Abstract
Background
An inverse correlation between serum 25-hydroxyvitamin D concentration and atopic dermatitis (AD) severity has been suggested.
Objective
To determine if a statistically significant relationship exists between serum 25-hydroxyvitamin D concentration and AD severity.
Methods
A cross-sectional study was conducted of patients with AD aged 1–18 years. An objective Severity Scoring of Atopic Dermatitis (SCORAD) and a serum 25-hydroxyvitamin D concentration were measured for each subject. Statistical analysis was performed using appropriate univariate tests and multivariable models.
Results
Ninety-four of 97 enrolled subjects were included in the analysis. Vitamin D deficiency (25-hydroxyvitamin D <20ng/ml) was present in 37 (39%), insufficiency (25-hydroxyvitamin D 21–29 ng/mL) in 33 (35%), and sufficiency (25-hydroxyvitamin D ≥30 ng/ml) in 24 (26%). The correlation between 25-hydroxyvitamin D concentration and SCORAD was not significant (r=−0.001, p=0.99). A multivariate model showed that a lower serum 25-hydroxyvitamin D concentration was significantly associated with age ≥3-years (p<0.0001), black race (p<0.0001), and winter season (p=0.0084).
Limitations
Limitations of this study include the inability to control for natural sunlight exposure, vitamin D intake and AD treatment, and that only a single time point was captured.
Conclusions
Serum 25-hydroxyvitamin D concentration is not significantly correlated with AD severity in our pediatric population.
Keywords: Atopic dermatitis, eczema, vitamin D, 25-hydroxyvitamin D, atopy, asthma
Introduction
Vitamin D is a fat-soluble vitamin primarily made in the skin but also derived from dietary sources and supplements. Ultraviolet light exposure triggers the initial steps of synthesis of vitamin D. Subsequently, vitamin D is hydroxylated in the liver into 25-hydroxyvitamin D (25(OH)D), the major circulating form, and serum concentrations of this metabolite are considered the primary indicator of vitamin D status. A second hydroxylation step in the kidney, as well as in other target tissues, generates the active metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D). 1,25(OH)2D binds the vitamin D receptor and exerts its effects on cells by binding to vitamin D responsive elements on DNA. In addition to regulation of calcium and phosphorus homeostasis, vitamin D plays a role in other physiologic processes and the vitamin D receptor is found on many target cells, including keratinocytes and almost all immune cells.1
Vitamin D plays a crucial role in normal cutaneous physiology and the immune response. It promotes cornified envelope formation and synthesis of the lipid permeability barrier.2 Vitamin D also stimulates the production of human cathelicidin, an antimicrobial peptide that is deficient in atopic dermatitis (AD).3, 4 As the pathogenesis of AD involves a complex interplay of epidermal barrier dysfunction and dysregulated immune response, and vitamin D is involved in both processes, it is reasonable to expect that vitamin D status could be associated with AD risk or severity.
The results from prior studies offer conflicting information for practicing clinicians. Both increased and decreased vitamin D concentrations have been implicated as risk factors for the development of AD, while one study found an inverse correlation between serum 25(OH)D and AD severity.5–7 Improvement of AD after oral supplementation with vitamin D has also been reported.8–10 We sought to clarify the relationship between serum 25(OH)D and AD severity in our urban pediatric AD population.
Methods
Study Population
Subjects were enrolled from the Children’s Hospital of Wisconsin dermatology clinic in Milwaukee. Inclusion criteria included: diagnosis of AD by a pediatric dermatologist and age 1 to 18 years old. Only children with a primary residence in Milwaukee County were enrolled in order to capture a high risk urban population and minimize the confounding variable of increased sunlight exposure in rural children.11 Exclusion criteria were: chronic systemic disease other than asthma or environmental allergies, hyperimmunoglobulin E syndrome, ichthyosis disorder other than ichthyosis vulgaris, prior systemic therapy or phototherapy for AD, ongoing or prior treatment for known vitamin D deficiency, and chronic systemic corticosteroid therapy for asthma.
Study Design
Institutional review board approval was obtained. Written informed consent was obtained from the parent or legal guardian, and subjects signed written assent when appropriate. AD disease severity was graded by the Severity Scoring of Atopic Dermatitis (SCORAD) index. The objective SCORAD has a range of 0–83 with an additional 20 points given for subjective symptoms of pruritus and sleep loss. An objective SCORAD score of <15 was classified as mild, 15–40 as moderate, and >40 as severe.12 Patient variables of age, height, weight, race, Fitzpatrick skin type, and asthma diagnosis were documented. Subject enrollment was categorized by season: winter (January 1 to March 31), spring (April 1 to June 30), summer (July 1 to September 30), and fall (October 1 to December 31).
