Abstract
Therapies targeting vascular endothelial growth factor (VEGF) are revolutionizing the treatment of diabetic retinopathy (DR) and diabetic macular edema (DME). In August 2012, ranibizumab, a monoclonal antibody fragment targeting VEGF designed for ocular use, became the first and only U.S. Food and Drug Administration–approved medical therapy for DME and the first approved treatment in over 25 years. This approval was based on strong preclinical data followed by numerous clinical trials that demonstrate an essential role of VEGF in vascular permeability and angiogenesis in both normal physiology and disease pathology. In this Perspective, we will examine the experimental studies and scientific data that aided in the success of the development of therapies targeting VEGF and consider how these approaches may inform the development of future therapeutics for diabetic eye disease. A multipoint model is proposed, based on well-established drug development principles, with the goal of improving the success of clinical drug development. This model suggests that to provide a validated preclinical target, investigators should demonstrate the following: the role of the target in normal physiology, a causal link to disease pathogenesis, correlation to human disease, and the ability to elicit clinically relevant improvements of disease phenotypes in animal models with multiple, chemically diverse interventions. This model will provide a framework to validate the current preclinical targets and identify novel targets to improve drug development success for DR.
For the last 20 years, managing the metabolic deregulation induced by diabetes has been the primary and most effective way to slow the development and progression of microvascular complications including diabetic retinopathy (DR) (1,2). After the appearance of clinically significant vascular lesions and macular edema, laser photocoagulation remains an effective approach to slow the loss of visual acuity (3,4). These established approaches have recently been extensively reviewed (rev. in 5). Unfortunately, ~20% of people with type 1 diabetes develop proliferative DR even under intense metabolic control by exogenous insulin (6), while others have inherent difficulties with maintaining proper euglycemia. Therefore, understanding the causative underlying mechanisms of DR remains of utmost importance in the treatment of this insidious disease. Basic and clinical research into the inflammatory cytokines and proangiogenic signals that drive DR has provided new therapeutic avenues for the treatment of diabetic eye disease. Importantly, anti–vascular endothelial growth factor (VEGF) therapy has revolutionized the treatment of diabetic macular edema (DME). The Diabetic Retinopathy Clinical Research Network suggested that ranibizumab improves visual acuity outcomes in patients with DME. Subsequently, in the RISE clinical trial, 44.8% of patients treated with 0.3 mg ranibizumab for 24 months gained ≥15 letters improvement in visual acuity vs. 18% of sham-treated patients. In the RIDE study, 45.7% of patients treated with 0.5 mg ranibizumab gained ≥15 letters vs. 12.3% of sham-treated patients. In addition to increases in visual acuity, improvements were observed in retinal thickness as measured by optical coherence tomography and reduced risk of further vision loss (7). This success has provided much-needed therapeutic options and a blueprint to discover novel treatments for diabetic ocular complications.
VEGF: dual role in physiology and pathology
Clinical success of anti-VEGF therapy is based on basic scientific research into the mechanisms of angiogenesis, neovascularization, and vascular permeability leading to a broad consensus from the scientific community on the significance and requirement of this growth factor to these defined processes. Exploration in tumor biology led to the hypothesis that diffusible factors provided angiogenic and permeabilizing signals to the tumor vasculature. This led to the seminal hypothesis by Dr. Judah Folkman that inhibition of angiogenesis may be a strategy to halt tumor growth (8). Protein purification and molecular cloning allowed two groups to discover the potent angiogenic and permeabilizing factor: one coining the term vascular permeability factor and the other VEGF (9,10). A review of the biology of VEGF and its receptors on angiogenesis, proliferation, migration, and vascular permeability was performed by Chung and Ferrara (11). Here, we provide a retrospective analysis of the significance of the seminal findings in the development of anti-VEGF therapies and propose a model to apply to the newest set of preclinical targets for DR.
Clear and compelling genetic studies revealed that VEGF contributes a critical and essential role in vascular biology (rev. in 11). Genetic loss-of-function experiments demonstrated that developmental expression of VEGF is required for vasculogenesis and angiogenesis, as single Vegf allele inactivation resulted in embryonic lethality with deficient vascularization of several organs (12,13). Moreover, gene targeting via the Cre-loxP system and administration of a soluble VEGF receptor chimeric protein led to significant increases in mortality and impaired organ development; however, this critical requirement for VEGF waned by 4 weeks of animal maturation (14). This was the first evidence that VEGF function may be altered in the adult without the detrimental effects observed in development.
