Abstract
Human type 1 diabetes (T1D) is an autoimmune disease associated with major histocompatibility complex polymorphisms, β-cell autoantibodies, and autoreactive T cells. However, there is increasing evidence that innate cells may also play critical roles in T1D. We aimed to monitor peripheral immune cells in early stages of T1D (i.e., in healthy autoantibody-positive subjects) and in more advanced phases of the disease (i.e., at disease onset and years after diagnosis). We found a mild but significant and reproducible peripheral neutropenia that both precedes and accompanies the onset of T1D. This reduction was not due to peripheral neutrophil cell death, impaired differentiation, or the presence of anti-neutrophil antibodies. Neutrophils were observed by electron microscopy and immunohistochemical analysis in the exocrine pancreas of multiorgan donors with T1D (both at onset and at later stages of the disease) and not in that of multiorgan donors with type 2 diabetes or nondiabetic donors. These pancreas-infiltrating neutrophils mainly localized at the level of very small blood vessels. Our findings suggest the existence of a hitherto unrecognized clinical phenotype that might reflect unexplored pathogenic pathways underlying T1D.
Type 1 diabetes (T1D) is an autoimmune disease that is associated with and predicted by β-cell autoantibodies (autoAbs) (1), where insulin-producing β-cells are thought to be destroyed by autoreactive T cells (2). These findings, together with the recognized major histocompatibility complex (MHC)-restricted genetic susceptibility (3), suggest a prominent role of adaptive immunity in the pathogenesis of T1D. However, there is increasing evidence that innate cells play critical roles in T1D (4,5).
RESEARCH DESIGN AND METHODS
Subjects and data collection.
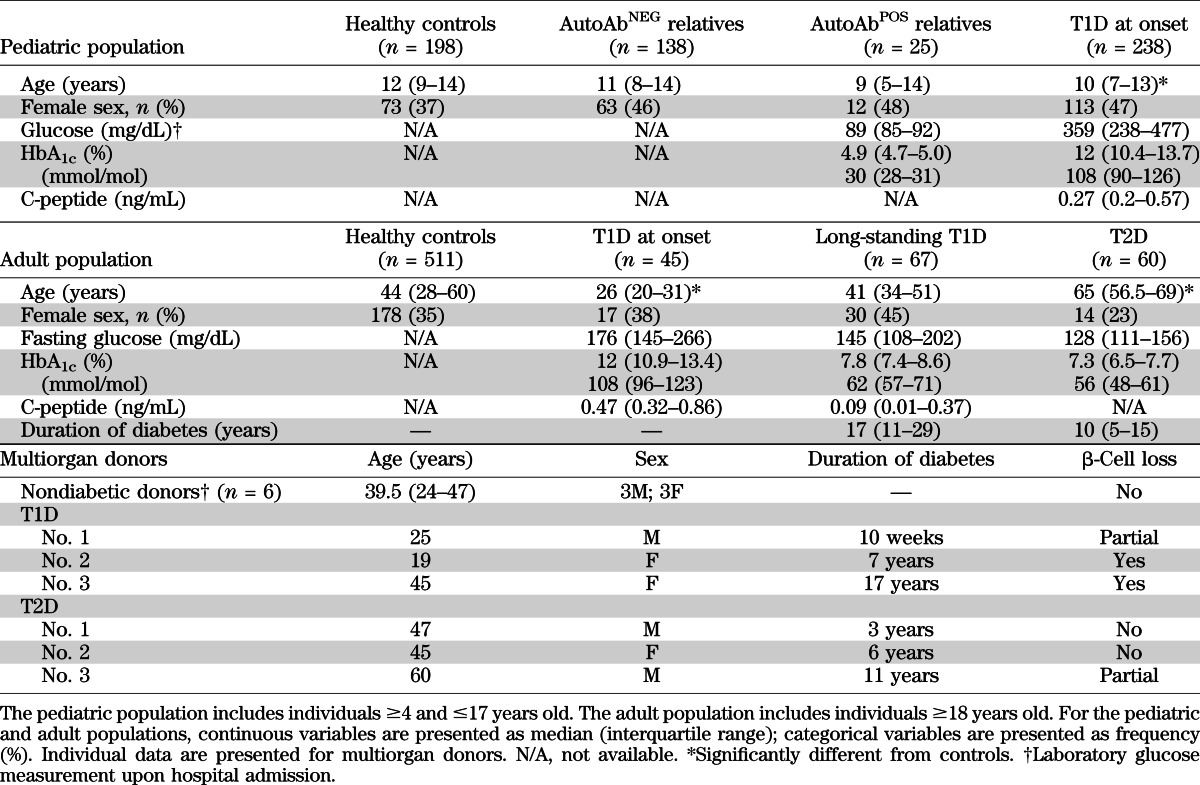
The study was approved by the San Raffaele Hospital Ethics Committee (protocol DRI-002). Cell blood counts (CBCs) performed by the Sysmex XE-2100 automated hematology analyzer (6) at the San Raffaele Hospital were collected retrospectively from pediatric (4–17 years of age) and adult individuals (≥18 years of age) in the groups described below and in Table 1.