Serum concentration of 25(OH)D was obtained for each patient on the day of enrollment. The serum concentration of 25(OH)D was determined using liquid chromatography tandem mass spectrometry (Quest Diagnostics Nichols Institute, San Juan Capistrano, California). A serum 25(OH)D concentration was categorized as deficient if ≤20 ng/ml, insufficient if 21–29 ng/ml, and sufficient if ≥30 ng/ml.13 Subjects with deficient or insufficient serum 25(OH)D concentrations were treated with vitamin D supplementation as medically appropriate.
Statistical Analysis
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Correlation between vitamin D and objective SCORAD was measured by Pearson correlation. The association of serum 25(OH)D concentration with categorical variables was assessed by one-way ANOVA and non-parametric Wilcoxon rank sum test or Kruskal-Wallis test. A multiple linear regression model was used to examine relationships between serum 25(OH)D concentration and objective SCORAD while controlling for other variables. A stepwise selection procedure was used to determine the significant variables among race, age, gender, body mass index, season and Fitzpatrick skin type, using a 5% significance level for entering the model. A non-parametric Friedman Rank test was also used to test for association between serum 25(OH)D concentration and objective SCORAD while controlling for race, age and season.
Results
Subject Characteristics
Ninety-seven patients were enrolled between November 2010 and February 2012. Serum 25(OH)D concentration was unavailable for 3 subjects; 94 subjects were included in the analysis (Table I). Forty (43%) were male, and 54 (57%) were female. Patient ages ranged between 1 and 16 years (mean, 5.0 years; median, 3 years). Seventy (74.5%) were black, 8 (8.5%) were white, 8 (8.5%) were Latino, and 8 (8.5%) self-identified as other race or ethnicity; there were no Asian or Native American subjects. Fourteen children (15.1%) had mild AD, 58 (62.4%) had moderate AD, and 21 (22.6%) had severe AD; the SCORAD evaluation was incomplete for one patient. Vitamin D deficiency was present in 32 children (34.0%), insufficiency in 38 children (40.4%), and sufficiency in 24 (25.5%). In the 70 subjects of black race, vitamin D deficiency was present in 34 (48.6%), insufficiency in 22 (31.4%), and sufficiency in 14 (20.0%). Thirty-seven subjects (39.4%) were enrolled in the winter, 12 subjects (12.8%) in the spring, 18 subjects (19.1%) in the summer, and 27 subjects (28.7%) in the fall.
