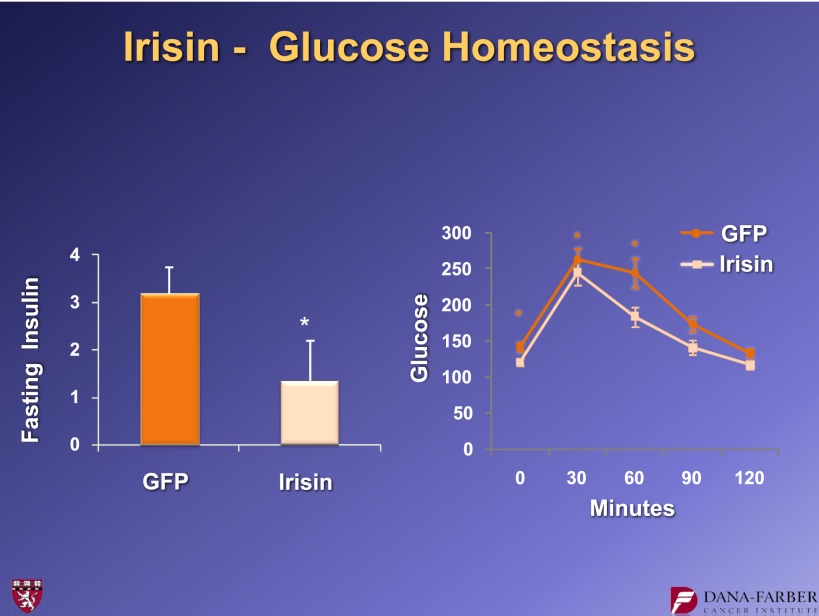
What does irisin do in vivo? We created adenoviral vectors that expressed full-length FNDC5 and injected them into the tail veins of mice. These viruses are taken up by the liver, which then cleaves and secretes the irisin. We see moderately elevated levels of circulating irisin (two- to threefold) in animals that received the FNDC5 virus. Ten days after the viral injection, we see more patches of UCP1-positive cells, again in the subcutaneous fat. We also observe the elevation of UCP1 protein levels and a robust increase in UCP1 mRNA (Fig. 12). When these experiments were done in the context of a high fat–fed, obese animal, we saw a reduction in fasting insulin and improvement in glucose tolerance (Fig. 13). This indicates that even a fairly moderate irisin elevation causes the browning of white fat and improvement in glucose homeostasis. That is exciting for a molecule that is increased with exercise.
FIG. 12.
Effects of adenoviral expression of FNDC5 on the browning of inguinal adipose tissue in mice. For further details, see ref. 15. Ad, adenovirus expression vector. Adipo, AdipoQ. *P < 0.05.
FIG. 13.
Fasting insulin levels and glucose tolerance in mice receiving adenoviral vectors expressing FNDC5. For further details, see ref. 15. *P < 0.05.
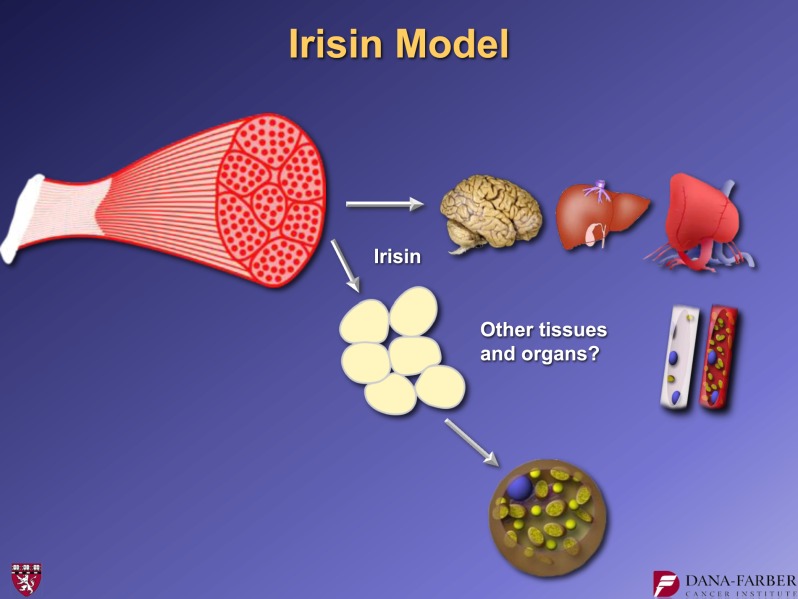
Taken together, these findings lead to a specific model: FNDC5 is a muscle surface protein that is proteolysed and shed to form the novel myokine, irisin (Fig. 14). This 112 amino acid polypeptide hormone is secreted into the blood and acts on the subcutaneous adipose cells to bring about a browning reaction that is protective against metabolic disease. It is of great interest to learn what effects irisin might have on other aspects of metabolic disease. It should be stressed that exercise provides great benefit to other tissues such as the brain, liver, heart, and skeletal muscle itself. Exercise is virtually the only thing known to cause neurogenesis in adult humans. It has also been shown to bring about some improvement in patients with Alzheimer’s, Parkinson’s, and some other neurodegenerative diseases. Obviously, in many cases, these conditions are so severe that it is difficult, if not impossible, for patients to exercise. In these and potentially other settings, it may be useful if we could provide some of the benefits of exercise for individuals who are not able to effectively exercise. This is currently a scientific mission of my research group.
FIG. 14.
Model of irisin secretion and actions.