TABLE 1.
Characteristics of the subjects recruited at the San Raffaele Hospital and of the multiorgan donors included in the study
Pediatric patients with T1D at onset.
Using the Pediatric Department registry, we identified children who were diagnosed with T1D between August 2006 and December 2011 and hospitalized. Of these patients, 89.4% had at least one islet-specific autoAb (ICA, IA2, GAD, or ZnT8). The CBCs closest to hospital discharge and the first CBC obtained at each time point during clinical follow-up after T1D diagnosis up to March 2012 were included in the analysis.
Healthy pediatric controls.
Using the Orthopedic Pediatric Surgery Registry, we identified all children with no concomitant diseases who had elective orthopedic surgery between August 2006 and February 2012. CBCs before surgery were included in the analysis.
Adult patients with T1D at onset.
Using the electronic records at San Raffaele Hospital, we identified all patients newly diagnosed with T1D and who were admitted to the Department of Internal Medicine between May 2006 and October 2011 to start insulin therapy. The diagnosis of T1D was made based on sustained hyperglycemia (documented by repeated glucose measurements and measurement of HbA1c), fasting C-peptide levels <1.0 ng/mL, or the presence of at least one islet-specific autoAb. The percentage of these patients with at least one islet-specific autoAb was 71.4%. The CBCs closest to hospital discharge were included in the analysis.
Adult patients with long-standing T1D and type 2 diabetes.
Patients with T1D and duration of disease ≥5 years and patients with type 2 diabetes (T2D) (median disease duration 10 years; interquartile range 5–15) were recruited from the Diabetes Clinics of the San Raffaele Hospital between April and August 2011. The CBCs obtained during a scheduled follow-up visit at the clinic in the absence of acute conditions were included in the analysis.
Healthy adult controls.
The list of all blood donors active between July 2006 and December 2010 was obtained from the blood bank of the San Raffaele Hospital (ABZero) (n = 7,903). From this pool of donors we randomly selected sex- and age-matched controls for patients with T1D or T2D. CBCs obtained before the first blood donation were included in the analysis.
Relatives of patients with T1D.
First-degree relatives (1–45 years of age) and second- and third-degree relatives (1–20 years of age) of patients with T1D were enrolled in the Type 1 Diabetes TrialNet Pathway to Prevention Trial (TN01 Trial, formerly the TrialNet Natural History Study) (7). The overall objective of this study is to perform baseline and repeated measurements to assess over time the immunologic and metabolic status of individuals at risk for T1D. The complete protocol is available online (8). Our local study was approved by the TrialNet Ancillary Studies Subcommittee, and we started to collect blood in February 2012. Detection of autoAbs was performed at a central core laboratory. Subjects with at least two positive tests for any one of the five islet-specific autoAbs (GADA, ICA512A, ICA, mIAA, and ZnT8) were defined as autoAb positive (autoAbPOS); subjects with negative or unconfirmed results for all autoAb tests (i.e., one positive test never confirmed in subsequent analyses) were defined as autoAb negative (autoAbNEG). AutoAbPOS subjects have a higher risk of developing T1D than do autoAbNEG subjects (7). The individuals enrolled in the TN01 Trial and included in our ancillary study were classified further as relatives at low risk (i.e., subjects positive for one autoAb and with a normal oral glucose tolerance test, n = 15), who have a 2.5% 5-year risk of developing diabetes, and relatives at high risk (i.e., subjects positive for ≥2 autoAb or a history of at least one abnormal oral glucose tolerance test, n = 10), who have a 32% 5-year risk of diabetes (9).
