Abstract
Background
Parenteral Nutrition (PN) increases risks of infections in critically injured patients. Recently, PN was shown to reduce intestine luminal levels of the Paneth cell antimicrobial molecule, secretory phospholipase A2 (sPLA2), and the goblet cell glycoprotein, MUC2. These molecules are critical factors for innate mucosal immunity and provide barrier protection. IL-4 and IL-13 regulate sPLA2 and MUC2 production through the IL-13 receptor. Since IL-25 stimulates IL-4 and IL-13 release and PN reduces luminal sPLA2 and MUC2, we hypothesized that adding IL-25 to PN would restore these innate immune factors and maintain barrier function.
Methods
2 days after venous cannulation, male ICR mice were randomized to receive Chow (n=12), PN (n=9), or PN + 0.7 μg of exogenous IL-25 (n=11) daily for 5 days. Small intestine wash fluid (SIWF) was collected for analysis of sPLA2 activity, MUC2, and luminal levels of IL-4 and IL-13. Small intestinal tissue was harvested for analysis of tissue sPLA2 activity or immediate use in an ex vivo intestinal segment culture (EVISC) to assess susceptibility of the tissue segments to enteroinvasive E. coli.
Results
PN reduced luminal sPLA2 (p<0.0001) and MUC2 (p<0.002)compared with chow while the addition of IL-25 to PN increased luminal sPLA2 (p<0.0001) and MUC2 (p<0.02) compared to PN. Tissue IL-4 and IL-13 decreased with PN compared to chow (IL-4: p<0.0001)(IL-13: p<0.002), while IL-25 increased both cytokines compared to PN (IL-4: p<0.03)(IL-13: p<0.02). Tissue levels of sPLA2 were significantly decreased in PN compared to Chow, while IL-25 significantly increased tissue sPLA2 levels compared to PN alone. Functionally, more bacteria invaded the PN treated tissue compared to Chow (p<0.01), and the addition of IL-25 to PN decreased enteroinvasion to Chow levels (p<0.01).
Conclusions
PN impairs innate mucosal immunity by suppressing luminal sPLA2 activity and MUC2 density compared to Chow. PN also increases bacterial invasion in ex vivo tissue. Administration of exogenous IL-25 reverses this dysfunction and increasing luminal sPLA2 and MUC2. PN tissue treated with IL-25 was significantly more resistant to bacterial invasion than PN alone, suggesting IL-25 induced effects augment the barrier defense mechanisms.
Keywords: Parenteral Nutrition; secretory phospholipase A2; small intestine; innate immunity, Paneth Cells, Gobelet Cells
Introduction
Parenteral nutrition (PN) allows nutritional support of patients unable to be fed enterally. Unfortunately, PN is associated with an increased risk of infectious complications 1-4. Compromise of the intestinal mucosal barrier is believed to be partly responsible for this increased risk 5. Multiple components of the intestinal mucosal barrier work in concert to prevent bacterial pathogens from attaching to and invading the host tissue 6. These barrier mechanisms include tight-junction proteins between epithelial cells, mucin glycoproteins secreted by goblet cells 5, 7, antimicrobial molecules released from Paneth cells 8-11, and the release of immunoglobulin-A (IgA) by the epithelium 12-14. PN, with lack of enteral stimulation, impairs multiple components of this barrier function, such as luminal IgA produced by acquired (adaptive) immunity through mechanisms previously reported 15-23. In addition to impairment of acquired immunity, we recently demonstrated PN reduced levels of the Paneth cell antimicrobial molecule, secretory phospholipase A2 (sPLA2), and the goblet cell glycoprotein mucin2 (MUC2), within the gut lumen 24.
Innate immunity is regulated by specialized epithelial cells which sustain mucosal defenses by providing a physical, protective barrier and by producing specialized bioactive molecules. The production and secretion of mucus by goblet cells plays an integral primary role by providing a physical barrier that expands over and protects the mucosa. Paneth cells produce, store and secrete various antimicrobial peptides which neutralize microbes. Importantly, secretory IgA and Paneth cell antimicrobial peptides localize within the mucus layer in high concentrations, which enhances the mucus layer and limits the exposure of microbes to the epithelium 6, 25.
The Th2 cytokines IL-4 and IL-13 are important mediators of goblet cell mucus production in the intestine 26-28. IL-13 binds to the IL-4 receptor (IL-4R) and the IL-13 receptor (IL-13R) to promote production and release of goblet cell products including MUC2. Mice develop a lethal colitis in the absence of MUC2 production 29. Paneth cells produce small antimicrobial proteins including sPLA2, defensins, and lysozymes which are stored in granules 8, 30-32. sPLA2 specifically provides antimicrobial activity against Gram-positive bacteria 33-35. IL-13 via the IL-13R, is necessary to maintain production of antimicrobial peptides including sPLA2 and various other cryptdins in Paneth cells 28. IL-13 knockout mice produce lower levels of these antimicrobial products compared to mice with intact IL-13 signaling 28. These products, in part, comprise the mucosal barrier and help prevent invasion of pathogenic bacteria into the bowel epithelium.