Table I.
Vitamin D and atopic dermatitis: demographic and clinical characteristics of subjects
| Age (year), median (min-max) | 3 (1–16) |
| Sex, N (%) | |
| Male | 40 (43.0) |
| Female | 54 (57.0) |
| Race/ethnicity, N (%) | |
| White | 8 (8.5) |
| Black | 70 (74.5) |
| Latino | 8 (8.5) |
| Asian | 0 |
| Native American | 0 |
| Other | 8 (8.5) |
| Season of enrollment, N (%) | |
| Winter | 37 (39.4) |
| Spring | 12 (12.8) |
| Summer | 18 (19.1) |
| Fall | 27 (28.7) |
| Mean serum 25-hydroxyvitamin D (ng/mL; min-max) | 24.84 (6–58) |
| Vitamin D status, N (%) | |
| Deficient (≤20 ng/ml) | 37 (39.4) |
| Insufficient (21–29 ng/ml) | 33 (35.1) |
| Sufficient (≥30 ng/ml) | 24 (25.5) |
| Mean objective SCORAD (min-max) | 31.48 (3.77–73.89) |
| Atopic dermatitis severity, N (%) | |
| Mild (SCORAD <15) | 14 (15.1) |
| Moderate (SCORAD 15–40) | 58 (62.4) |
| Severe (SCORAD >40) | 21(22.6) |
N, number; SCORAD, Severity Scoring of Atopic Dermatitis
Vitamin D Associations
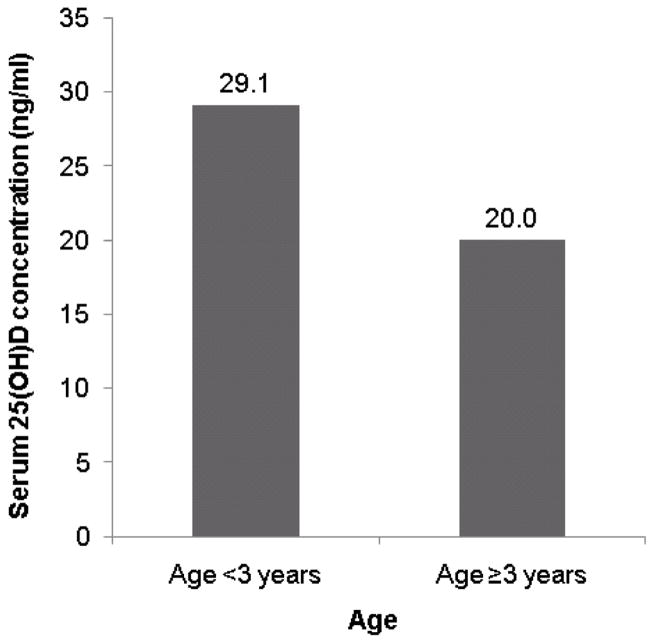
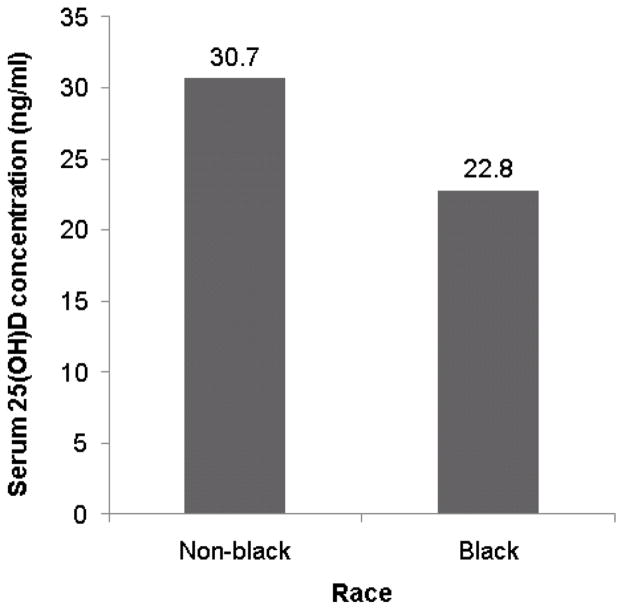
On univariate analysis, lower serum 25(OH)D concentration was associated with age ≥3 years (p<0.0001), female sex (p=0.0088), black race (p=0.0019), Fitzpatrick skin type VI (p=0.038), diagnosis of asthma (p=0.030), and winter season (p=0.042) (Fig 1). There was a trend toward an association of lower serum 25(OH)D concentration with body mass index ≥25 (p=0.13). On multivariate analysis, lower serum 25(OH)D concentration was significantly associated with age ≥3-years (p<0.0001), black race (p<0.0001), and winter season (p=0.0084) (Table II).
Figure 1.
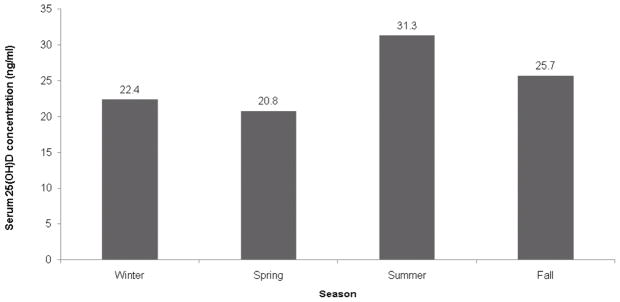
Mean serum 25-hydroxyvitamin D (25(OH)D) concentrations in pediatric atopic dermatitis subjects. (A) Lower concentration in older subjects (B) Lower concentration in black subjects (C) Lowest concentration in winter and spring (25(OH)D concentrations were lowest in the spring on univariate analysis but lowest in the winter on multivariate analysis).
Table II.