Phenotype and apoptosis analysis by flow cytometry.
Fresh whole blood was stained with specific monoclonal antibodies (mAbs; all from BD Pharmingen, Franklin Lakes, NJ) and samples were analyzed with the BD FACSCanto-II flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Rainbow calibration particles (Spherotech Inc., Lake Forest, IL) were used to calibrate and normalize acquisition settings in each experiment. Apoptosis was defined by staining fresh whole blood with annexin V mAb and 7-aminoactinomycin D (BD Pharmingen), and analyzed within 1 h with the BD FACSCanto-II flow cytometer. Analyses of flow cytometry data were performed with FCS Express 4.0 software (De Novo Software, Los Angeles, CA).
Immunohistochemical and electron microscopical studies of pancreata from multiorgan donors.
Whole pancreases were obtained from three multiorgan donors with T1D, three with T2D, and six Caucasoid multiorgan donors (33.2 ± 14.4 years of age; three men, three women; BMI 24.9 ± 1.3 kg/m2) with no family history of T1D or T2D. Characteristics of multiorgan donors are described in Table 1. Length of stay in the intensive care unit, perfusion protocol, and time of cold ischemia were not different among the three groups of donors analyzed. Pancreatic specimens were fixed in formalin and embedded in paraffin for immunohistochemical investigations. Immunoperoxidase staining for neutrophils on pancreatic paraffin sections was performed using rabbit antihuman myeloperoxidase mAb (Abcam, Cambridge Sciences Park, Cambridge, UK) followed by horseradish peroxidase (HRP) conjugated swine antirabbit secondary antibody (Dako Corporation, Carpinteria, CA). For electron microscopy, pancreatic samples were fixed, dehydrated, transferred to propylene oxide, and embedded in epon-araldite. Ultrathin sections (60–80 nm thick) were cut with a diamond knife.
Statistical analysis.