IL-25 is a powerful cytokine which amplifies Th2 responses 36. Exogenous administration of IL-25 up-regulates production of the Th2 cytokines IL-4, IL-5, IL-9, and IL-13 37 and attenuates colitis in animal models. Others have demonstrated these cytokines promote Paneth cell and goblet cell hyperplasia, hypertrophy, and secretion 28, in addition to smooth muscle contraction 38, resulting in what is described as a “weep and sweep” effect on the mucosa that promotes the clearance of pathogens 39. Because PN is known to reduce the Th2 cytokines IL-4 and IL-13 and the Paneth and goblet cell products, we hypothesized that adding IL-25 to PN would restore the Th2 response, stimulate innate luminal factors including goblet cell MUC2 and Paneth cell sPLA2, and restore tissue levels of IL-4 and IL-13. We further hypothesized that loss of these products in PN would result in increased susceptibility to bacterial invasion in an ex vivo intestinal segment culture (EVISC) and that administration of IL-25 during PN would rescue this phenotype.
MATERIALS AND METHODS
Animals
All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison, and the William S. Middleton Memorial Veterans Hospital, Madison. Male Institute of Cancer Research (ICR) mice were purchased from Harlan (Indianapolis, IN) and housed 5 per covered/filtered box under controlled temperature and humidity conditions with a 12:12 hour light:dark cycle in an American Association for Accreditation of Laboratory Animal Care accredited conventional facility. Animals were fed standard mouse chow (Rodent Diet 5001; LabDiet, PMI Nutrition International, St. Louis, MO) and water ad libitum for 1 week prior to initiation of study protocol.
Experimental Design
Male ICR mice, ages 6 to 8 weeks, were randomized to Chow with an intravenous catheter (Chow, n = 12), PN (n=12), or PN with 0.7 μg/mouse/day exogenous IL-25 (R&D Systems) delivered IV for 5 days (PN+IL-25, n=12). Chow and PN animals also received a saline vehicle control to the IL-25 treatment vehicle. Animals were anesthetized by intramuscular injection, weighed, and underwent placement of silicon rubber catheter (0.012-inch I.D./0.025-inch O.D.; Helix Medical, Inc., Carpinteria, CA) in the vena cava through the right external jugular vein. The catheter was tunneled subcutaneously and exited at the midpoint of the tail. The animals were housed individually and partially immobilized by tail restraint in metabolic cages with wire floors to prevent coprophagia and bedding ingestion to protect the catheter during infusion. This technique has proven to be an acceptable method of nutritional support and does not produce physical or biochemical evidence of stress. The catheterized mice were connected to infusion pumps and received saline (0.9%) at 4 mL/day and ad libitum chow (Agway Inc., Syracuse, NY) and water during 48 hours of recovery. After 48 hours, Chow mice continued to receive 0.9% saline at 4 mL/day as well as ad libitum chow and water. PN animals received PN solution at rates 4 mL/d (day 1), 7 mL/d (day 2) and 10 mL/d (day 3 to 5) because a graded infusion period has been demonstrated to be necessary for the mice to adapt to the glucose and fluid loads. The PN solution contains 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, containing 1440 kcal/L and a non-protein calories/nitrogen ratio of 128:1. These values were calculated to meet the nutrient requirements of mice weighting 25 to 30 g.
After 5 days of feeding (7 days post-catheterization), mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and acepromazine (10 mg/kg), and exsanguinated via left axillary artery transection. The small intestine was removed and the lumen rinsed with 20 mL Hanks Balanced Saline Solution (HBSS, Bio Whittaker, Walkersville, MD). The luminal wash was centrifuged at 2,000 x g for 10 min and supernate aliquots were frozen at −80°C for analysis of sPLA2 activity, MUC2 densitometry, and luminal IL-4 and IL-13 levels. Tissue samples were taken by removing ileal segments (3 cm) excluding Peyer’s patches. These samples were frozen in liquid nitrogen and stored at −80°C until processing for tissue analysis or used immediately in the ex vivo intestinal segment culture methodology to determine bacterial enteroinvasion.
Continuous Fluorescent Assay for sPLA2 Activity
Fluorescent assay for sPLA2 activity was performed as described previously by Tsao et al 24,with some modification to substrate preparation. This assay uses a specific probe, Bis-BODipy FL, which is designed to fluoresce when the Sn2 position of the phospholipid glyceraldehyde backbone is cleaved. This method was established as a high throughput method to rapidly analyze sPLA2 activity, and we have reliably utilized this technique to measure sPLA2 activity in the small intestinal tissue and luminal wash fluid. After the reactions reached equilibrium temperature, the reaction curve was fit to a second-order polynomial equation and the first-degree coefficient was taken as the initial rate of reaction (expressed as change in Fluorescence (FL)/min/uL sample). Blank wells containing only substrate and buffer were used to find coefficient rates determined as background activity.
Western Blot for MUC2 in the Small Intestinal Wash Fluid
Tissue culture media (5 uL) was denatured at 95 °C for 10 min with sodium dodecylsulfate and β-mercaptoethanol and separated in a 4-15 % polyacrylamide gel (Ready Gel, Bio-Rad Laboratories, Hercules, CA) by electrophoresis at 150 V for 65 min. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane using the standard transfer buffer (Tris-glycine buffer plus 20% methanol) at 80 V for 60 min. Membranes were blocked with 5 % nonfat dry milk prepared in TBS-Tween (Tris-buffered saline with 0.5% Tween-20) for 1 hour with continuous stirring. The membranes were incubated with the primary antibody, mouse anti-human MUC2 (ab-11197, Abcam Inc, Cambridge, MA) diluted 1:2500 overnight at 4 °C with constant rocking. Membranes were washed and incubated with stabilized goat anti-mouse IgG-HRP conjugate (sc-2005, Santa Cruz Biotechnology, CA) diluted 1:20,000 for 1 hour at room temperature with constant agitation. After washing with TBS-tween, membranes were incubated with HRP substrate (Super Signal West Femto maximum sensitivity substrate; Pierce, Rockford, IL) for 5 min and bands were detected using photographic film. Densitometric analysis of molecular weight bands at 260kDa and 500 kDa were used to measure MUC2 concentration.