Multivariate analysis of factors associated with serum 25-hydroxyvitamin D concentration in 94 children with atopic dermatitis.
| Variables | Mean* serum 25(OH)D concentration | p-value |
|---|---|---|
| Objective SCORAD (overall) | 0.60 (df=2) | |
| Objective SCORAD (mild) | 25.55 | 0.32 |
| Objective SCORAD (moderate) | 26.86 | 0.49 |
| Objective SCORAD (severe) | 28.31† | |
| Age <3 years | 31.07 | <0.0001 |
| Age ≥3 years | 22.74† | |
| Non-black race | 31.13 | <0.0001 |
| Black race | 22.68† | |
| Season (overall) | 0.0084 (df=3) | |
| Winter | 23.89 | 0.04 |
| Spring | 24.12 | 0.15 |
| Summer | 31.38 | 0.22 |
| Fall | 28.24† |
least squares mean;
reference value; 25(OH)D, 25-hydroxyvitamin D; SCORAD, Severity Scoring of Atopic Dermatitis; df, degrees of freedom
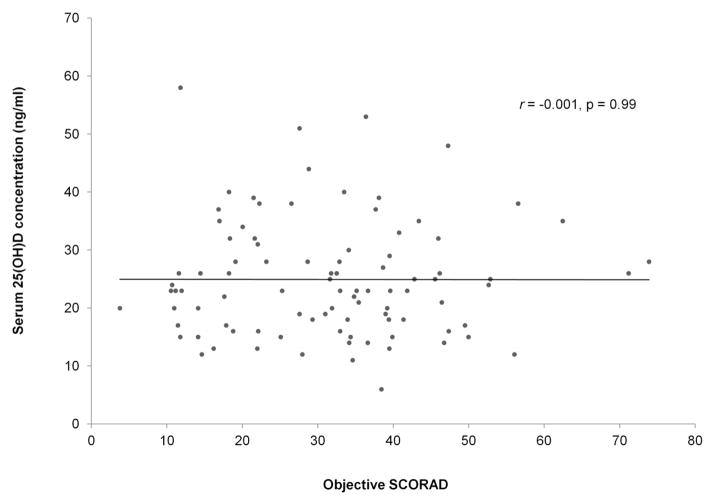
The Pearson correlation coefficient between serum 25(OH)D concentration and objective SCORAD was r= −0.001 (p=0.99) (Fig 2), and total SCORAD was r = −0.02 (p = 0.86). The serum 25(OH)D concentration was lower in mild AD (mean, 23.0 ng/ml) compared to moderate AD (mean, 25.1 ng/ml) and severe AD (mean, 25.5 ng/ml; p=0.74). When comparing 25(OH)D concentration between AD severity classes while controlling for race, age, and season, the Friedman Rank test p-value was 0.61.
Figure 2.
Correlation between 25-hydroxyvitamin D (25(OH)D) concentration and objective Severity Scoring of Atopic Dermatitis (SCORAD) in 94 children with atopic dermatitis.
Discussion
There are multiple studies showing an association between vitamin D deficiency and adverse outcomes beyond its well-known role in bone health. Although these relationships have been the focus of intense research, the full implications of vitamin D deficiency remain controversial and many claims remain unproven. Controversy even exists as to the optimal serum concentration of 25(OH)D and recommended dietary intake of vitamin D, especially in children. The American Academy of Pediatrics recommends a daily intake of 400 IU of vitamin D for infants and children, while the Institute of Medicine and The Endocrine Society recommend 400 IU daily for infants and 600 IU daily for children 1–18 years old.14–16 The American Academy of Pediatrics and The Endocrine Society use serum 25(OH)D concentrations of 20 ng/ml as the cut off for deficiency, but the Institute of Medicine proposes that 16 ng/ml is the appropriate cut-off level.14–16 The Endocrine Society recommends the additional classification of 21–29 ng/ml as vitamin D insufficiency.16 At the time of study conception, we used the existing Endocrine Society recommendations for categorizing serum 25(OH)D concentrations.
As reported by other authors, our study confirmed an association between serum 25(OH)D concentration and season, race, and age.17–24 Since ultraviolet light exposure is necessary for vitamin D synthesis, it is not surprising that race and season have a significant impact on vitamin D status. The reason for the inverse relationship between serum 25(OH)D concentration and age is unclear, though some authors have theorized that older children may have decreased oral supplementation or spend less time playing outdoors.21, 24
Female sex has been associated with lower serum 25(OH)D in some studies, but this relationship was seen only on univariate analysis in our study.17, 19 Body mass index has been inversely correlated with serum 25(OH)D although this association did not reach statistical significance in our study.17, 18, 20, 21 In our population, a diagnosis of asthma was associated with significantly lower serum 25(OH)D concentrations than in patients without asthma, though the difference did not achieve statistical significance on multivariate analysis. Multiple studies have associated vitamin D deficiency and lower 25(OH)D with higher asthma risk.25–29 Serum 25(OH)D concentration has also been inversely correlated with asthma severity.30–33 Our study was not specifically designed and powered to address these other correlations, perhaps explaining the lack of a statistically significant association on multivariate analysis.