How can we move forward into therapy for metabolic diseases and related syndromes? We have created a form of the irisin protein that is bioactive and stable in the blood. Fusion proteins between the Fc fragment of immunoglobulin and other proteins tend to take on some of the stability properties in the blood of the immunoglobulin. Of course, these may or may not retain the bioactivity of the native protein. We created two orientations, Fc with irisin at the C-terminus and irisin at the N-terminus of the Fc fragment. We made these in large quantities from CHO cells and purified them to near homogeneity. They were again applied to cultured subcutaneous murine adipose cells. We observed that Fc-irisin has little to no activity in inducing UCP1 mRNA, while, in contrast, irisin-Fc induces UCP1 and other thermogenic genes (16).
These are proof of concept molecules. Thus, they are not necessarily optimal, nor may they be appropriate for clinical use in humans. We and our colleagues still have much work to do to identify the most effective protein version of irisin that will permit us to maximize therapy. At the current time, we have a bioactive substance that can be used by us and other investigators to study this new hormone.
Recall that there are two kinds of brown fat cells: the classical brown fat (as exemplified by the interscapular depot in rodents) and the brown fat cells that appear in patches in the white adipose tissues. We first showed that these brownish cells in white fat do not even come from the same lineage as the classical brown fat cells (8). This indicated that the beige cells must be a different cell type from brown fat cells. But no one had isolated those cells. We therefore aimed to clone and isolate the beige adipose cells in order to characterize them and understand their molecular and physiological properties (16).
We began with a strain of mouse (129) that is known to have robust browning of its white fat. We took the stromal-vascular fraction, the precursor fibroblastic faction, from the subcutaneous adipose tissue. We then created 3T3-like immortalized cultures of these cells. By limiting dilution, we did single-cell cloning and created 305 new clonal cell lines, of which 23 underwent adipose differentiation very well.
We used Affymetrix analyses of gene expression from these 23 lines, and these datasets were then subjected to unsupervised clustering. These cell lines fell easily into two groupings. One group clustered closely with classical brown fat cells, which we also cloned at the same time. We hypothesized that these were more likely to be the beige cells, and that the other cluster was most likely the white cells of the adipose tissue.