Tissue and Luminal Cytokine Quantitative Analysis
Concentrations of IL-4 and IL-13 were measured in the small intestinal tissue or in the small intestinal wash fluid (SIWF) using a solid phase sandwich ELISA (BD Biosciences, San Diego, CA) as previously described 40. The mass amounts were determined by plotting sample absorbance values on a 4-parameter logistic fit standard curve, as calculated with SOFTmax PRO software (Molecular Devices) and were normalized to total protein content.
Bacterial Preparation
Escherichia coli 5011-Lux containing ampicillin resistance were grown in 40 mL lysogeny broth medium (LB) for 48 hours at 37 °C under 5% CO2, then a surface sample was passed to a new 40 mL LB broth and grown for 24 hours at 37°C under 5 % CO2. The surface sample is used since bacteria growing near the culture surface express virulence. Bacteria were spun down at 1780 x g for 11 min, the supernatant was poured off, and the pellet was re-suspended in 40 mL LB to wash twice, and then re-suspended in 1 mL DPBS at 4 °C as a bacteria stock solution. A 1:100 dilution of the bacterial stock solution was made and read on a spectrophotometer (DU640B, Beckman) at 450 nm wavelength. Bacterial concentrations were adjusted based on growth curves previously established.
Ex Vivo Intestinal Segment Culture
Intestinal Segments were placed on a sterile surface covered in a light layer of RPMI and carefully opened apical side up. Tissue glue (Dermabond, Ethicon, Cornelia, GA) was lightly covered on one side of a plastic tissue ring and lowered over the intestinal segment. Tissue rings were manufactured from polystyrene with a 9 mm diameter and 6 mm internal aperture. Once the tissue glue had set (approximately 10 seconds) the tissue disc and intestinal segment were turned over. A second tissue disc was lightly covered with tissue glue and placed on the serosal side of the intestinal segment. Once the second tissue disc was adherent, a light layer of tissue glue was applied to the bottom of the serosal disc and the intestinal segment sandwiched between tissue discs were lowered into a cell culture insert (Cat 3292, 3.0 μM pore, 12 well format, BD bioscience, NJ). Gentle pressure was applied to ensure adherence of the bottom tissue disc to the cell culture insert. Cell culture inserts were placed into 12 well plates prefilled with 1 mL RPMI + Ampicillin per well.
400 μL of bacterial inoculums (1×108 Colony Forming Units (CFU)/mL) in RPMI + Ampicillin was placed into the wells for 1 hour at 37°C. Then the bacterial inoculum was collected, centrifuged at 14,000g for 2 minutes to pellet bacteria, and the supernatant stored for analysis of mucosal secretions. The wells were rinsed gently with 500 μL of DPBS 3 times. 600 μL RPMI + Gentamicin was added to each well for 1 hour at 37°C. This was done to kill any remaining bacteria in the well or adherent to the mucosal surface. RPMI + Gentamicin were then removed and the wash step repeated. Then 500 μL of 0.1% Triton-X in PBS was added to each well. The 12 well plates were placed on an orbital shaker and agitated (175 rpm; New Brunswick Scientific Classic Series C1 Shaker) for 30 minutes at room temperature. Serial dilutions (101-107) of the cell lysate in DPBS were plated on LB + Ampicillin agar plates and grown for 18 hours at 37°C. Enteroinvasion was assessed by counting CFUs. The RPMI + Ampicillin added to the 12 well plate was also plated to detect cell culture insert leaks. If leaks were detected from the apical to serosal side, the well was discarded from the experiment.
Statistical analysis
The data are expressed as means ± standard error of the mean. Statistical significance was determined using ANOVA with Fisher’s protected least significant difference post hoc test or Student’s t-test. Differences were considered to be statistically significant at p<0.05. All statistical calculations were performed with StatView (Abacus Concepts, Berkeley, CA).
RESULTS
Analysis of Intestinal Fluid sPLA2
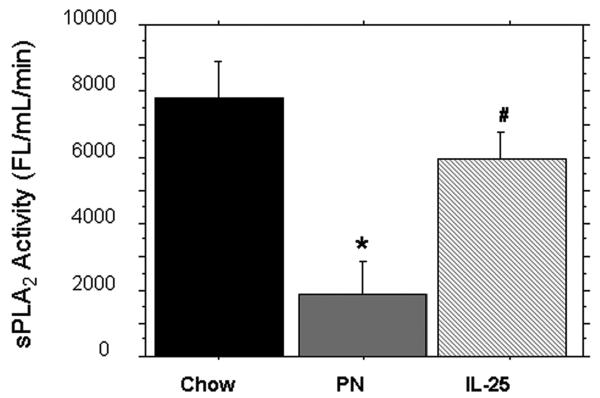
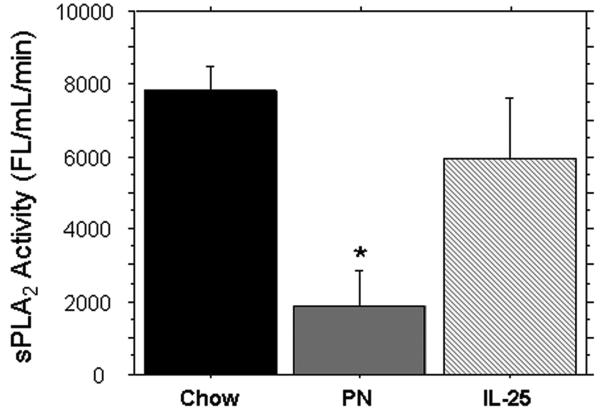
The sPLA2 activity (FL/min/uL) within the small intestinal wash fluid was significantly lower in PN compared to Chow (1895 ± 418 vs. 7811 ± 467, p < 0.0001). The addition of IL-25 to PN significantly increased sPLA2 activity compared to PN alone (5954 ± 824 vs. 1895 ± 418, p < 0.0001); sPLA2 was still significantly lower in PN+IL-25 vs. Chow fed mice (5954 ± 824 vs. 7811 ± 467, p = 0.03) (Figure 1).