VEGF clearly contributes to vascular homeostasis, and further research confirmed that excess VEGF and aberrant VEGF signaling induces pathological angiogenesis and permeability. Pharmacological targeting of VEGF began in the cancer field prior to its application to ocular disease. The first preclinical evidence of anti-VEGF therapy was performed using targeted monoclonal antibody (mAb) technology that successfully prevented the growth of tumors in animal models (15). The results were verified by targeted deletion of the VEGF-A gene, which suppresses tumor angiogenesis in a well-established pancreatic islet carcinoma cancer model (16). Along with numerous other preclinical studies, these data suggest that pharmacological manipulation of VEGF may be effective for the treatment of malignancies, and the U.S. Food and Drug Administration (FDA) granted approval for anti-VEGF therapy for several cancer indications after several successful clinical trials. Nonetheless, there is concern that systemic anti-VEGF treatment may lead to an increased occurrence of cardiovascular side effects (17).
Years prior to the discovery of VEGF, the oncology and ophthalmology fields were united when Dr. Isaac Michaelson proposed a bold hypothesis in 1948 that a diffusible “Factor X” produced by the retina was responsible for retinal and iris neovascularization that occurred in proliferative DR (18). VEGF was later identified as a likely candidate for this retinal “Factor X” and helped to connect the underlying pathological angiogenic and permeability responses in numerous retinopathies (19). Analysis of human vitreous from patients with severe DR demonstrated that VEGF concentrations were increased in proliferative DR (20,21). Clearly, DR demonstrates both severe angiogenic and permeabilizing features closely related to VEGF’s biological function. Initial animal studies using intraocular VEGF injections and various VEGF inhibitors demonstrated the causal role of VEGF as a mediator of ischemia-induced intraocular neovascularization and retinal permeability (22–24).
These preclinical experiments led to several clinical trials evaluating the efficacy of anti-VEGF therapy in the eye and exemplify how evaluation of normal physiology and pathology may establish a clinical target. Genetic analysis of the physiologic role of VEGF and its receptor led to hypothesis-driven studies examining the role of the growth factor in models of retinal permeability and angiogenesis. Meanwhile, clinical studies revealed a close correlation of VEGF expression in proliferative DR. It is also noteworthy that the models used provided dramatic responses of ischemia-induced angiogenesis and permeability. To our knowledge, only one study demonstrated blockade of VEGF effects after 1-week induction of experimental diabetes and vascular permeability (25). Diabetic rodents likely reflect an early or background retinopathy with moderate permeability rather than the severe response in humans targeted by clinicians, which includes robust edema or neovascularization—both features lacking in the current rodent diabetic models.
VEGF as an ocular therapy: obtaining FDA approval
Generating novel therapies for the indication to treat DR is extremely difficult owing to the long and incremental organ failure associated with diabetes. The first ocular indication for anti-VEGF therapy (FDA approved in 2006) was for neovascular age-related macular degeneration (wet-AMD). The pathogenesis of wet-AMD involves significant choroidal neovascularization and vascular permeability, and strong preclinical data demonstrate that VEGF mediates these effects. The Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular De-generation (MARINA) trial showed that ranibizumab treatment led to a 95% rate of visual stabilization, and nearly 40% of treated patients had a ≥15 letter gain of visual acuity at 12 months. Importantly, these benefits were associated with significant decreases in foveal center point thickness in the ranibizumab-treated patients (26). Additionally, the ANCHOR Study (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-related Macular Degeneration) demonstrated superior efficacy in treating choroidal neovascularization over photodynamic therapy with verteporfin (27,28). Notably, ocular anti-VEGF therapy was well tolerated with low risk of ocular adverse events and no systemic side effects as seen with intravenous administration for cancer treatment (29). FDA approval in 2010 for macular edema after retinal vein occlusion was granted after the Branch Retinal Vein Occlusion (BRAVO) and Central Retinal Vein Occlusion (CRUISE) phase III trials that demonstrated that 50% of patients gained ≥15 letters in visual acuity after treatment with ranibizumab (30,31).
Initial studies in persons with diabetes began in 2005 with pegaptanib, an aptamer targeting VEGF. This phase II trial demonstrated greater visual acuity improvements after treatment with pegaptanib compared with sham injection patients (32). After this study, numerous clinical trials have demonstrated success of anti-VEGF therapies including ranibizumab, bevacizumab, and afliberacept, which are extensively reviewed elsewhere (33). Subsequent studies examining dosing regimens and dose responses all arrived at the same conclusion; namely, multiple independent pharmacological agents targeting VEGF exhibit robust clinical efficacy. After the RIDE/RISE results, ranibizumab gained approval on 10 August 2012 for DME after evidence of significant visual acuity gains of ≥15 letters for 35–50% of the patients treated with ranibizumab, which correlated with dramatic improvements in retinal edema as measured by optical coherence tomography (7). Although some patients fail to respond to anti-VEGF therapy, this medical treatment has revolutionized ophthalmic care. Clearly, increased vascular permeability and retinal edema contribute to the pathology of DR and loss of visual acuity (34), and various studies targeting VEGF with chemically diverse therapies demonstrate that blocking excess VEGF signaling prevents loss of vision in DR.