Comparisons of general characteristics and different blood cell counts between groups were conducted using ANOVA after rank transformation, when necessary. Pairwise comparisons were conducted using the Tukey wholly significant difference (WSD) test when the ANOVA showed a significant difference between at least two group means. Comparisons of neutrophil and platelet counts in pediatric patients at onset of T1D and at 1 year (±60 days) after diagnosis were conducted using the Mann-Whitney U test. Neutrophil and platelet counts obtained during the clinical follow-up of pediatric patients after T1D diagnosis were analyzed using a mixed-effect model, with time as the fixed effect. An unstructured variance-covariance matrix was used to model the correlation between repeated measurements within each patient. Data management and statistical analyses were conducted with the Stata statistical software, version 11.0 (Stata Corp., College Station, TX).
RESULTS
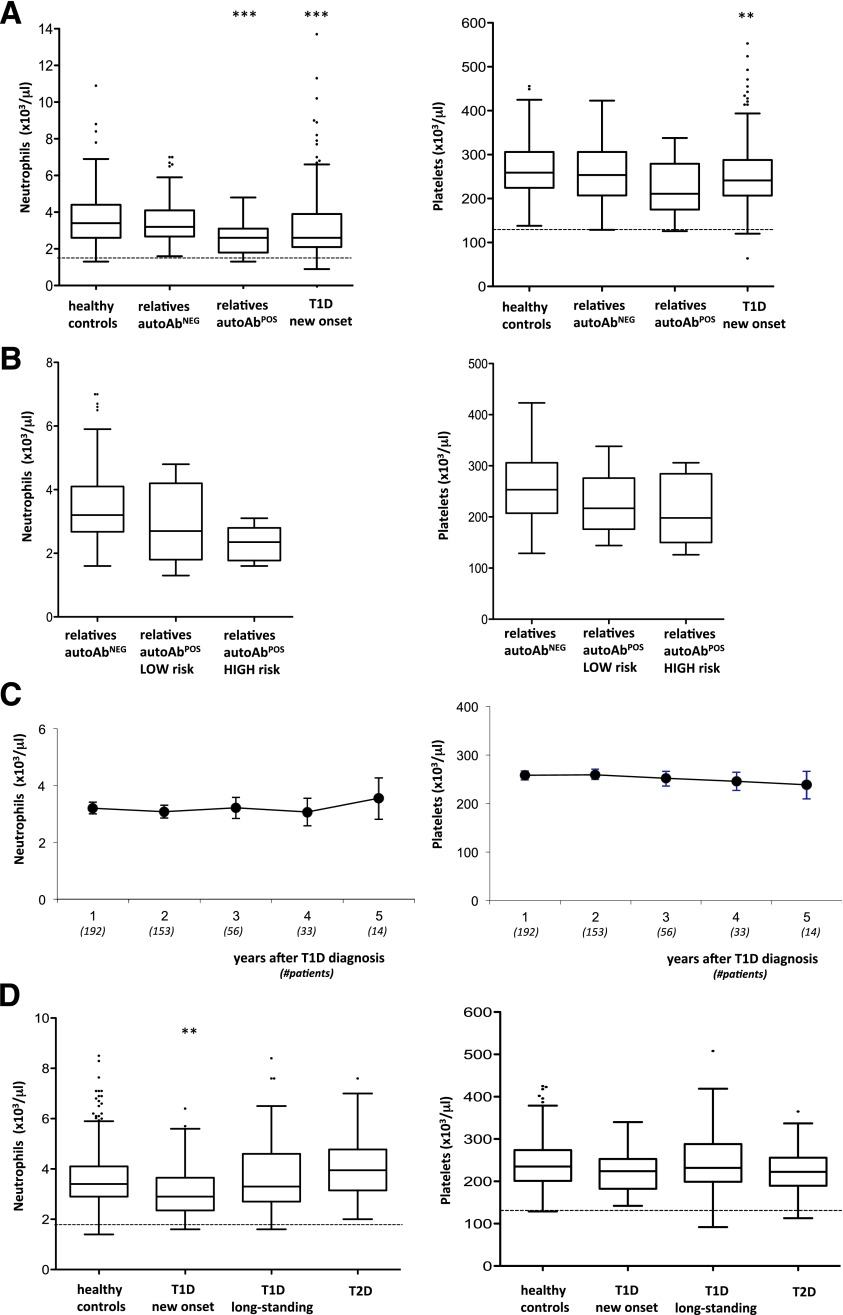
A retrospective analysis of CBCs performed in pediatric patients newly diagnosed with T1D demonstrated that circulating neutrophils and platelets were significantly lower than levels in healthy control subjects (Table 2). This finding was also confirmed in a subset of 123 patients with T1D at onset who were tested 1 year (±60 days) after diagnosis (data not shown). Given that the reduced neutrophil and platelet counts might derive from the metabolic derangements that often characterize patients with T1D, we analyzed CBCs of the subjects enrolled in the TN01 Trial (7). Both neutrophils and platelets were already significantly reduced in healthy pediatric autoAbPOS relatives of patients with T1D but not in autoAbNEG relatives (Fig. 1A). Neutrophil and platelet reductions were proportionate to the risk of developing T1D (i.e., the more pronounced the neutrophil decline, the higher the risk of developing T1D) (see research design and methods for risk assessment) (Fig. 1B). All CBCs obtained after diagnosis of T1D then were analyzed, and neutrophils seemed to return to normal levels 5 years after diagnosis, whereas platelets remained low (Fig. 1C).
TABLE 2.