Figure 1.
sPLA2 activity from the Small intestinal wash fluid (SIWF). Parenteral nutrition (PN) significantly decreased the luminal levels of sPLA2 compared to chow. IL-25 significantly increased sPLA2 activity similar to chow levels. Data are represented as mean ± SEM. *p < 0.0001 vs Chow and IL-25. #p = 0.03 vs Chow.
Analysis of Intestinal Fluid MUC-2
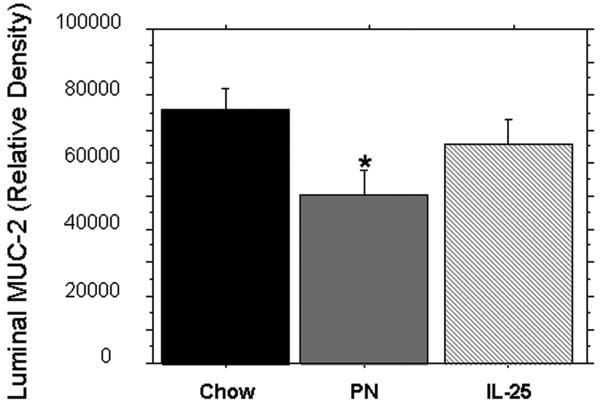
The luminal MUC2 (relative density) within the small intestinal wash fluid was significantly depressed in PN mice compared to Chow (50366 ± 4059 vs. 75912 ± 6181, p = 0.001). PN supplementation with IL-25 significantly increased the relative luminal level of MUC2 compared to PN (65448 ± 4625 vs. 50366 ± 4059, p = 0.02). There were no significant differences between chow and PN+IL-25 (75912 ± 6181 vs. 65448 ± 4625, p = 0.16) (Figure 2).
Figure 2.
Densitometric analysis of MUC-2 in the Small intestinal wash fluid (SIWF). Intravenous parenteral nutrition (PN) significantly decreased the luminal density of MUC-2 compared to PN. IL-25 stimulated PN significantly increased luminal MUC-2 levels compared to PN alone. Data are presented as mean ± SEM. *p < 0.02 vs Chow and IL-25.
Analysis of Tissue IL-4 and IL-13 Levels
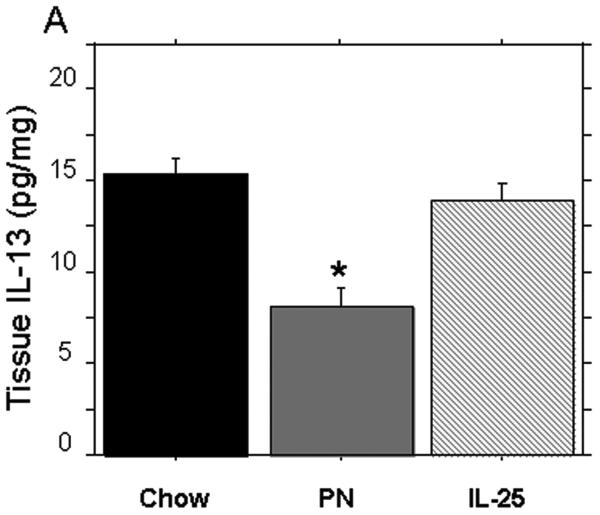
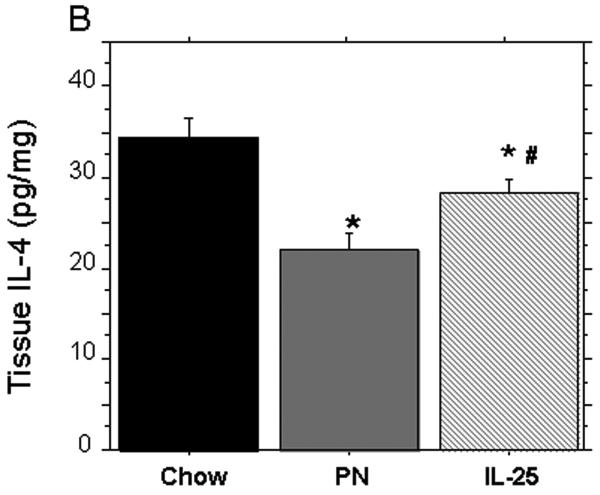
PN significantly reduced tissue levels of IL-13 (pg/mg) compared to chow (PN: 7.9 ± 1.1 vs. Chow: 15.1 ± 1.3, p < 0.02) (Figure 3A). The addition of IL-25 to PN significantly increased IL-13 tissue levels compared to PN alone (PN+ IL-25: 13.8 ± 1.5 vs. PN: 7.9 ± 1.1, p < 0.02) and the IL-13 levels were similar to Chow levels (PN+ IL-25: 15.1 ± 1.3 vs. Chow: 13.8 ± 1.3, p = NS). Consistent with our previous studies, PN significantly decreased the tissue levels of IL-4 compared to chow fed mice (PN: 22.4 ± 0.8 pg/mg protein vs. Chow: 33.9 ± 2.5 pg/mg protein, p < 0.03) (Figure 3B). The addition of IL-25 to PN significantly increased tissue IL-4 compared to PN alone (PN+ IL-25: 28.6 ± 1.6 pg/mg protein vs. PN: 22.4 ± 0.8 pg/mg protein, p = 0.03); however, the IL-4 levels remained significantly lower than Chow alone (PN+ IL-25: 28.6 ± 1.6 pg/mg protein vs. Chow: 33.9 ± 2.5 pg/mg protein, p < 0.05).
