Abstract
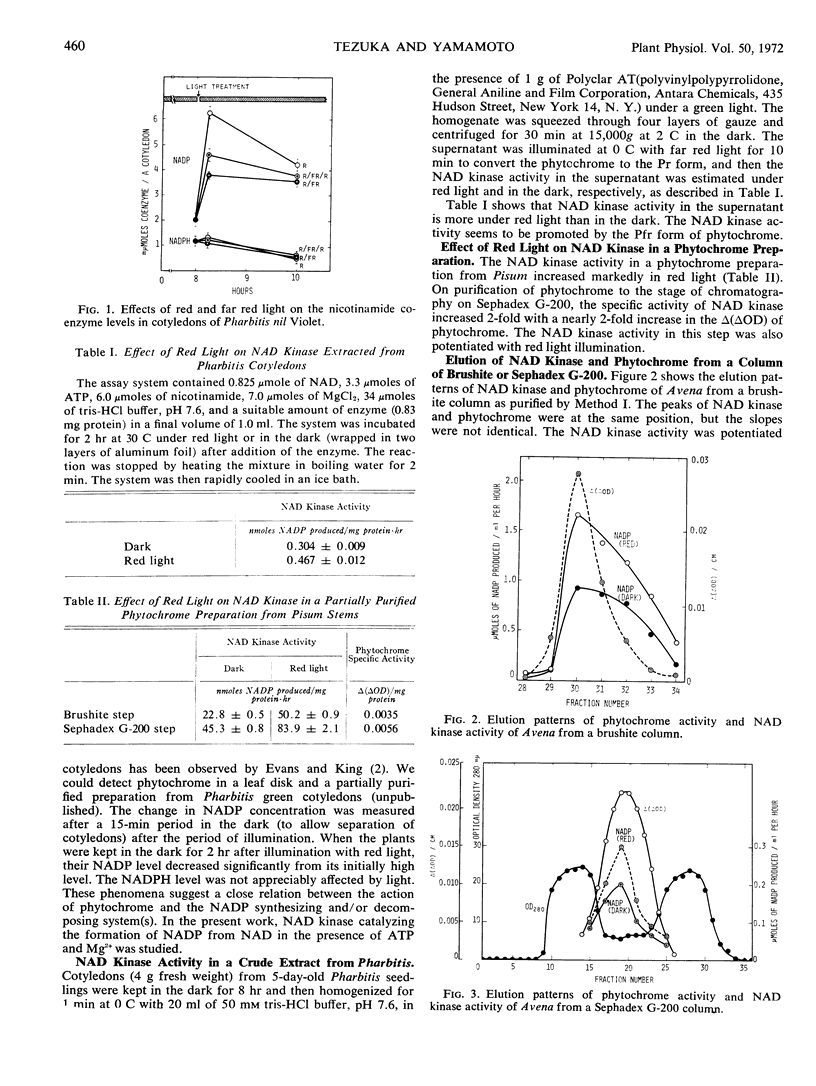
This article gives evidence that NAD kinase activity is controlled by the action of phytochrome. The NADP level rapidly increased in the cotyledons of seedlings of Pharbitis nil strain Violet (a short day plant), when the inductive dark for flowering was interrupted with a 5-minute illumination of red light. Illumination with far red light immediately after illumination with red light counteracted partly the effect of the latter.