Cell blood counts (×103/μL)
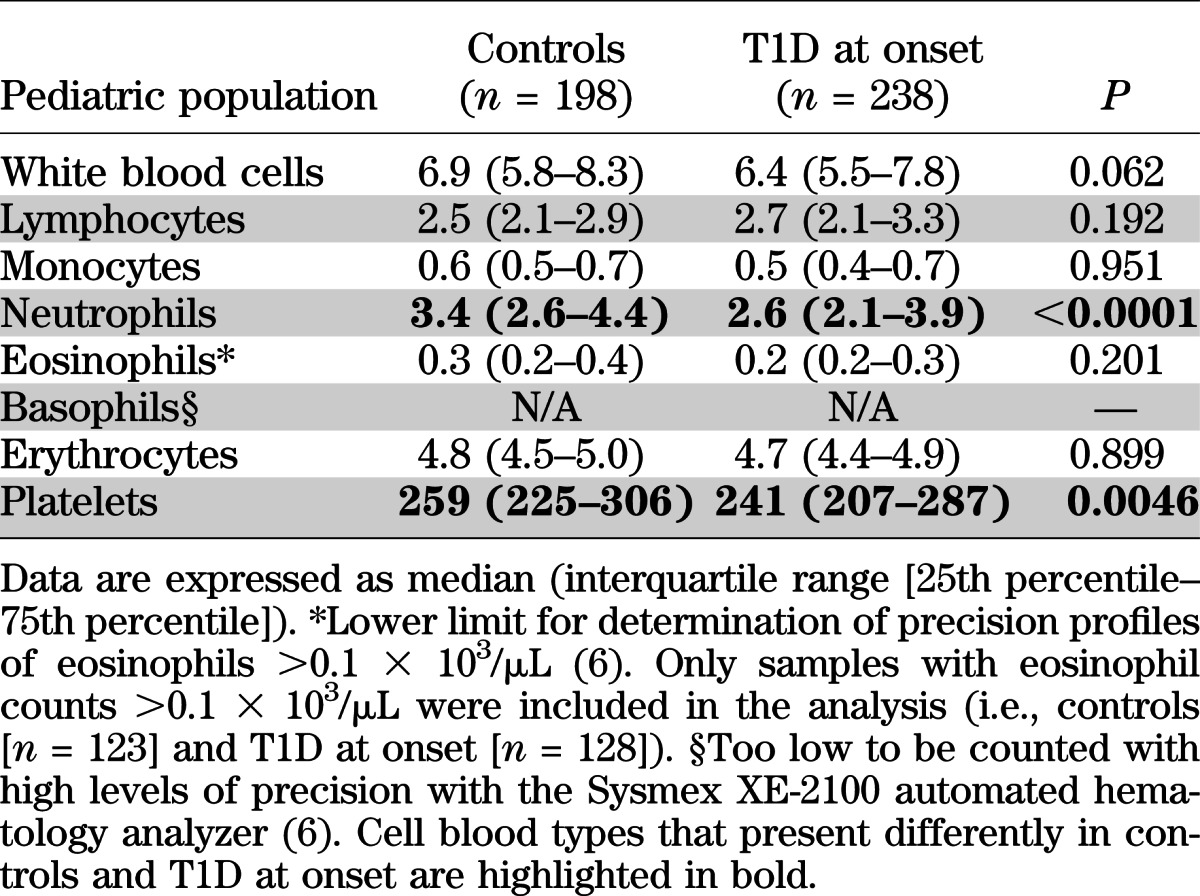
FIG. 1.
A: Neutrophil and platelet counts in pediatric healthy controls (n = 198) and autoAbNEG (n = 138) and autoAbPOS (n = 25) relatives of patients with T1D, and patients with T1D at onset (n = 238); **P < 0.005, ***P < 0.0001 vs. healthy controls, ANOVA and Tukey WSD test. The lower normal laboratory range is shown by the dotted line. B: Neutrophil and platelet counts in pediatric autoAbNEG relatives (n = 138) and autoAbPOS relatives at low (n = 15) and high risk (n = 10), as defined in the research design and methods section. The nonparametric test for trend is significant for both neutrophil (P < 0.001) and platelet (P = 0.023) counts. Means and SDs of neutrophil and platelet counts for each group also are reported. C: Neutrophil and platelet counts in pediatric patients studied at the onset of T1D and measured during clinical follow-up. Filled circles represent mean values, and bars represent 95% CIs. Numbers in parentheses on the x-axis represent the number of patients analyzed. D: Neutrophil and platelet counts in adult healthy controls (n = 511), patients with T1D at onset (n = 45), individuals with ≥5 years of T1D (n = 67), and individuals with T2D (n = 60) aged ≥18 years; **P < 0.005 vs. healthy controls, ANOVA and Tukey WSD test. The lower normal laboratory range is shown by the dotted line. Square dots that appear above the whiskers are outliers, i.e., individual observations that are above the upper fence of the distribution (i.e., above the 75th percentile).