Figure 3.
Tissue levels of IL-4 and IL-13 after exogenous IL-25. (A) IL-13 was significantly depressed in PN compared to Chow and IL-25. The addition of IL-25 to PN significantly increased IL-13 similar to chow levels. (B) IL-4 was significantly decreased in PN compared to Chow and IL-25. The addition of IL-25 to PN significantly increased IL-4 compared to PN but failed to restore IL-4 to Chow levels. Data are presented as mean ± SEM. *p < 0.02 vs Chow. #p = 0.03 vs PN.
Analysis of small intestinal wash levels of IL-4 and IL-13
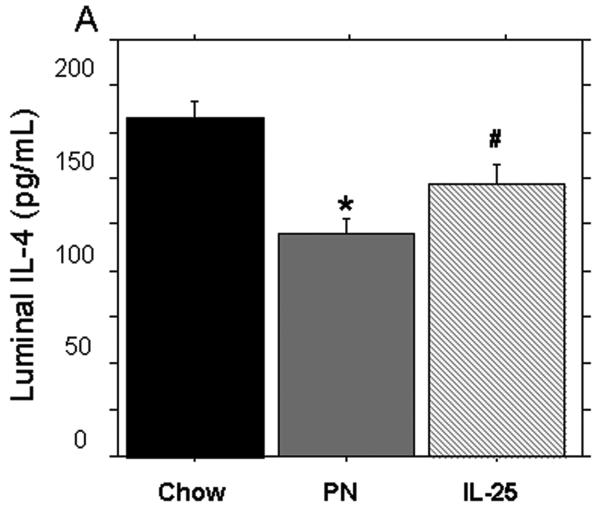
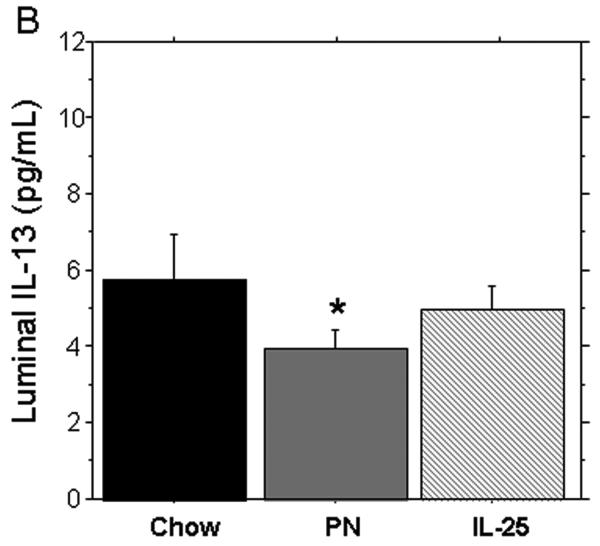
PN significantly decreased the levels of IL-4 and IL-13 (pg/mL) in the small intestinal washes compared to Chow (IL-4: 119.3 ± 8.1 vs. 174.8 ± 9.1, p = 0.16) (IL-13: 3.9 ± 0.4 vs. 5.8 ± 0.9, p < 0.05). The addition of IL-25 to PN significantly increased IL-4 compared to PN alone (146.4 ± 8.7 vs119.3 ± 8.1, p = 0.02) but levels were still significantly lower than Chow levels (146.4 ± 8.7 vs. 174.8 ± 9.1, p = 0.04). PN + IL-25 significantly increased the small intestinal wash levels of IL-13 compared to PN (5.1 ± 0.4 vs. 3.9 ± 0.4, p < 0.05) and the IL-13 was statistically similar to the Chow group (5.1 ± 0.4 vs. 5.8 ± 0.9, p = 0.2) (Figure 4A and 4B).
Figure 4.
IL-4 concentration in small intestinal wash fluid (SIWF). (A) Parenteral nutrition (PN) significantly decreased the expression of IL-4 compared to Chow. The addition of IL-25 to PN animals significantly increased IL-4 compared to PN alone. (B) IL-13 concentration in the small intestinal wash fluid (SIWF). Parenteral nutrition (PN) significantly suppressed luminal levels of IL-13 compared to Chow. The addition of IL-25 to PN significantly increased the IL-13 similar to chow levels. Data are presented as mean ± SEM. *p < 0.05 vs Chow and IL-25. #p = 0.04 vs Chow.