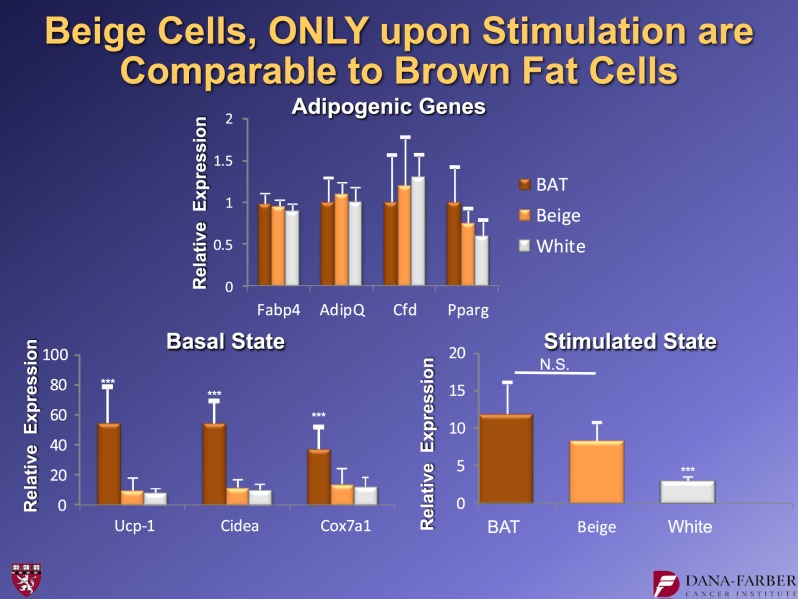
We performed some very basic characterization of the beige versus the white cells. The clones we chose differentiated equally well. Figure 15 shows genes like aP2 that serve as markers of adipose differentiation per se. Brown, beige, and white all differentiated well in these experiments. However, in an unstimulated state (with no cyclic AMP stimulation, a classical thermogenic agent), the brown fat cells had a high basal level of UCP1, while the beige and white cells had identical very low levels of UCP1 and other markers of beige or brown cells, such as cidea and COX7A. This suggested that in an unstimulated state, the beige cells behave like white fat cells in many routine aspects. However, when stimulated with cyclic AMP, the beige cells can robustly induce UCP1, whereas the white cells cannot. Thus, the beige cells can “hide” as white fat cells because they have essentially no UCP1 expression at baseline, but they can turn it on very robustly under appropriate conditions.
FIG. 15.
Molecular characteristics of beige adipocytes. Note that these cells have very low basal UCP1 mRNA expression, but induce it robustly upon cAMP stimulation. For further details, see ref. 16. BAT, brown adipose tissue. N.S., not significant. ***P < 0.05.
These findings led to a key question: Is the brown fat in adult humans that has been identified through PET scanning closer to the classical brown fat of rodents or is it similar to beige fat?
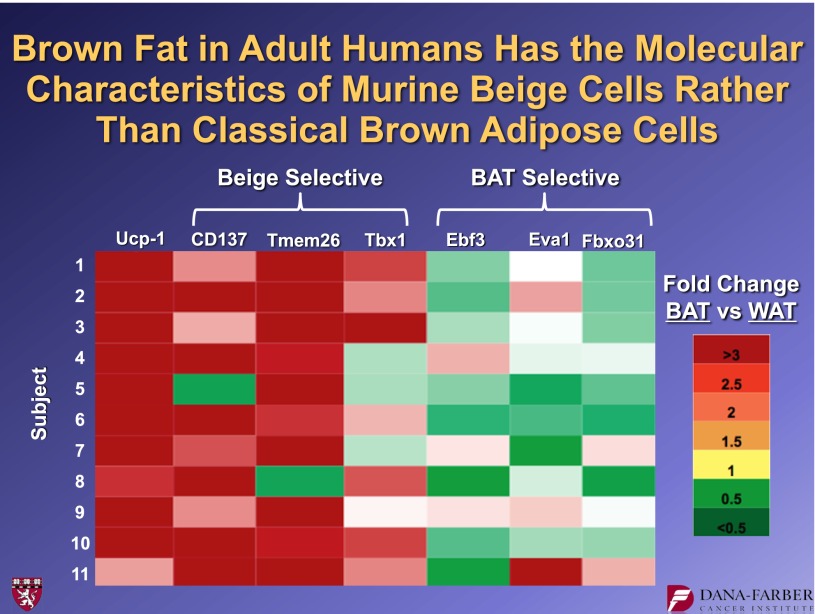
In collaboration with our Dutch and Scandinavian colleagues, we obtained biopsy material from healthy human volunteers. Surgeons harvested material from the PET-identified hot spots, as well as material from nearby regions of white fat that were not PET-positive. Using our brown and beige fat cells lines, we identified several molecules that were expressed in beige fat cells, but not brown ones. Conversely, we also found molecules that were expressed in brown cells, but not in beige ones. Figure 16 shows these data. The human brown fat, which is all UCP1-positive, is also positive for the three beige markers, CD137, Tmem26, and Tbx1. The three markers that we identified that were selective for the classical brown fat (Ebf3, Eva1, and Fbxo31) were not preferentially expressed in these human “brown” hot spots. Thus, these data argue strongly that those brown-like cells in adult humans are not equivalent to the interscapular brown fat of a rodent, but that they are much more similar to murine beige fat cells.
