Substantial evidence supports a role for chronic subclinical inflammation in the pathogenesis of insulin resistance (1,2). The inflammation induces a number of local and systemic responses, including extracellular matrix (ECM) remodeling. ECM is normally one of the most important regulators of cellular and tissue function in the body since it acts as a scaffold for cell migration, a reservoir for cytokines and growth factors, and a binding site for various cellular receptors. A disturbed ECM is therefore linked to cellular dysfunction, and emerging studies show that insulin-resistant skeletal muscle is characterized by increased ECM (3–5). However, the functional connection between insulin resistance and ECM expansion has received little attention.
In the current issue of Diabetes, Kang and colleagues (6) show that feeding rodents a high-fat diet leads to accumulation of the ECM molecule hyaluronan (HA) in skeletal muscle, which contributes to insulin resistance. HA is a large nonbranched anionic glycosaminoglycan that is important for holding water in tissues and thereby functions as a space-filling molecule. It also binds to cell-surface receptors and influences cellular responses such as cell migration and proliferation (7,8). In contrast to other glycosaminoglycans, HA is not linked to a core protein, and it is synthesized at the plasma membrane rather than in the endoplasmic reticulum and Golgi. HA is synthesized by a family of enzymes known as HA synthases and is extruded into the ECM while still elongating (7).
Compared with other ECM molecules, HA has a surprisingly rapid turnover, which is regulated by hyaluronidases, HA-degrading enzymes (7). The hyaluronidase PH20 has enzymatic activity at neutral pH and can thus be used in therapeutic approaches to degrade HA (9). Although the native PH20 is rapidly degraded in serum, a pegylated variant of recombinant PH20 (PEGPH20) is stable and can be administered intravenously to tissues to degrade HA. Using this approach, Kang and colleagues (6) tested the hypothesis that reduction of HA in the muscle ECM by the long-acting PEGPH20 reverses high-fat diet–induced muscle insulin resistance. Treatment with PEGPH20 reduced the HA content in skeletal muscle by 80% and, most importantly, reversed the insulin resistance. This treatment also reversed insulin resistance in other insulin-sensitive tissues, including liver and adipose tissue, and decreased inflammation in adipose tissue. Hence, Kang and colleagues (6) provide evidence for a causal relationship between HA expression and insulin resistance (Fig. 1).
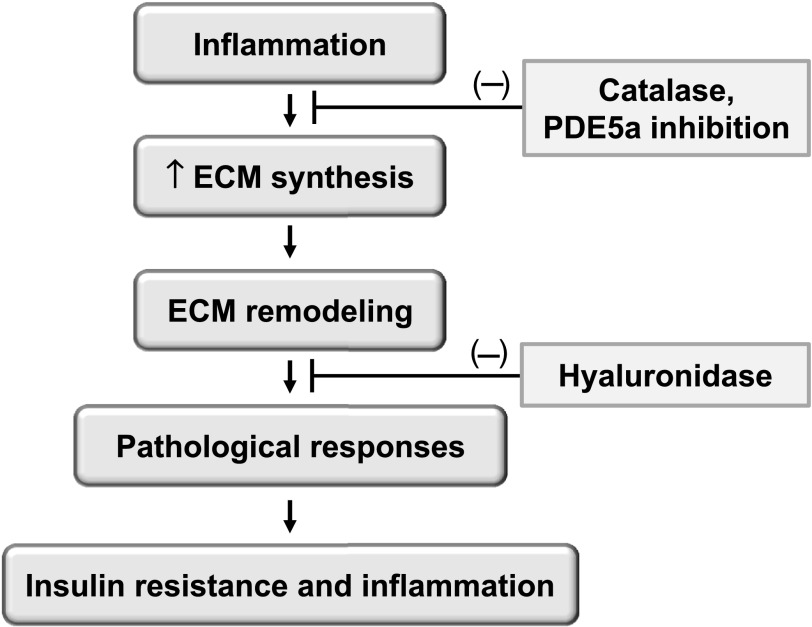
FIG. 1.
Proposed model for how ECM remodeling is linked to high-fat diet–induced muscle insulin resistance. High-fat diet potentially leads to insulin resistance by several mechanisms, including inflammation that is closely linked to ECM expansion and remodeling. Interruptions of the pathway at several steps rescue insulin resistance. In the current study, Kang and colleagues (6) used hyaluronidase to degrade the HA in skeletal muscle. Earlier results from the same group have shown that overexpression of catalase in the mitochondria, which reduces oxygen free-radical production, prevented muscle inflammation, diminished ECM expansion, and rescued insulin resistance (5). Likewise, inhibition of phosphodiesterase 5A (PDE5a) has been shown to rescue insulin resistance by preventing ECM expansion (5).