While empiricism has gained great advances in therapies with limited mechanistic understanding (e.g., aspirin, penicillin), we propose that the approach used in understanding the role of VEGF in vascular biology provides a learning model for future drug development for DR. An analysis of the development of anti-VEGF therapies may be distilled to a set of principles that may expedite the validation and development of preclinical drug targets for DR prior to large clinical trials in humans. This model has five principles:
1) Physiology: knowledge of the target’s role in normal physiology from biochemical and genetic manipulation.
2) Pathology: demonstration of the target’s causal link to pathological features as evident from biochemical and genetic gain-of-function or loss-of-function experiments.
3) Relevance: evidence demonstrating a correlation with disease progression or severity in humans.
4) Reversibility: pharmacological agents targeting the factor must be able to elicit a biologically significant response in vivo in preclinical screens that are clinically meaningful.
5) Repeatability: structurally distinct probes that regulate the target, or another target on the same pathway, demonstrate a similar effectiveness.
VEGF clearly obtained broad scientific consensus as a valid target for vascular angiogenesis and vascular permeability. VEGF was shown to contribute to pathological vascular permeability and retinal edema as well as angiogenesis in preclinical studies, while clinical studies demonstrated a clear correlation with disease. Furthermore, multiple pharmacological agents successfully targeting VEGF showed dramatic improvements in vascular permeability and retinal edema in a range of disease models and clinical studies. Approaches independent of this model have demonstrated success in identifying compounds with great potential for clinical efficacy such as the discovery of the use of fenofibrate for DR (35). Indeed, future studies may provide important mechanistic understanding of the use of these compounds but will not be discussed here. Rather, the model provided by anti-VEGF therapies will be discussed as providing a framework for preclinical research that may improve drug development success and outcomes.
Novel targets to support or replace anti-VEGF agents
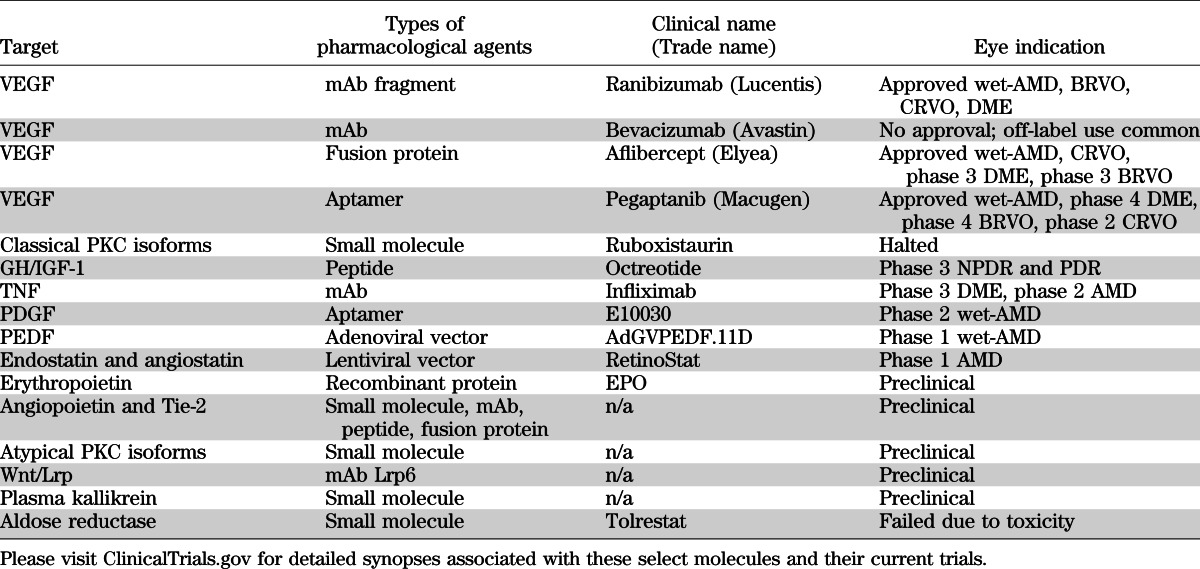
Although significant success is apparent with anti-VEGF therapy, a number of limitations exist including the need for repeat intraocular injections, the potential for disease rebound upon discontinuing treatment, and the fact that not all patients respond to the treatment, collectively lead to the justification for the investigation of novel therapies for DR. In addition to VEGF, numerous factors have been identified as potential candidates mediating the vascular dysfunction observed in DR. The pathology of the diabetic retina is complex and includes regions lacking vascular perfusion and degeneration as well as regions of vascular growth and responsiveness to VEGF. Figure 1 reveals the cells associated with the retinal capillary and the proposed factors that may contribute to DR. The first category includes targets that are downstream of VEGF signaling. Factors in this category may provide additive benefits to current anti-VEGF strategies through improved pharmacokinetic or drug delivery options such as an orally available alternative to repeat intraocular injections of anti-VEGF mAbs. The second category includes novel proangiogenic factors distinct from VEGF with unique signaling mechanisms that contribute to blood-retinal barrier dysfunction and angiogenesis. Finally, extracellular inflammatory factors that contribute to vascular permeability or vascular degeneration are discussed. Importantly, drugs for DR may be considered for systemic or local delivery. Local delivery provides a number of potential advantages including the potential for reduced side effects and prolonged drug half-life with the current disadvantage of the need for intraocular injection. The route of drug delivery may have a profound impact on the success of drug development for DR. We apologize in advance to our colleagues with exciting new targets not discussed, as limited examples will be highlighted (Table 1).