A similar reduced level of circulating neutrophils also was confirmed in a group of adult patients newly diagnosed with T1D but not in those with T2D, whereas platelets were not reduced, in contrast with the findings for pediatric subjects. As in the pediatric populations, neutrophils were normal in adult patients ≥5 years after diagnosis (Fig. 1D). Similar results were observed in an independent group of adult patients followed in the Outpatient Diabetes Care Unit at Siena University Hospital (Supplementary Fig. 1).
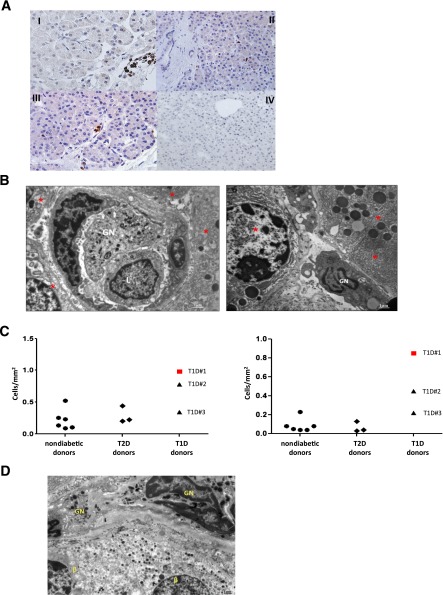
Reduced circulating neutrophils can be caused by one or more of the following: 1) impairment in neutrophil output from the bone marrow, differentiation of neutrophils, or both; 2) increase in peripheral consumption/destruction; 3) tissue sequestration. A bone marrow defect, an impaired peripheral differentiation, or both are unlikely because the phenomenon is mild and transitory (from preclinical phase to a few years after clinical onset) and there was no evidence of peripheral accumulation of immature forms, such as banded cells (Supplementary Fig. 2A). Alternatively, an increase in the consumption/destruction of peripheral neutrophils might be caused by augmented neutrophil apoptosis (10) or anti-neutrophil–specific antibodies (11). Both hypotheses were tested and neither was supported by experimental evidence. Neutrophil apoptosis, determined by 7-aminoactinomycin D and annexin V stainings, was very low in all donors tested (i.e., 11 autoAbNEG relatives, 3 autoAbPOS relatives, 3 patients with T1D at onset, and 2 patients with long-term T1D; data not shown). In addition, the expression levels of CD11b and CD16 in neutrophils were similar in all cohorts analyzed, a finding that further confirms the lack of any specific neutrophil activation, which might specifically account for increased neutrophil death (Supplementary Fig. 2B). Anti-neutrophil cytoplasmic antibodies (ANCA)—both perinuclear (pANCA) and cytolpasmic (cANCA)—or surface antibodies (human neutrophil antigens [HNA]) were not consistently observed in patients with T1D and their relatives (Supplementary Table 1). Finally, the hypothesis of tissue sequestration at pancreatic level was tested in pancreatic tissue specimens from adult organ donors with T1D and T2D and from nondiabetic donors (12–14) (donor characteristics are described in Table 1). Increased numbers of neutrophils were detected specifically in the exocrine pancreas of the three patients with T1D who were analyzed but were not detected in the pancreas of donors with T2D and nondiabetic donors. These pancreas-infiltrating neutrophils mainly localize at the level of very small blood vessels and, to a lesser extent, adjacent to acinar cells (Fig. 2A and B). These data, albeit generated in a limited number of donors, were consistent between individuals and independent of the disease stage (from onset to long-standing) (Fig. 2C). A low number of neutrophils was observed close to β islets, but only in the patients recently diagnosed with T1D (Fig. 2D).
FIG. 2.