Analysis of Tissue sPLA2
sPLA2 activity (Fl/min/uL) within the ileum tissue was significantly reduced in PN compared to Chow (1895 ± 418 vs. 7811 ± 467, p < 0.0001). The addition of IL-25 to PN significantly increased tissue sPLA2 activity compared to PN (5954 ± 845 vs. 1895 ± 418, p < 0.0001) but IL-25 was still significantly decreased compared to Chow (5954 ± 845 vs. 7811 ± 467, p = 0.03) (Figure 5).
Figure 5.
sPLA2 activity from Ileum Tissue. Parenteral nutrition significantly decreased the tissue levels of sPLA2 compared to Chow. IL-25 significantly increased the tissue levels of sPLA2 compared to PN, but the levels were still depressed compared to chow alone. Data are represented as mean ± SEM. *p < 0.0001 vs Chow and IL-25.
Bacterial Invasion after EVISC
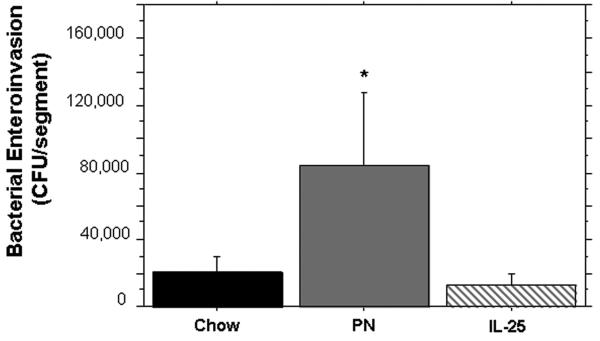
Ileum segments from PN mice were significantly more susceptible to bacterial invasion (CFUs/segment) than Chow segments (20450 ± 4290 CFU vs 84851± 24671, p < 0.01). The addition of IL-25 to PN significantly decreased bacterial invasion compared to PN alone (13497 ± 4400 CFU vs 84851± 24671, p < 0.01) and was statistically similar to bacterial invasion in chow mice.
DISCUSSION
Parenteral Nutrition (PN) prevents progressive malnutrition in patients with contraindications to enteral nutrition. Unfortunately, lack of enteral feeding is associated with an increased risk of infectious complications, a risk suspected to be due in-part to alterations in the intestinal mucosal barrier. The mucosa is protected by both adaptive (acquired) immunity and innate immunity. Adaptive immunity functions to produce and release IgA at mucosal surfaces. Innate immunity of the mucosal barrier includes the release of antimicrobial molecules from Paneth cells and secreted mucin glycoproteins by goblet cells that cover the epithelial surface. Under normal circumstances these and other mechanisms successfully defend a surface area of roughly 300 meters2 against dietary and environmental antigens and enormous numbers of bacteria 5. We previously demonstrated that PN feeding suppresses luminal levels of IgA and the Paneth cell antimicrobial compound, sPLA2 24, 41, 42. In addition, we observed PN with lack of enteral stimulation and PN reduces the goblet cell derived mucin layer, which is largely composed of the MUC2 glycoprotein 5, 7. The current study investigated whether deleterious changes in innate immunity from PN can be corrected via administration of the Th2 cytokine IL-25 during PN feeding.
The Th2 cytokines IL-4 and IL-13 are mediators of goblet cells and mucus production in the intestine 26-28, 43. IL-13 interacts with the IL-4 receptor (IL-4R) and the IL-13 receptor (IL-13R) to promote production and release of the glycoprotein MUC2. IL-13 is also a modulator of Paneth cell function, where its interaction with IL-13R is necessary to stimulate production of antimicrobial peptides including sPLA2 and cryptdins. Indeed, IL-13 knockout mice produce low levels of these antimicrobial products 28. IL-25 is a powerful cytokine which amplifies Th2 responses and appears to be produced by the epithelium itself 36. Recent experiments demonstrate exogenous administration of IL-25 attenuates colitis in animal models and up-regulates production of the Th2 cytokines IL-4, IL-5, IL-9, and IL-13 37. Collectively, these studies show the Th2 profile promotes Paneth and goblet cell hyperplasia, hypertrophy, and secretion, in addition to smooth muscle contraction 38, 39. These effects result in the so called “weep and sweep” effect on the mucosa that promotes the clearance of pathogens 39.
We studied the effects of PN and PN + IL-25 on the innate immune products released by two types of secreting epithelial cells, Paneth cells and goblet cells, which provide innate defenses for the intestinal mucosa. goblet cells produce mucin glyoproteins that provide a physical barrier between organisms within the lumen and the epithelial cell layer. The most abundant mucin secreted into the small intestine is MUC2. This large glycoprotein provides a visoelastic surface over which the intestinal contents pass. Paneth cells reside at the base of the intestinal crypts where they produce, store and secrete an array of antimicrobial products, including sPLA2, lysozyme, and the defensins, that provide a chemical defense against luminal microorganisms 31, 32, 44. sPLA2 attacks Gram positive bacteria cell membranes where it catalytically cleaves phospholipids, inducing membrane disruption and lysis 44. Interestingly, the many Paneth cell antimicrobial molecules, including sPLA2, are cationic and concentrate within the highly anionic MUC2 layer. Together, these molecules form an immunological barrier preventing attachment of bacteria and compromise of the mucosal epithelium. The importance of these mechanisms is demonstrated in mice lacking either Paneth or goblet cell products. These mice have impaired clearance of infectious organisms and succumb to epithelial inflammation and colitis that may be lethal 29.