FIG. 1.
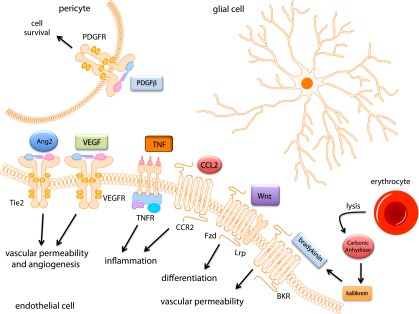
Extracellular signaling implicated in the pathogenesis of DR. This cartoon illustration of the neurovascular retina provides a summary of the factors implicated in DR and highlighted in this Perspective. Receptors for permeabilizing factors, such as VEGF, are expressed on the endothelial cells. The factors may be secreted in a paracrine fashion by the surrounding glial cells or from microglia or inflammatory cells (not shown). Proangiogenic factors such as Ang2 contribute to pathological angiogenesis and vascular permeability along with VEGF. Inflammatory factors, such as TNF and CCL2, represent another class of factors that may contribute to the increased retinal vascular endothelial permeability. Wnt signaling during endothelial maturation promotes differentiation, while aberrant Wnt signaling increases vascular permeability. In proliferative DR, vascular hemorrhage and erythrocyte lysis increase extracellular carbonic anhydrase in the blood vessel lumen and activate the kinin-kallikrein system promoting vascular permeability. In addition, PDGF signaling in pericytes is imperative for pericyte survival, and hyperglycemia-induced defects in PDGFRβ signaling in pericytes lead to blood-retinal barrier dysfunction. BKR, bradykinin receptor.
TABLE 1.
List of preclinical targets and their current state of development from ClinicalTrials.gov
Targets downstream of VEGF signaling.
VEGF requires classical protein kinase C (PKC) and, specifically, PKCβ for signal transduction in vascular endothelium. Both diabetes and VEGF increase retinal PKCβ activity, and an orally active PKCβ-specific inhibitor, ruboxistaurin (RBX), can block VEGF-induced permeability and angiogenesis in rodents (36). PKCβ contributes a critical role in mediating proliferation through retinoblastoma-associated protein phosphorylation (37) and permeability through phosphorylation-induced endocytosis of the tight junction protein occludin (38). Clinical trials to determine the effect of RBX on visual loss in patients with DR demonstrated that the drug was well tolerated and reduced the progression of macular edema, the need for laser, and the risk of sustained moderate visual loss by ~50% (39). However, currently, RBX has not received FDA approval for treatment of DR. PKCβ has been one of the most well characterized DR therapeutic targets since VEGF, and further clinical efficacy studies with appropriately targeted drugs may be warranted.
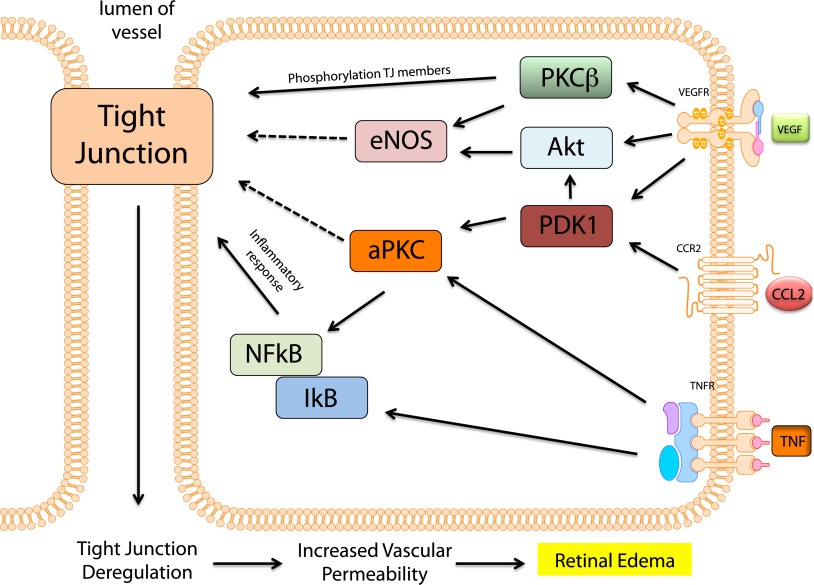
Recent evidence suggests that atypical PKC (aPKC) isoforms also contribute to VEGF-induced permeability. aPKC contributes to normal developmental physiology by mediating various processes including cellular polarity and barrier establishment (40). Targeting aPKC isoforms both genetically and pharmacologically with siRNA and novel small-molecule inhibitors demonstrates a requirement for aPKC activity in VEGF-induced retinal endothelial permeability. These small molecule inhibitors also prevent VEGF-induced permeability in the rodent retina (41). aPKC may provide an ideal target, as other inflammatory factors such as thrombin, tumor necrosis factor (TNF), and chemokine ligand 2 (CCL2), discussed in more detail below, also signal through aPKC to mediate their effects on endothelial permeability (42–44). Although additional genetic data are needed to validate this target, aPKC represents a common signaling node required for multiple permeabilizing factors and may provide a target that offers improved efficacy over current monotherapies. Signal transduction pathways involved in permeability are summarized in Fig. 2.