Although the exact mechanism was not determined, the study gives important clues. First, treating cultured muscle cells with PEGPH20 did not affect the cellular insulin response, indicating that PEGPH20 does not achieve its in vivo effect by acting directly on the skeletal muscle cells. Second, acute degradation of HA did not improve insulin sensitivity, which suggests that HA does not simply act as a physical barrier for insulin in the muscle ECM. Third, the authors showed that HA degradation caused increased vascularization in the muscle tissue, which may be the main explanation for the improved insulin sensitivity. For example, increased vascularization may improve insulin sensitivity by reducing tissue inflammation (10) and/or improving the transport of insulin from blood to skeletal muscle cells (11).
Recent genetic association studies have linked CD44 (the main cell-surface receptor for HA) with type 2 diabetes (12). Studies with genetically modified mice further revealed that a genetic deletion of CD44 reduces high-fat diet–induced insulin resistance and adipose tissue inflammation; similar results were achieved with anti-CD44 antibody treatment (12). CD44 is a cell-surface glycoprotein that is involved in a wide variety of cellular functions including cell–cell interactions, cell adhesion, migration, lymphocyte activation, regulation of angiogenesis, and endothelial cell proliferation (13,14). It is therefore possible that increased HA–CD44 interactions regulate insulin sensitivity by influencing the endothelial/vascular function. Interestingly, an increased level of HA in the arterial wall is also a recognized feature of the diabetic macroangiopathy (15,16), and studies in vitro support an important role for CD44 and its isoform, CD44v3, in the smooth muscle cell response to the metabolic and hormonal disorders of diabetes (16). To directly test if the beneficial effect of PEGPH20 is linked to CD44, it would be interesting to elucidate if PEGPH20 treatment further improves high-fat diet–induced insulin resistance and adipose tissue inflammation in cd44 null mice.
HA not only binds CD44 but also several other receptors such as HA-mediated motility receptor (17). This receptor, which is less well-studied than CD44, was originally discovered as a soluble protein that altered migratory cell behavior (17). Evidence suggests that HA also plays a role in innate immunity and that HA degradation products transduce their inflammatory signal through Toll-like receptors 2 and 4 in macrophages and dendritic cells (18). Studies are required to determine if HA mediates its effect on insulin resistance through these or other signaling pathways.
In summary, the study by Kang and colleagues (6) opens up a fascinating field of research by highlighting the role of ECM remodeling after administration of a high-fat diet. Further studies elucidating the mechanism(s) that link increased HA to the pathogenesis of insulin resistance are necessary and could reveal novel therapies for type 2 diabetes and associated metabolic disturbances.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1888.
REFERENCES
- 1.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson DK, Kashyap S, Bajaj M, et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 2005;280:10290–10297 [DOI] [PubMed] [Google Scholar]
- 4.Berria R, Wang L, Richardson DK, et al. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 2006;290:E560–E565 [DOI] [PubMed] [Google Scholar]
- 5.Kang L, Ayala JE, Lee-Young RS, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes 2011;60:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang L, Lantier L, Kennedy A, et al. Hyaluronan accumulates with high fat feeding and contributes to insulin resistance. Diabetes 2013;62:1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology 2003;13:105R–115R [DOI] [PubMed] [Google Scholar]
- 8.Toole BP. Hyaluronan is not just a goo! J Clin Invest 2000;106:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bookbinder LH, Hofer A, Haller MF, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release 2006;114:230–241 [DOI] [PubMed] [Google Scholar]
- 10.Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep 2011;11:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett EJ, Eggleston EM, Inyard AC, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009;52:752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodama K, Horikoshi M, Toda K, et al. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci USA 2012;109:7049–7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao G, Savani RC, Fehrenbach M, et al. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol 2006;169:325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33–45 [DOI] [PubMed] [Google Scholar]
- 15.Chajara A, Raoudi M, Delpech B, Leroy M, Basuyau JP, Levesque H. Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler Thromb Vasc Biol 2000;20:1480–1487 [DOI] [PubMed] [Google Scholar]
- 16.Schultz K, Rasmussen LM, Ledet T. Expression levels and functional aspects of the hyaluronan receptor CD44. Effects of insulin, glucose, IGF-I, or growth hormone on human arterial smooth muscle cells. Metabolism 2005;54:287–295 [DOI] [PubMed] [Google Scholar]
- 17.Turley EA. Purification of a hyaluronate-binding protein fraction that modifies cell social behavior. Biochem Biophys Res Commun 1982;108:1016–1024 [DOI] [PubMed] [Google Scholar]
- 18.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 2002;195:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]