FIG. 16.
Human adult “brown fat” has the molecular signature of beige adipose cells. Biopsies of human PET-positive brown fat and neighboring white fat depots were taken, and RNA was prepared. qPCR was performed for UCP1 and beige and brown selective mRNAs. For further details, see ref. 16. BAT, brown adipose tissue. WAT, white adipose tissue.
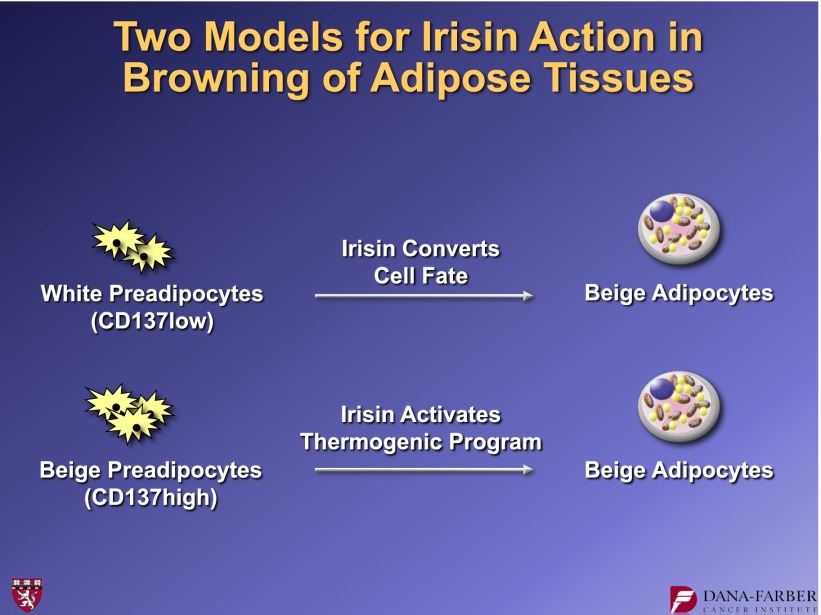
These observations allowed us to address another important question: What is irisin doing when it browns white fat? One of the markers of beige fat is CD137, a cell surface protein. We developed a fluorescence-activated cell sorter approach to isolate primary cells from the stromal-vascular fraction of inguinal adipose tissues as either white or beige preadipocytes. This led to two models: Does irisin convert cell fate, driving a white preadipose cell into a beige pathway? Or does it take cells predetermined to the beige phenotype and activate their thermogenic gene program (Fig. 17)?
FIG. 17.
Alternative models for how irisin can bring about thermogenic actions on beige cells.