FIG. 2.
Intracellular mechanisms of retinal vascular permeability in DR. Signaling mechanisms downstream of VEGF including PKCβ, Akt, and aPKC lead to retinal vascular permeability. VEGF activates PKCβ, which in turn phosphorylates the tight junction proteins occludin and induces the endocytosis of several tight junction–containing proteins leading to retinal vascular permeability. VEGF also activates eNOS and aPKC, which lead to barrier destabilization through currently unknown mechanisms. TNF is known to activate NF-κB and induce an inflammatory response leading to decreases in tight junction proteins ZO-1 and claudin-5. Importantly, aPKC contributes to the permeabilizing mechanisms of VEGF, CCL2, and TNF and represents a common signaling node for all three permeabilizing factors. PDK1, phosphoinositde-dependent kinase 1.
Angiogenesis targets distinct from VEGF signaling.
From the success of anti-VEGF therapy, other factors that regulate angiogenesis and permeability have been examined as potential therapeutic targets to treat DR. Angiopoietins (Ang) have a clearly defined role in vascular development and physiology. The angiopoietins consists of several ligands and receptors, but the focus has been primarily on Ang1 and Ang2 and the receptor Tie2. Importantly, Ang1 and Ang2 have antagonistic roles upon receptor binding, as Ang1 is an agonist for Tie2, while Ang2 acts as a competitive antagonist of the Tie2 receptor (45). The signaling axis of Ang-Tie2 is essential for angiogenesis as Ang2 promotes vessel destabilization and initiates angiogenesis. Alternatively, Ang1 stabilizes vessels following angiogenesis, and has been implicated in pathological angiogenesis (45). Importantly, double blockade of VEGF and Ang2 with a chimeric decoy receptor has added benefit over VEGF and Ang2 blockade alone in inhibition of tumor angiogenesis and vascular leakage (46). Clinically, Ang2 concentrations are elevated in the vitreous of patients with PDR (47), while neutralizing antibody that blocks Ang2 prevents ocular angiogenesis in animal models of oxygen-induced retinopathy (48). In addition, Ang1 is a retinal vasoprotectant and exhibits anti-inflammatory activity in animal models of diabetes (49). Strong preclinical genetic and biochemical data supporting a role for Ang-Tie2 axis in physiological and pathological angiogenesis and permeability support targeting this pathway to promote vascular stabilization and reduce vascular leak. Evidence for changes in this pathway in human disease progression would support further drug development. The human protein tyrosine phosphatase β (HPTPβ) has emerged as a regulator of Tie2 signaling and provides a potential point of therapeutic intervention (50).
Platelet-derived growth factor (PDGF) has been suggested as a potent proangiogenic molecule, which is also important for the establishment of the blood-retinal barrier. The literature regarding PDGF’s role in retinal angiogenesis and DR pathogenesis is complex and even contradictory. Whole-body genetic loss-of-function studies demonstrate that PDGF and PDGFRβ are required for vascular development (51). Importantly, for blood-retinal barrier development, PDGF deficiency leads to pericyte loss, microaneurysm formation, and blood-retinal barrier dysfunction mimicking the DR phenotype (52). This work suggests that reduced pericyte density is sufficient to cause a vascular retinopathy phenotype in mice. This observation was mechanistically verified in a diabetic mouse model in which hyperglycemia induces loss of PDGF signaling in pericytes through the activation of PKCδ/Src homology phosphatase-1, leading to pericyte apoptosis and vascular dysfunction (53). These data suggest that PDGF supplementation may be protective in DR. Conversely, studies have demonstrated elevated vitreous concentration of PDGFAB in diabetic patients (54). Indeed, in mouse retina, excessive PDGFA from neuronal cells leads to proliferative disease (55). PDGF-specific antibody can enhance anti-VEGF therapy in models of ocular neovascularization and cause vessel regression, as some neovessels become refractory to sustained VEGF deprivation (56). Together, these studies suggest that PDGF is a well-validated preclinical target according to our model; however, additional studies are needed to determine how to effectively modulate the PDGF axis to prevent the pathogenesis of DR, as both supplementation and inhibition of PDGF may improve the DR phenotype depending on the specific vascular microenvironment.