Nearly 40% of our subjects were vitamin D deficient, a higher rate than found in a National Health and Nutrition Examination Survey in which an estimated 18% of children ages 1–11 years had serum 25(OH)D concentrations <20ng/ml.34 Our study population had a high proportion of black subjects, nearly 75%, of whom 48.6% had a serum 25(OH)D concentration ≤20 ng/ml, again higher than the national rate of 34%.34 These numbers are in contrast to a study of Japanese schoolchildren that found children with AD had serum 25(OH)D concentrations comparable to their peers.26 Further studies are necessary to determine whether vitamin D deficiency is more prevalent in children with AD, or if other factors such as race or geography contributed to the high rates seen in our population.
The evidence linking vitamin D to atopy and AD has been conflicting. One study suggested that increased vitamin D intake during infancy predisposed to the development of AD in later childhood.6 Miyake et al found higher levels of maternal vitamin D intake protective against the development of AD, while there was no effect of maternal vitamin D intake on AD risk in a study by Camargo et al.35, 36 In contrast, a study in obese adults found that those who were vitamin D deficient were 5 times more likely to have AD than those who were vitamin D replete.5
Vitamin D has also been suggested as a treatment for AD. A small randomized controlled trial of 11 children found that oral ergocalciferol 1000 IU daily reduced AD severity more than placebo, though the results were not statistically significant.8 Vitamin D treatment resulted in statistically significant decreases in the SCORAD index in adult AD patients in two additional randomized, placebo-controlled, double-blinded trials.9, 10 Larger studies are needed before vitamin D can be recommended as a treatment option for AD.
Although an association between serum 25(OH)D concentration and AD severity has been reported in other studies, we were unable to replicate the findings in our urban population. Peroni et al employed a similar cross-sectional study design with 37 children with AD and found a negative correlation between serum 25(OH)D concentration and total SCORAD (r = −0.49, p=0.002).7 Vitamin D deficiency was present in 21%, lower than the 34% in our cohort. In their study, serum 25(OH)D concentration was also significantly lower in children with severe AD (20.5 ± 5.9 ng/ml) compared to those with moderate (27.5 ± 8.3 ng/ml) and mild AD (36.9 ± 15.7 ng/ml). Interestingly, our subjects with mild AD had decreased mean serum 25(OH)D concentrations compared to the moderate and severe AD groups, although these differences were not found to be statistically significant. The Peroni study excluded children who had used topical corticosteroids within the past 4 weeks and topical calcineurin inhibitors within 2 weeks prior to study enrollment, which may have improved the accuracy of the SCORAD in determining AD severity. Additionally, environmental differences such as natural sunlight exposure and population differences such as race may explain the differences in serum 25(OH)D concentration and rates of vitamin D deficiency.
The main limitation of this study is that we were unable to control for AD treatments as most patients evaluated, even new patients, had been prescribed topical therapies. Compliance with topical corticosteroid use is poor, and controlling for self-reported steroid use may not have been accurate.33 The fact that most subjects had moderate to severe AD suggested under- treatment or non-adherence to the prescribed therapy, and we believe the SCORAD accurately reflected AD severity at the time of evaluation. Confounding variables of sunlight exposure and dietary vitamin D intake were not assessed as reliable quantitative measures were not felt to be feasible.
A cross-sectional study design was chosen to best correlate serum 25(OH)D concentration with AD severity across a study population of AD patients. As a result of the cross-sectional nature, only a single time point was captured. Other study designs could explore the relationship between vitamin D and AD and answer the correlation question more definitively. A cohort study would better assess correlation between SCORAD and serum 25(OH)D concentration over time for a given individual. A case control study could evaluate the correlation between 25(OH)D and SCORAD while controlling for confounding variables, although recruitment for such a study would be difficult.
