Abstract
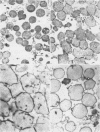
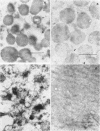
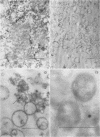
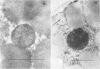
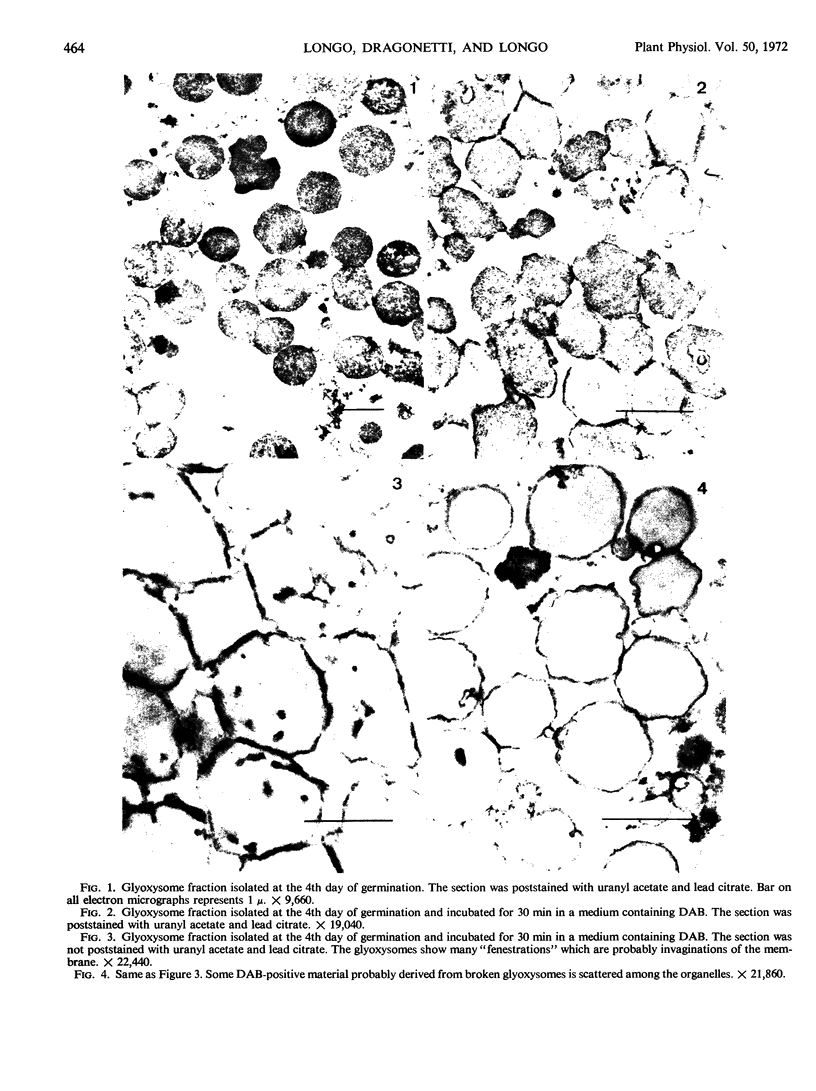
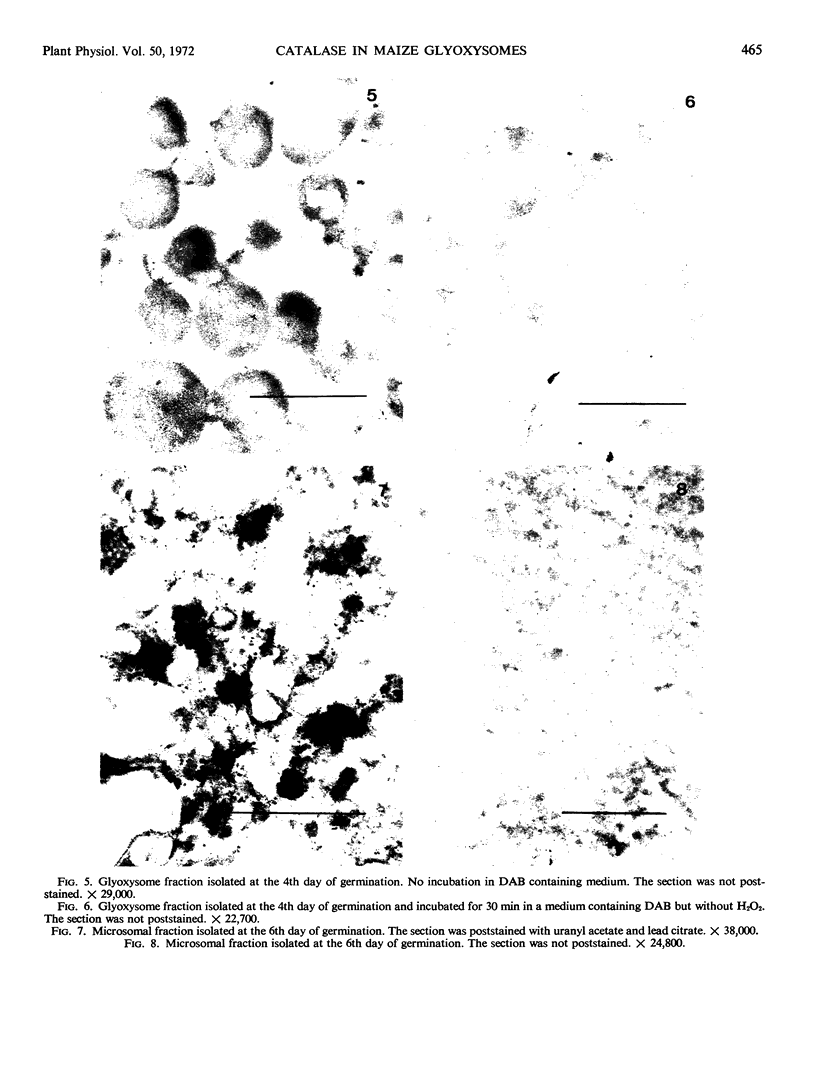
The localization of catalase in isolated maize scutellum glyoxysomes was investigated by means of the diaminobenzidine histochemical reaction. Only the membranes of the glyoxysomes become heavily stained after incubation with diaminobenzidine and H2O2. If the glyoxysomes are lysed with Tricine buffer at pH 9, 70% of the catalase is solubilized, while the remaining 30% is tightly bound to an insoluble fraction formed mostly by glyoxysomal membranes. This suggests that catalase may be present also in the matrix of the glyoxysomes. The lack of staining of the matrix with diaminobenzidine is probably due to the high concentration of catalase in the membranes of the organelles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard M. E., Novikoff A. B. Distribution of peroxisomes (microbodies) in the nephron of the rat: a cytochemical study. J Cell Biol. 1969 Aug;42(2):501–518. doi: 10.1083/jcb.42.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D. Cytochemical localization of peroxidase activity in rat hepatic microbodies (peroxisomes). J Histochem Cytochem. 1968 Aug;16(8):547–550. doi: 10.1177/16.8.547. [DOI] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Lazarow P. B., De Duve C. The synthesis and turnover of rat liver peroxisomes. I. Fractionation of peroxisome proteins. J Cell Biol. 1969 May;41(2):521–535. doi: 10.1083/jcb.41.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Longo C. P. The development of glyoxysomes in maize scutellum: changes in morphology and enzyme compartmentation. Plant Physiol. 1970 Oct;46(4):599–604. doi: 10.1104/pp.46.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Vigil E. L. Intracellular localization of catalase (peroxidatic) activity in plant microbodies. J Histochem Cytochem. 1969 Jun;17(6):425–428. doi: 10.1177/17.6.425. [DOI] [PubMed] [Google Scholar]