Several laboratories have suggested that the Wnt signaling axis contributes a fundamental role in both normal and disease physiology and therefore represents an exciting pharmacological target for diabetic eye disease. Wnts are a large family of secreted proteins that have a wide range of functions including the regulation of endothelial gene expression and angiogenesis. Upon Wnt ligand binding to the Frizzled receptor and recruitment of LDL receptor–related peptide (Lrp)5/6, β-catenin is stabilized and trans-locates to the nucleus, where it binds TCF/Lef transcription factors and induces specific gene transcription. Gain- and loss-of-function experiments clearly demonstrate that normal vascular development requires Wnt signaling, specifically in the retina, where several targeted mutations of this signaling axis have drastic retinal vascular defects (57). Wnt also contributes to the pathophysiology of ischemic retinopathies. Deletion of Lrp5 and downstream signaling molecule dishevelled2 decreases the formation of pathological neovascularization in the oxygen-induced retinopathy model (58). Several endogenous antiangiogenic inhibitors of Wnt signaling have become potential therapies to inhibit aberrant Wnt signaling. SERPINA3K binds to Lrp6 and antagonizes Wnt signaling preventing the vascular permeability and inflammation seen in diabetic rats (59). Inhibition with an mAb to Lrp6 also prevents vascular leakage and inflammation of the retina of diabetic animals (60). Evidence suggests that pigment epithelium–derived factor (PEDF) acts as an antiangiogenic factor in the retina of rodents (61). The effect of PEDF may be attributed to antagonism of Wnt signaling through binding of Lrp6 preventing Lrp6–Frizzled receptor dimerization and signaling (62). Restoring proper balance of Wnt signaling represents an exciting novel target in normalizing retinal vessels in diabetes.
Other proangiogenic growth factors such as growth hormone (GH) and IGF-1 are linked to DR pathogenesis. Clinical trials suggest a delay in time to progression to DR and trends to improve visual acuity with somatostatin or the analog octreotide, presumably by inhibiting GH secretion (63). Conversely, recent work suggests a protective role for ischemia-induced neovacularization by IGF-1–binding protein 3 (64). Additional studies are needed to identify the pathophysiological role of IGF-1/GH signaling and the relationship to disease progression. In addition to GH/IGF-1, erythropoietin (Epo) is elevated in the vitreous of patients with proliferative DR (47). Epo enhances pathological retinal angiogenesis in mice in the oxygen-induced retinopathy model (65), while small interfering RNA–mediated knockdown of Epo suppresses retinal neovascularization (66), suggesting a pathogenic nature for Epo. Conversely, intravitreal injection of Epo inhibits retinal barrier breakdown in diabetic animals (67). Clearly, there is a lack of consensus on the pathogenicity of Epo, and further preclinical studies are needed to validate these targets.
Blood-retinal barrier dysfunction induced by inflammatory cytokines and proteases.
In addition to proangiogenic signals, inflammatory cytokines represent novel targets for DR. Experimental evidence indicates that cytokines, in addition to VEGF, also contribute to vascular permeability in DR. A series of elegant experiments demonstrated that leukostasis occurs during streptozotocin-induced diabetes in mouse and rat models and that deletion of the gene for the adhesion protein ICAM or its leukocyte-binding partner CD18 ameliorated leukostasis (68). Furthermore, in an acute (1 week) model of diabetes, vascular permeability, leukostasis, CD18, and ICAM expression, as well as nuclear factor-κB activation, were all normalized by high-dose aspirin or the cyclooxygenase-2 inhibitor meloxicam and by a soluble TNF receptor/Fc hybrid (entanercept), suggesting that TNF and cyclooxygenase contribute to DR (69). A recent report provides insight into how VEGF and TNF may both contribute to different phases of vascular dysfunction in DR. Using mice with TNF gene deletion, the investigators demonstrate that diabetes-induced permeability was unaffected at 1 month; at 3 months, changes in permeability are partially reduced, while at 6 months the permeability defect is ameliorated (70). Furthermore, in humans elevated interleukin (IL)-1β and TNF levels have been associated with proliferative DR (71,72) and TNF with nonproliferative DR (73). In addition, anti-TNF therapies led to significant improvement in visual acuity compared with placebo-treated eyes in a controlled trial involving patients refractory to other treatments (74). ILs and other inflammatory factors may also contribute to DR; monocyte chemotactic protein-1 (MCP)-1 (or CCL2) along with IL-6 and IL-8 were found elevated in patients with retinopathies associated with inflammation including DR (75), and MCP-1 contributes important roles in leukocyte infiltration and vascular inflammation after retinal detachment (76). The contribution of inflammatory cytokines and chemokines to DR is complex and early in the discovery phase. What factors are relevant to human DR and at what phase of the disease process remain to be elucidated, but targeting inflammatory cytokines and chemokines offers an inviting option to control DR. However, the effectiveness of monotherapies targeting specific inflammatory cytokines in the face of the wide array of factors changed in DR remains to be determined.