In conclusion, although further studies may elucidate whether a relationship exists between AD and vitamin D, we found no statistically significant correlation between vitamin D status and AD severity, as measured by serum 25(OH)D and SCORAD respectively, in our urban pediatric AD population. There were statistically significant associations between serum 25(OH)D concentration and age, race, and season. Vitamin D deficiency and insufficiency were prevalent in children with AD but whether this represents a higher prevalence rate than in the general population is unclear.
Capsule Summary.
Low serum vitamin D concentration has been associated with atopic dermatitis (AD) severity and risk.
In a cross sectional study of 94 children with AD, we found high rates of vitamin D deficiency but no correlation between serum vitamin D concentration and AD severity.
Further studies are needed before vitamin D supplementation can be recommended as a treatment option for AD.
Acknowledgments
Funding Sources: This project was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1RR031973. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: Dr. Galbraith serves on an Advisory Board for Valeant Pharmaceuticals.
Prior Presentation: This work has previously been presented in poster format at the 38th Annual Society for Pediatric Dermatology Meeting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–9. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 4.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 5.Oren E, Banerji A, Camargo CA., Jr Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121:533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Back O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89:28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 7.Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011;164:1078–82. doi: 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 8.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 9.Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22:144–50. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 10.Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, et al. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol. 2012;11:327–30. [PubMed] [Google Scholar]
- 11.Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y. Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr. 2001;74:494–500. doi: 10.1093/ajcn/74.4.494. [DOI] [PubMed] [Google Scholar]
- 12.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 15.Dietary reference intakes for calcium and vitamin D. Washington, DC: Institute of Medicine; 2010. [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Andiran N, Celik N, Akca H, Dogan G. Vitamin D deficiency in children and adolescents. J Clin Res Pediatr Endocrinol. 2012;4:25–9. doi: 10.4274/jcrpe.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoian CA, Lyon M, Cox RG, Stephure DK, Mah JK. Vitamin D concentrations among healthy children in Calgary, Alberta. Paediatr Child Health. 2011;16:82–6. doi: 10.1093/pch/16.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolppanen AM, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-hydroxyvitamin D(3) and D(2) concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab. 2012;97:1202–10. doi: 10.1210/jc.2011-2516. [DOI] [PubMed] [Google Scholar]
- 20.Sioen I, Mouratidou T, Kaufman JM, Bammann K, Michels N, Pigeot I, et al. Determinants of vitamin D status in young children: results from the Belgian arm of the IDEFICS (Identification and Prevention of Dietary- and Lifestyle-Induced Health Effects in Children and Infants) Study. Public Health Nutr. 2011:1–7. doi: 10.1017/S1368980011002989. [DOI] [PubMed] [Google Scholar]
- 21.Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N. Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One. 2011;6:e22179. doi: 10.1371/journal.pone.0022179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bener A, Al-Ali M, Hoffmann GF. Vitamin D deficiency in healthy children in a sunny country: associated factors. Int J Food Sci Nutr. 2009;60 (Suppl 5):60–70. doi: 10.1080/09637480802400487. [DOI] [PubMed] [Google Scholar]
- 23.Kemp FW, Neti PV, Howell RW, Wenger P, Louria DB, Bogden JD. Elevated blood lead concentrations and vitamin D deficiency in winter and summer in young urban children. Environ Health Perspect. 2007;115:630–5. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–8. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 25.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156:948–52. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito Y, Adachi Y, Yoshida K, Akasawa A. No association between serum vitamin D status and the prevalence of allergic diseases in Japanese children. Int Arch Allergy Immunol. 2012;160:218–20. doi: 10.1159/000339962. [DOI] [PubMed] [Google Scholar]
- 27.Alyasin S, Momen T, Kashef S, Alipour A, Amin R. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res. 2011;3:251–5. doi: 10.4168/aair.2011.3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Oeffelen AA, Bekkers MB, Smit HA, Kerkhof M, Koppelman GH, Haveman-Nies A, et al. Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol. 2011;22:784–93. doi: 10.1111/j.1399-3038.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 29.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J 2011. 2011;38:1320–7. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
- 30.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158:437–41. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–8. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, et al. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J. 2011;37:1366–70. doi: 10.1183/09031936.00044710. [DOI] [PubMed] [Google Scholar]
- 33.Krejci-Manwaring J, Tusa MG, Carroll C, Camacho F, Kaur M, Carr D, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol. 2007;56:211–6. doi: 10.1016/j.jaad.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 34.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35:1228–34. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 36.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]