A: Neutrophils are detected by immunoperoxidase in the exocrine pancreas. Representative neutrophil stainings of sections from donor no. 1 with T1D (I and II), donor no. 3 with T1D (III), and a nondiabetic donor (IV) are shown. These pancreas-infiltrating neutrophils localize mainly at the level of very small blood vessels (panels I and III) and, to a lesser extent, adjacent to acinar cells (panel II). B: Representative sections of the pancreas collected from donor no. 1 with T1D, analyzed using electron microscopy, and showing a granulocyte (GN) and a lymphocyte (L) in a microvessel of the exocrine tissue (left panel), and a GN adjacent to acinar cells (right panel). In both panels, red stars identify pancreatic acinar cells. C: The frequency of pancreatic neutrophils is determined by immunoperoxidase on pancreatic paraffin sections. The numbers of myeloperoxidase positive cells per square millimeter in the pancreas (left panel) and within the small blood vessels (right panel) are shown. At least 20 fields per donor have been examined. D: A representative section of the pancreas collected from donor no. 1 with T1D, analyzed using electron microscopy and showing GNs adjacent to β-cells (β).
DISCUSSION
These findings demonstrate an association between human T1D and a hitherto unrecognized neutropenia. Although minor, this neutropenia is not negligible; neutrophil reduction varies from 7 to 27%. This abnormality manifests from the preclinical phase of the disease to onset and persists for some years before long-term resolution. During the preclinical phase, neutrophil reduction is greatest in the subjects with the highest risk of developing T1D, a finding that possibly reflects the severity of the underlying autoimmune process. After disease onset, the persistence of mild neutropenia for a few years, and its subsequent resolution, seems to mirror the continuing destruction of β-cell mass during its residual survival. Hence, reduced circulating neutrophils seem to be a phenotype that accompanies the phase of active, ongoing, destructive β-cell–specific autoimmunity. Conversely, the lack of this phenotype in patients with T2D and the lack of correlation between glucose levels/HbA1c and neutrophil counts in both pediatric and adult subjects (data not shown) excludes hyperglycemia and associated metabolic abnormalities as the cause of this reduction in T1D.
Platelet levels seem to be reduced exclusively in patients with disease onset during childhood and to differ in trend from neutrophil levels (i.e., the former remain low beyond 5 years after diagnosis). However, this difference might be ascribed to the small group of adults analyzed compared with the group of pediatric patients. Future longitudinal studies including more adult subjects and with a well-designed sampling time frame should allow us to draw definitive conclusions about neutrophil and platelet counts in T1D.
A specific reduction in circulating neutrophils in T1D might be indirect evidence of a chronic viral infection, which has been long suspected as a trigger in susceptible hosts. However, despite decades of research, the body of evidence supporting a relationship between viral infections and initiation or acceleration of islet autoimmunity remains largely circumstantial (15).
The occurrence of neutrophils in inflamed tissues targeted by an autoimmune process is not surprising from an immunological point of view (16), but, to our knowledge, this finding in humans has been reported only once (several years ago) (17) and has never been confirmed in subsequent studies (18). However, we recognize that our data were generated from a limited amount of donors and are far from being conclusive.
While neutrophils play a crucial role in several autoimmune diseases (e.g., systemic lupus erythematosus, rheumatoid arthritis) (19) and, as recently demonstrated, in a murine model of T1D (4), their function in human T1D has been ignored to date. There are several likely reasons for this neglect: the perception of neutrophils as terminally differentiated, short-lived immune cells; the lack of appropriate methods of molecular manipulation of neutrophils; and the inability to test the role of such cells in a given disease mechanism. However, more recent studies indicate that neutrophils are capable of performing a large number of functions that are critical for the autoimmune disease process, including antigen presentation, regulation of the activity of other cell types (20,21), and direct tissue damage (16). Our new evidence suggests that neutrophils might also be key in the pathogenesis of T1D.
Supplementary Material
ACKNOWLEDGMENTS
M.B. is supported by the Juvenile Diabetes Research Foundation (JDRF), the Italian Ministry of Health, and the European Union. F.D. is supported by the European Union (Collaborative Projects NAIMIT and PEVNET in the Framework Program 7 [FP7]) and by the Italian Ministry of Research. Type 1 Diabetes TrialNet is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources (NCRR), the JDRF, and the American Diabetes Association (ADA). E.Bo. has received the JDRF grant 6-2012-18 in support of the TrialNet TN01 study at the San Raffaele Hospital.
This work was performed in partial fulfillment of the requirements for a PhD degree in Molecular Medicine for A.V.
No potential conflicts of interest relevant to this article were reported.