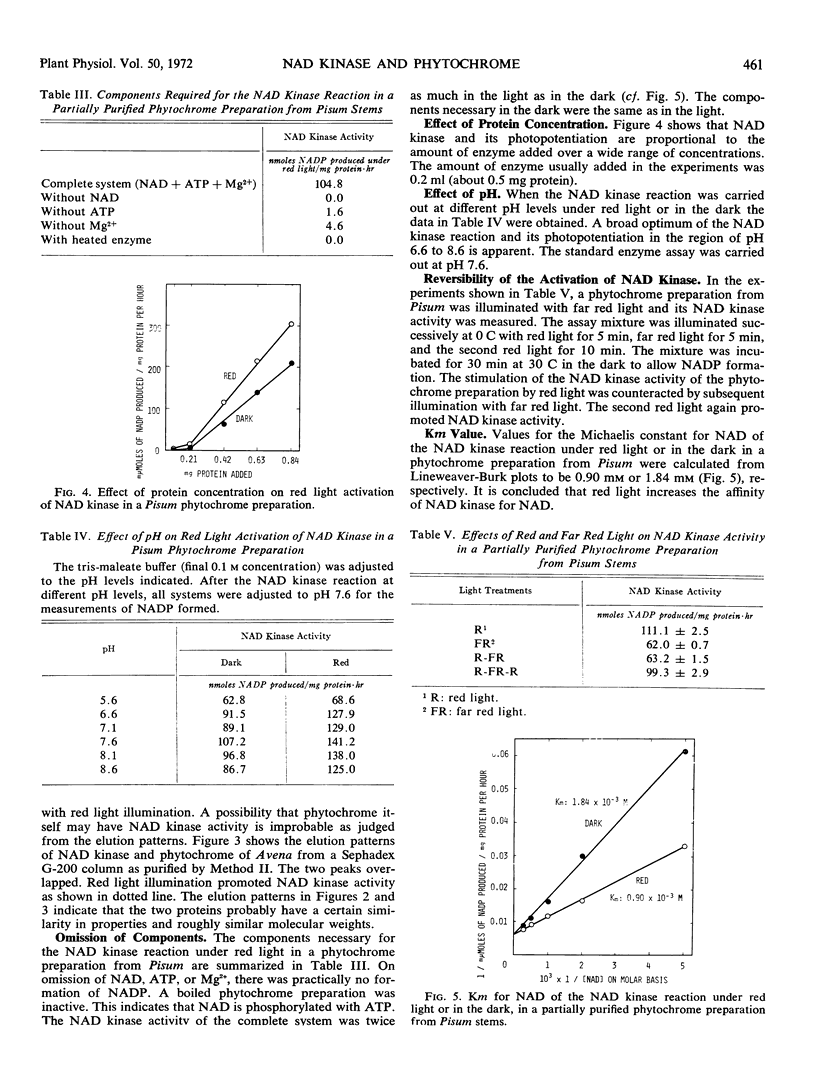
A partially purified phytochrome preparation from the uppermost internodes including the hook of Pisum sativum var. Alaska contained NAD kinase activity, and this increased greatly on illumination with red light. The effects of red and far red light on NAD kinase activity were reversible. The Km value of NAD kinase in the phytochrome preparation for NAD was 1.84 mm in the dark and 0.90 mm under red light. A broad optimum of the NAD kinase reaction and its photopotentiation appeared in the region of pH 6.6 to 8.6.
The NAD kinase activity in a phytochrome preparation from the coleoptiles of Avena sativa was also activated by red light.
It was suggested that the Pfr type of phytochrome may contribute to the decrease of the Km value of NAD kinase reaction by causing a conformational alteration of NAD kinase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Jenner E. L., Mumford F. E. Temperature and pH studies on phytochrome in vitro. Biochemistry. 1969 Mar;8(3):1182–1187. doi: 10.1021/bi00831a052. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARRE E., BIANCHETTI R. Metabolic responses to auxin. VI. The effect of auxin on the oxidation-reduction state of triphosphopyridine nucleotide. Biochim Biophys Acta. 1961 Apr 15;48:583–585. doi: 10.1016/0006-3002(61)90056-7. [DOI] [PubMed] [Google Scholar]
- OH-HAMA T., MIYACHI S. Effects of illumination and oxygen supply upon the levels of pyridine nucleotides in Chlorella cells. Biochim Biophys Acta. 1959 Jul;34:202–210. doi: 10.1016/0006-3002(59)90248-3. [DOI] [PubMed] [Google Scholar]
- Ogren W. L., Krogmann D. W. Studies on pyridine nucleotides in photosynthetic tissue. Concentrations, interconversions, and distribution. J Biol Chem. 1965 Dec;240(12):4603–4608. [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. Modification of metabolic pattern by variation of nicotinamide adenine dinucleotide phosphate level. Plant Physiol. 1969 Mar;44(3):407–412. doi: 10.1104/pp.44.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. NAD Kinase in Higher Plants. Plant Physiol. 1966 Mar;41(3):523–528. doi: 10.1104/pp.41.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Variation of nicotinamide adenine dinucleotide phosphate level in bean hypocotyls in relation to o(2) concentration. Plant Physiol. 1966 Mar;41(3):519–522. doi: 10.1104/pp.41.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