The results from this current study are consistent with our previous work which demonstrated PN significantly reduces luminal levels of the goblet cell product, MUC2, and the Paneth cell product, sPLA2 24. PN also decreases the expression of the Paneth cell product sPLA2 in tissue homogenates, suggesting Paneth cell function is affected. Since Paneth and goblet cell products are stimulated in-part through the Th2 cytokines IL-4 and IL-13, we also assayed these molecules in the tissue and showed their levels were significantly lower following PN, consistent with our previous reports. This study also shows a reduction in the luminal expression of the Th2 cytokines, IL-4 and IL-1316, 45. Interestingly, the luminal cytokine levels mirror the tissue levels, suggesting a link between the two compartments. Other groups have concluded luminal cytokines may provide paracrine signals to the epithelium under inflammatory conditions 46-48. To our knowledge, this is the first report of changes to luminal cytokines following PN feeding, and we are currently investigating the implications and functions of these and other luminal cytokines in maintenance of immune function.
To determine the stimulatory role of Th2 cytokines upon tissue and luminal levels of these cell products, we exogenously administered the Th2 amplifying cytokine, IL-25, during PN. The administration of IL-25 with PN significantly increased tissue sPLA2 levels, implicating a stimulatory effect on Paneth cells. IL-25 administration to PN also significantly increased the tissue levels of Th2 cytokines IL-4 and IL-13 compared with PN alone. Finally, the addition of IL-25 to PN also significantly increased luminal levels of sPLA2 and MUC2 compared to PN alone. This suggests Th2 cytokines are important for the modulation of these Paneth and goblet cell products. Consistent with the tissue cytokines, luminal IL-4 and IL-13 were also higher in the wash fluid after exogenous IL-25 treatment of PN, further supporting a possible functional role for luminal cytokines.
To determine if IL-25 administration to PN has functional benefit to barrier function, we challenged the small bowel tissue using an ex vivo intestinal segment culture model designed to quantify bacterial invasion. Consistent with the impaired sPLA2 and MUC2 levels following PN feeding, small bowel segments from PN animals had significantly greater enteroinvasion compared to chow. Strikingly, compared with PN alone, the addition of exogenous IL-25 to PN significantly decreased bacterial invasion to levels observed in chow segments. Since IL-25 increased luminal levels of sPLA2 and MUC2, it is likely that these molecules are protective in the ex vivo model. In this study, sPLA2 served as a surrogate marker for Paneth cell production in tissue and lumen, since sPLA2 is a constitutively expressed molecule. However, there are other antimicrobial compounds produced and secreted by Paneth cells, including Lysozyme, RegIII, Ang-4, and the cryptidins in addition to sPLA2. Further studies are underway to investigate this possibility and further elucidate protective mechanisms of barrier function.
In summary, this study supports the hypothesis that PN with the lack of enteral stimulation induces dysfunctional mucosal barrier responses, as measured through the loss of MUC2, sPLA2, and increased susceptibility to Escherichia coli enteroinvasion. Further, this study demonstrates that the Th2 amplifying cytokine, IL-25, stimulates production of the cytokines IL-4 and IL-13 and restores the luminal concentrations of sPLA2 and MUC2, changes which coincide with restored barrier function in our ex vivo enteroinvasion challenge. Further work is underway to determine contributions of MUC2, sPLA2, and other Paneth cell antimicrobials molecules in protection of the mucosal barrier.
Parenteral nutrition impairs mucosal immunity and increases risks of infection in part via reduced production and secretion of both Paneth cell and goblet cell levels at mucosal surfaces. This work examines the mechanisms which control the production and secretion of these innate molecules within the epithelium and on mucosal surfaces.
Figure 6.
Recovered CFU’s after ex vivo intestinal segement culture (EVISC). Parenteral nutrition significantly increased the recovered CFU’s compared to Chow. IL-25 significantly decreased the recovered CFU’s compared to PN and was statistically similar to CFU’s recovered from Chow. Data are represented as mean ± SEM. *p < 0.0001 vs Chow and IL-25.
Acknowledgments
This research is supported by National Institute of Health (NIH) Grant R01 GM 53439. The project described was supported by Award Number I01BX001672 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development.