A.V., G.M.G., M.S., and M.B. conceived the study, designed the experiments, analyzed and interpreted data, and wrote the manuscript. A.S. processed samples and performed in vitro experiments. P.G. and E.Bi. collected samples. G.S. and M.M. performed analyses on pancreas sections. N.M. contributed to experimental design and analyzed and interpreted data. L.Po. performed anti-neutrophil antibodies assay. R.B. and F.M. recruited pediatric patients with T1D. M.D.P. and A.L. recruited nondiabetic pediatric patients. S.R. recruited nondiabetic adults. L.Pi. contributed to experimental design and interpreted data. P.M. collected pancreas samples from multiorgan donors. F.D. contributed to experimental design and interpreted data. E.Bo. analyzed and interpreted data and wrote the manuscript. M.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all members of M.B.’s laboratory (especially Georgia Fousteri and Cristina Morsiani, for excellent technical help/support); all nurses from the pediatric department at the San Raffaele Hospital for help with blood collection; Sonny Michael Assennato (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico) and Elena Bazzigalupi (Laboraf) for performing the antineutrophil antibody tests; Giulio Frontino and Andrea Rigamonti (Department of Paediatrics and Neonatology, San Raffaele Hospital) and Andrea Laurenzi (Department of Internal Medicine, San Raffaele Hospital) for patient recruitment; Isabella Spagnuolo and Aurora Patti (University of Siena) for excellent contributions to the morphometric counting of neutrophils in human pancreatic sections; Gaetano Di Terlizzi, Ferruccio Ceriotti, and Massimo Locatelli (Laboraf) for technical support; Davide Di Napoli (San Raffaele Hospital) for providing patient information; and Luca G. Guidotti and Angelo Manfredi (San Raffaele Scientific Institute) for suggestions and critical discussion.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1345/-/DC1.
A.V., G.M.G., and M.S. contributed equally to this study.
S.R. is currently affiliated with the Department of Transfusion Medicine, Niguarda Ca’ Granda Hospital, Milan, Italy.
See accompanying commentary, p. 1821.
REFERENCES
- 1.Pietropaolo M, Eisenbarth GS. Autoantibodies in human diabetes. Curr Dir Autoimmun 2001;4:252–282 [DOI] [PubMed] [Google Scholar]
- 2.Roep BO, Peakman M. Diabetogenic T lymphocytes in human type 1 diabetes. Curr Opin Immunol 2011;23:746–753 [DOI] [PubMed] [Google Scholar]
- 3.Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet 2011;12:781–792 [DOI] [PubMed] [Google Scholar]
- 4.Diana J, Simoni Y, Furio L, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013;19:65–73 [DOI] [PubMed] [Google Scholar]
- 5.Grieco FA, Vendrame F, Spagnuolo I, Dotta F. Innate immunity and the pathogenesis of type 1 diabetes. Semin Immunopathol 2011;33:57–66 [DOI] [PubMed] [Google Scholar]
- 6.Walters J, Garrity P. Performance evaluation of the Sysmex XE-2100 Hematology Analyzer. Lab Hematol 2000;6:83–92 [Google Scholar]
- 7.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. TrialNet Natural History Committee. Type 1 Diabetes TrialNet Study Group The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 8.Type 1 Diabetes TrialNet. TN-01 natural history – protocol synopsis & assessment schedule [Internet], 2011. Available from http://www.diabetestrialnet.org/documents/biobank/6NaturalHistoryProtocolSynopsisAssessmentSchedule.pdf Accessed 21 January 2013
- 9.Sosenko JM, Skyler JS, Mahon J, et al. Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care 2011;34:1785–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett 2001;487:318–322 [DOI] [PubMed] [Google Scholar]
- 11.Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther 2005;7:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roep BO, Kleijwegt FS, van Halteren AG, et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 2010;159:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med 2012;2:a007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012;30:459–489 [DOI] [PubMed] [Google Scholar]
- 17.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 [DOI] [PubMed] [Google Scholar]
- 18.Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK. Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 2011;33:9–21 [DOI] [PubMed] [Google Scholar]
- 19.Németh T, Mócsai A. The role of neutrophils in autoimmune diseases. Immunol Lett 2012;143:9–19 [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11:519–531 [DOI] [PubMed] [Google Scholar]
- 21.Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 2012;122:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.