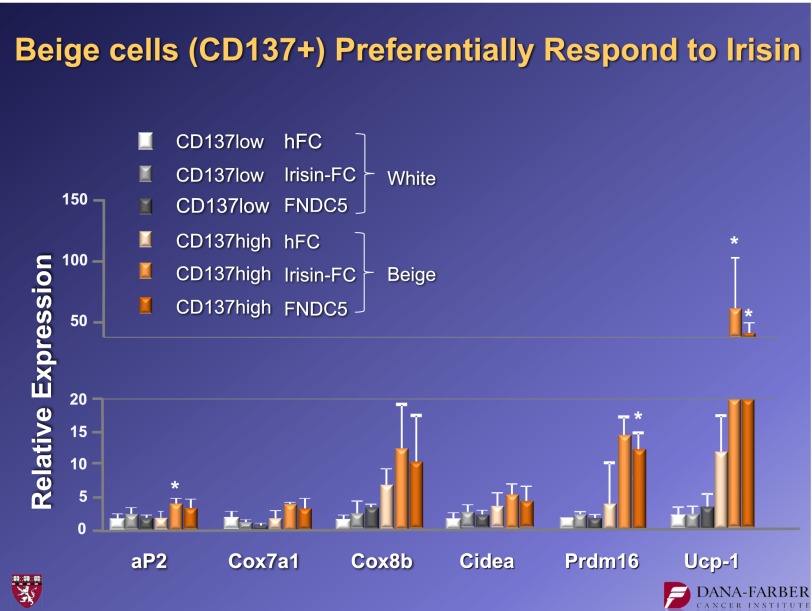
The data resolving this question are shown in Fig. 18. If the CD137 low cells (white preadipose) are taken from the subcutaneous depot and irisin is applied, there is no induction of UCP1. Thus, white primary cells do not appear to respond to irisin. On the other hand, the beige cells high in CD137 respond to irisin with a robust induction of UCP1. The same is true for the other molecular markers of thermogenesis. These findings support the idea that irisin works on the beige cell precursors by activating the thermogenic progam. The irisin molecule induces them to follow the pathway for which they are preprogrammed. It does not cause transdifferentiation and does not cause the white preadipose cells to “jump” cell lineage and become beige cells.
FIG. 18.
Irisin functions preferentially on beige cells and does not cause a change in cell fate. hFC, human FC fragment of IgG. *P < 0.05.
In conclusion, recent work shows that FNDC5 is an exercise- and PGC1-α–induced protein that is cleaved to release a peptide termed irisin. Irisin can be identified in the plasma of both mice and humans and increases with exercise. Irisin potently induces the browning of white adipose tissues. While it does not work on classical brown fat, its actions result in elevated thermogenesis. It should be noted that these studies were done in relatively short timeframes of 5- to 7-day treatments. The results of longer treatments are still unknown.
A very important conclusion from our studies is that while most or all humans have brown fat, it is closely related to—if not identical to—rodent beige fat rather than the classical brown fat. If we want to study hormone sensitivity and bioenergetics of human brown fat, we probably need to model it with the rodent beige fat. Fortunately, we now have the tools available to do this. Our work, which cloned immortalized beige fat cells, also identified a way to isolate primary white and beige cells. Despite this progress, many questions remain: What is the function of the markers of this cell type? What are the hormone sensitivities? What are the biogenetic parameters? What is the developmental lineage of beige cells? I am optimistic that in the next decade or two, we and the entire field can use this information to develop a new generation of therapeutics. Irisin is an attractive example, but I am certain it will not be the only example. We are in a position now to bring this area of cell biology into the realm of human diabetes prevention and perhaps also therapy for obesity.
ACKNOWLEDGMENTS
This work has been funded by the National Institutes of Health (the National Institute of Diabetes and Digestive and Kidney Diseases specifically) and by The JPB Foundation.
B.M.S. is a cofounder, consultant, and shareholder in Ember Therapeutics.
B.M.S. thanks the people in his own laboratory who participated in the studies described in this article, as well as collaborators. B.M.S. acknowledges Pontus Boström, Jun Wu, Kyle Rasbach, Jonathan Long, Christiane Wrann, Anisha Korde, Sondra Calhoun, Steve Gygi (Harvard), Mark Jedrychowski, Kurt Hojlund (Denmark), Hua Tu (LakePharma), Sven Enerbach Gothenberg, Patrick Schrauwen (Maastricht), and Wouter van Marken Lichtenbelt.
REFERENCES
- 1.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 2.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994;79:1147–1156 [DOI] [PubMed] [Google Scholar]
- 3.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–839 [DOI] [PubMed] [Google Scholar]
- 4.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007;6:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 6.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002;16:1879–1886 [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002;418:797–801 [DOI] [PubMed] [Google Scholar]
- 13.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–735 [DOI] [PubMed] [Google Scholar]
- 14.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 2012;23:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science 2010;328:1113–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]