Aberrant protease signaling is a relatively new area of investigation for DR. Some of the factors recently identified are actually well-known molecules in human physiology. One promising target is the kinin-kallikrein system, which contributes an essential role in inflammation, blood pressure control, and pain through bradykinin activation. Importantly, complement component C1-inhibitor is an important physiological inhibitor of plasma kallikrein. Deficiency of C1-inhibitor leads to plasma kallikrein and bradykinin activation and robust vascular permeability responses (77). Gao et al. (78) detected carbonic anhydrase (CA-1) and multiple components of the complement and kinin-kallikrein system in the vitreous of patients with DR. Importantly, carbonic anhydrase leads to alkalization of the vitreous initiating bradykinin-kallikrein-system and retinal permeability that may be prevented by a systemically administered PK inhibitor (79). The bradykinin-kallikrein system fits our model of a well-validated target linked to human disease, as prior literature has implicated this system in both normal and disease physiology, and multiple pharmacological agents effectively reverse aspects of DR in relevant animal models. Current clinical trials are underway to determine efficacy of kallikrien inhibition in humans to treat DME and vascular hemorrhage.
Conclusions
In this Perspective, a model for preclinical target identification and drug development is proposed to validate clinical trials based on the discovery and success of anti-VEGF therapy for diabetic eye disease. This model encompasses five principles, amassed by extensive preclinical research and supported by numerous independent laboratories that collectively provide compelling evidence for a drug target in human disease. This model aims to help guide preclinical experiments for potential drug targets and reduce the failure rate of agents entering clinical trials. Knowingly, many of the principles discussed in this Perspective have been integral to drug development for some time, but reviewing how these approaches have aided in the success of the development of the anti-VEGF therapies may provide a blueprint for the development of novel approaches to treat DR. Indeed, there are different approaches that may lead to validation of a preclinical target; however, in our perspective, the elucidation of physiologically relevant factors and defining their contribution to the pathogenesis of DR are clearly worth the efforts.
ACKNOWLEDGMENTS
The authors are supported by funding from the National Institutes of Health (EY-012021), the JDRF, and Research to Prevent Blindness (all held by D.A.A.).
No potential conflicts of interest relevant to this article were reported.
P.M.T. and D.A.A. wrote, reviewed, and edited the manuscript. D.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Drs. Steve Abcouwer (University of Michigan) and Elia Duh (Johns Hopkins University) for their comments and views.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE. Are individuals with diabetes seeing better? A long-term epidemiological perspective. Diabetes 2010;59:1853–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991;98(Suppl.):766–785 [PubMed] [Google Scholar]
- 4.Neubauer AS, Ulbig MW. Laser treatment in diabetic retinopathy. Ophthalmologica 2007;221:95–102 [DOI] [PubMed] [Google Scholar]
- 5.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366:1227–1239 [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen QD, Brown DM, Marcus DM, et al. RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801 [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–1186 [DOI] [PubMed] [Google Scholar]
- 9.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246:1306–1309 [DOI] [PubMed] [Google Scholar]
- 10.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309–1312 [DOI] [PubMed] [Google Scholar]
- 11.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 2011;27:563–584 [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439 [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–442 [DOI] [PubMed] [Google Scholar]
- 14.Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149–1159 [DOI] [PubMed] [Google Scholar]
- 15.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 2002;16:438–440 [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell 2002;1:193–202 [DOI] [PubMed] [Google Scholar]
- 17.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 2012;14:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc U K 1948;68:137–180 [Google Scholar]
- 19.Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994;145:574–584 [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–1487 [DOI] [PubMed] [Google Scholar]
- 21.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994;118:445–450 [DOI] [PubMed] [Google Scholar]
- 22.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995;92:10457–10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol 2002;120:338–346 [DOI] [PubMed] [Google Scholar]
- 24.Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol 1996;114:66–71 [DOI] [PubMed] [Google Scholar]
- 25.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001;42:2408–2413 [PubMed] [Google Scholar]
- 26.Kaiser PK, Blodi BA, Shapiro H, Acharya NR, MARINA Study Group Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007;114:1868–1875 [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 28.Group ToA-RMDWPTS Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999;117:1329–1345 [PubMed] [Google Scholar]
- 29.Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol 2009;54:372–400 [DOI] [PubMed] [Google Scholar]
- 30.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102.e1–1112.e1 [DOI] [PubMed]
- 31.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1124.e1–1133.e1 [DOI] [PubMed]
- 32.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. Macugen Diabetic Retinopathy Study Group A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747–1757 [DOI] [PubMed] [Google Scholar]