Footnotes
The contents of this article do not represent the views of the Veterans Affairs or the United States Government.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabian T, Cicala R, Croce M, et al. A prospective evaluation of myocardial contusion: correlation of significant arrhythmias and cardiac output with CPK-MB measurements. J Trauma. 1991;31(5):653–9. discussion 659-60. [PubMed] [Google Scholar]
- 2.Moore F, Moore E, Jones T, et al. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29(7):916–22. doi: 10.1097/00005373-198907000-00003. discussion 922-3. [DOI] [PubMed] [Google Scholar]
- 3.Moore E, Jones T. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986;26(10):874–81. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–11. doi: 10.1097/00000658-199205000-00013. discussion 511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson ME, Ambort D, Pelaseyed T, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68(22):3635–41. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuckin MA, Lindén SK, Sutton P, et al. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–78. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom KS, Kissoon-Singh V, Gibson DL, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10(11):1280–9. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 9.Ouellette AJ, Greco RM, James M, et al. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108(5):1687–95. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harwig SS, Tan L, Qu XD, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95(2):603–10. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harwig SS, Eisenhauer PB, Chen NP, et al. Cryptdins: endogenous antibiotic peptides of small intestinal Paneth cells. Adv Exp Med Biol. 1995;371A:251–5. doi: 10.1007/978-1-4615-1941-6_53. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39(1):44–51. doi: 10.1097/00005373-199507000-00006. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 13.King B, Li J, Kudsk K. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132(12):1303–9. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 14.Zarzaur B, Kudsk K. The mucosa-associated lymphoid tissue structure, function, and derangements. Shock. 2001;15(6):411–20. doi: 10.1097/00024382-200115060-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fukatsu K, Lundberg A, Hanna M, et al. Route of nutrition influences intercellular adhesion molecule-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134(10):1055–60. doi: 10.1001/archsurg.134.10.1055. [DOI] [PubMed] [Google Scholar]
- 16.Fukatsu K, Kudsk K, Zarzaur B, et al. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–22. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 17.Renegar K, Johnson C, Dewitt R, et al. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166(2):819–25. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 18.Fukatsu K, Kudsk KA. Nutrition and gut immunity. Surg Clin North Am. 2011;91(4):755–70. doi: 10.1016/j.suc.2011.04.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermsen J, Sano Y, Kudsk K. Food fight! Parenteral nutrition, enteral stimulation and gut-derived mucosal immunity. Langenbecks Arch Surg. 2009;394(1):17–30. doi: 10.1007/s00423-008-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermsen J, Gomez F, Sano Y, et al. Parenteral feeding depletes pulmonary lymphocyte populations. JPEN J Parenter Enteral Nutr. 2009;33(5):535–40. doi: 10.1177/0148607109332909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudsk K, Li J, Renegar K. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223(6):629–35. doi: 10.1097/00000658-199606000-00001. discussion 635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudsk K. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183(4):390–8. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 23.Lan J, Heneghan AF, Sano Y, et al. Parenteral nutrition impairs lymphotoxin β receptor signaling via NF-κB. Ann Surg. 2011;253(5):996–1003. doi: 10.1097/SLA.0b013e31821224eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierre JF, Heneghan AF, Tsao FH, et al. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. JPEN J Parenter Enteral Nutr. 2011;35(6):748–56. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57(6):764–71. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie GJ, Bancroft A, Grencis RK, et al. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8(6):339–42. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie GJ, Emson CL, Bell SE, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9(3):423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 28.Steenwinckel V, Louahed J, Lemaire MM, et al. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182(8):4737–43. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 29.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Beers SA, Buckland AG, Koduri RS, et al. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277(3):1788–93. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 31.Laine VJ, Grass DS, Nevalainen TJ. Protection by group II phospholipase A2 against Staphylococcus aureus. J Immunol. 1999;162(12):7402–8. [PubMed] [Google Scholar]
- 32.Inada M, Crowl RM, Bekkers AC, et al. Determinants of the inhibitory action of purified 14-kDa phospholipases A2 on cell-free prothrombinase complex. J Biol Chem. 1994;269(42):26338–43. [PubMed] [Google Scholar]
- 33.Foreman-Wykert AK, Weinrauch Y, Elsbach P, et al. Cell-wall determinants of the bactericidal action of group IIA phospholipase A2 against Gram-positive bacteria. J Clin Invest. 1999;103(5):715–21. doi: 10.1172/JCI5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinrauch Y, Elsbach P, Madsen LM, et al. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996;97(1):250–7. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinrauch Y, Abad C, Liang NS, et al. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J Clin Invest. 1998;102(3):633–8. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 37.Monteleone G, Pallone F, Macdonald TT. Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Factor Rev. 2010;21(6):471–5. doi: 10.1016/j.cytogfr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhao A, Urban JF, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135(1):217–225.e1. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madden KB, Yeung KA, Zhao A, et al. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172(9):5616–21. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 40.Zarzaur B, Wu Y, Fukatsu K, et al. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery. 2002;131(1):59–65. doi: 10.1067/msy.2002.118319. [DOI] [PubMed] [Google Scholar]
- 41.Janu P, Li J, Renegar K, et al. Recovery of gut-associated lymphoid tissue and upper respiratory tract immunity after parenteral nutrition. Ann Surg. 1997;225(6):707–15. doi: 10.1097/00000658-199706000-00008. discussion 715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson C, Kudsk K, Fukatsu K, et al. Route of nutrition influences generation of antibody-forming cells and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;237(4):565–73. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fallon PG, Jolin HE, Smith P, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17(1):7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 44.Weiss J, Inada M, Elsbach P, et al. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994;269(42):26331–7. [PubMed] [Google Scholar]
- 45.Heneghan AF, Pierre JF, Kudsk KA. IL-25 Improves IgA During Parenteral Nutrition Through the JAK-STAT Pathway. Annals Surgery. 2012 doi: 10.1097/SLA.0b013e318277ea9e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long KZ, Santos JI, Rosado JL, et al. Vitamin A supplementation modifies the association between mucosal innate and adaptive immune responses and resolution of enteric pathogen infections. Am J Clin Nutr. 2011;93(3):578–85. doi: 10.3945/ajcn.110.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumy KL, McCormick BA. The role of neutrophils in the event of intestinal inflammation. Curr Opin Pharmacol. 2009;9(6):697–701. doi: 10.1016/j.coph.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Splichal I, Rychlik I, Gregorova D, et al. Susceptibility of germ-free pigs to challenge with protease mutants of Salmonella enterica serovar Typhimurium. Immunobiology. 2007;212(7):577–82. doi: 10.1016/j.imbio.2007.05.001. [DOI] [PubMed] [Google Scholar]