- 33.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010;248:915–930 [DOI] [PubMed] [Google Scholar]
- 34.Gardner TW, Larsen M, Girach A, Zhi X, Protein Kinase C Diabetic Retinopathy Study (PKC-DRS2) Study Group Diabetic macular oedema and visual loss: relationship to location, severity and duration. Acta Ophthalmol (Copenh) 2009;87:709–713 [DOI] [PubMed] [Google Scholar]
- 35.Keech AC, Mitchell P, Summanen PA, et al. FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687–1697 [DOI] [PubMed] [Google Scholar]
- 36.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 1997;46:1473–1480 [DOI] [PubMed] [Google Scholar]
- 37.Suzuma K, Takahara N, Suzuma I, et al. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA 2002;99:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase cβ phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes 2012;61:1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aiello LP, Davis MD, Girach A, et al. PKC-DRS2 Group Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology 2006;113:2221–2230 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci 2006;119:979–987 [DOI] [PubMed] [Google Scholar]
- 41.Titchenell PM, Lin CM, Keil JM, Sundstrom JM, Smith CD, Antonetti DA. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem J 2012;446:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010;59:2872–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab 2006;26:797–810 [DOI] [PubMed]
- 44.Minshall RD, Vandenbroucke EE, Holinstat M, et al. Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res 2010;80:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung K, Lee D, Lim HS, et al. Double anti-angiogenic and anti-inflammatory protein Valpha targeting VEGF-A and TNF-alpha in retinopathy and psoriasis. J Biol Chem 2011;286:14410–14418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2005;139:476–481 [DOI] [PubMed] [Google Scholar]
- 48.Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C, Bates DO. A human neutralizing antibody specific to Ang-2 inhibits ocular angiogenesis. Microcirculation 2011;18:598–607 [DOI] [PubMed] [Google Scholar]
- 49.Joussen AM, Poulaki V, Tsujikawa A, et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol 2002;160:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yacyshyn OK, Lai PF, Forse K, Teichert-Kuliszewska K, Jurasz P, Stewart DJ. Tyrosine phosphatase beta regulates angiopoietin-Tie2 signaling in human endothelial cells. Angiogenesis 2009;12:25–33 [DOI] [PubMed] [Google Scholar]
- 51.Hoch RV, Soriano P. Roles of PDGF in animal development. Development 2003;130:4769–4784 [DOI] [PubMed] [Google Scholar]
- 52.Enge M, Bjarnegård M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 2002;21:4307–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 2009;15:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freyberger H, Brocker M, Yakut H, et al. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes 2000;108:106–109 [DOI] [PubMed] [Google Scholar]
- 55.Andrews A, Balciunaite E, Leong FL, et al. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1999;40:2683–2689 [PubMed] [Google Scholar]
- 56.Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 2006;168:2036–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 2010;107:943–952 [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Stahl A, Krah NM, et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 2011;124:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang B, Abreu JG, Zhou K, et al. Blocking the Wnt pathway, a unifying mechanism for an angiogenic inhibitor in the serine proteinase inhibitor family. Proc Natl Acad Sci USA 2010;107:6900–6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K, Hu Y, Ding L, et al. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 2012;61:2948–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 2006;20:323–325 [DOI] [PubMed]
- 62.Park K, Lee K, Zhang B, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol 2011;31:3038–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grant MB, Caballero S., Jr The potential role of octreotide in the treatment of diabetic retinopathy. Treat Endocrinol 2005;4:199–203 [DOI] [PubMed] [Google Scholar]
- 64.Chang KH, Chan-Ling T, McFarland EL, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA 2007;104:10595–10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest 2008;118:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci 2009;50:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Wu Y, Jin Y, et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 2008;49:732–742 [DOI] [PubMed] [Google Scholar]
- 68.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450–1452 [DOI] [PubMed]
- 69.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 2002;16:438–440 [DOI] [PubMed]
- 70.Huang H, Gandhi JK, Zhong X, et al. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci 2011;52:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–1369 [DOI] [PubMed] [Google Scholar]
- 72.Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci 2012;53:5906–5911 [DOI] [PubMed] [Google Scholar]
- 73.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia 2005;48:370–378 [DOI] [PubMed] [Google Scholar]
- 74.Sfikakis PP, Grigoropoulos V, Emfietzoglou I, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care 2010;33:1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009;4:e8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakazawa T, Hisatomi T, Nakazawa C, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci USA 2007;104:2425–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., 3rd Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest 2002;109:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res 2008;7:2516–2525 [DOI] [PubMed] [Google Scholar]
- 79.Clermont A, Chilcote TJ, Kita T, et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes 2011;60:1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]