Abstract
Objective
Some obese individuals appear to be protected from developing type 2 diabetes mellitus and cardiovascular disease (CVD). This has led to characterizing body size phenotypes based on cardiometabolic risk factors specifically as obese or overweight, and as metabolically healthy (MH) or metabolically abnormal (MA) based upon blood pressure, lipids, glucose homeostasis and inflammatory parameters. The aim of this study was to measure the prevalence of and describe fat distribution across these phenotypes in a minority population.
Design and Methods
Hispanic participants (N=1054) in the IRAS Family Study were categorized into different body size phenotypes. Computed tomography (CT) abdominal scans were evaluated for measures of non-alcoholic fatty liver disease (NAFLD) and abdominal fat distribution. Statistical models adjusting for familial relationships were estimated.
Results
Seventy percent (70%) of the Hispanic cohort was overweight (32%) or obese (38%). Forty-one percent (n=138) of overweight participants and 19% (n=74) of obese participants met criteria for MH. Adjusted analyses showed the MH phenotype was associated with lower visceral adipose tissue (VAT) and higher liver density (indicating lower fat content) in obese participants (p=0.0005 and p=0.0002, respectively), and lower VAT but not liver density in overweight participants (p=0.008 and p=0.162, respectively) compared to their MA counterparts. Odds of NAFLD were reduced in MH obese (OR=0.34, p=0.0007) compared to MA obese. VAT did not differ between MH obese or overweight and normal weight groups.
Conclusions
These findings suggest that lower levels of visceral and liver fat, despite overall increased total body fat, may be a defining feature of MH obesity in Hispanic Americans.
INTRODUCTION
Recent evidence suggests that not all obese individuals contribute to the epidemic of type 2 diabetes, cardiovascular disease (CVD) and other co-morbid conditions (1–2). Although obesity remains an important risk factor in the development of these conditions, it has become apparent over the last decade that a subset of obese individuals seems to be protected against any subsequent complications. That is, significant variation exists in cardiometabolic risk factors among individuals of similar body mass index (BMI) and waist circumference (WC), while a person's CVD risk may depend jointly on body size and metabolic profile (3–7). Since BMI and WC are imprecise measures of obesity, investigators have identified a relatively new phenomenon of body size phenotypes (3–7). Specifically, obese individuals can be characterized as either being phenotypically metabolically abnormal (MA) or metabolically healthy (MH) (2). The MA obese phenotype is typically defined as BMI ≥ 30 kg/m2 and having ≥ 2 cardiometabolic abnormalities, whereas the MH obese phenotype is defined as BMI ≥ 30 kg/m2 and having no or one cardiometabolic abnormality(3). The definition of cardiometabolic abnormalities is partially based on the National Cholesterol Education Program’s Adult Treatment Panel III report for metabolic syndrome (7). In general, the MH obese individual is characterized by BMI ≥ 30 kg/m2 in the absence of pre-diabetes/type 2 diabetes, dyslipidemia, and hypertension (6). More specifically, these individuals are characterized by having favorable metabolic profiles including being normotensive, having high insulin sensitivity, low triglyceride levels, high HDL-C levels, and low levels of subclinical inflammation (2).
Several studies using primarily Caucasian/Northern European populations have tried to identify, characterize, and estimate the prevalence of MH obesity (1, 5, 6). In a recent study of 314 German participants the prevalence of MH obesity was measured to be approximately twenty-five percent (25%). (1). In a separate study, the prevalence of MH obesity ranged from 3.3 and to 32.1% in men and between 11.4 and 43.3% in women in a population based sample of 2803 Swiss women and 2557 Swiss men (5). In an attempt to examine a more heterogeneous population, a cross-sectional study of 5440 participants of the NHANES 1999–2004 was conducted to determine the prevalence of body size phenotypes in the US (7). On average, among US adults 20 years and older, 31.7% (approximately 19.5 million adults) of obese adults were metabolically healthy and 51.3% (approximately 35.9 million adults) of overweight adults were metabolically healthy (7). Similar prevalence patterns were noted for all races/ethnicities sampled including non-Hispanic whites, non-Hispanic blacks, and Mexican Americans (7). No other studies to date have examined the prevalence of MH obesity in minority populations representative of the general US population.
Following the recognition of the existence of body size phenotypes based on initial prevalence studies, further research has been undertaken to describe how body composition and/or body fat distribution may be related to metabolic health. Several studies have shown that the MH obese phenotype is characterized by significantly lower visceral adipose tissue (VAT) levels despite similar subcutaneous adipose tissue (SAT) levels when compared to their MA obese counterparts (8–11). Coincident to differences in body composition and body fat distribution, it has been observed that MH obese individuals may have lower levels of ectopic liver fat and possibly a lower risk of NAFLD (1, 12–16). However, these studies have been performed in Caucasian/Northern European populations and have yet to be replicated in minority US populations.
Given the sparse data and poor understanding of the role that body size phenotypes have in minority US populations, the primary aims of this study are s to assess the prevalence of different body size phenotypes in a Hispanic cohort and to characterize the clinical and metabolic factors associated with MH obesity or MH overweight including fat distribution in visceral, subcutaneous, and liver depots as measured by computed tomography (CT) across these different body size phenotypes. To accomplish these goals, we used data from the IRAS Family Study which contains detailed and extensive standardized phenotypes of all our measures of interest (17). To date, it is the only existing large multi-center trial that encompasses multi-generational pedigrees from individuals of Hispanic ethnicity in the United States.
METHODS AND PROCEDURES
Study Population
The IRAS Family Study is an epidemiologic cohort study of men and women specifically designed to investigate the genetics of insulin resistance and visceral adiposity (17). Multigenerational families of Hispanic background were enrolled using probands of the original IRAS study supplemented from the general population (18). Briefly, two sites recruited and examined family members of Hispanic ethnicity (San Antonio, TX, and San Luis Valley, CO) from 1999–2002. Insulin resistance was measured using the intravenous glucose tolerance test, and abdominal obesity was measured using CT. Cardiometabolic disease risk factors were also assessed. Follow-up examinations were conducted in 2005–2006; liver density scans were done during this period. Eligibility criteria included (i) self-reported Hispanic ethnicity, (ii) 18 years of age or older, (iii) under 350 pounds (because of CT size limitations), and (iv) not having conditions that interfere with measurement of insulin resistance or any cardiometabolic (CM) risk factor (19). This current study included all Hispanic individuals for whom we had the data necessary to estimate the MH phenotype (n=1054). All studies were conducted using protocols approved by the Institutional Review Boards at each participating institution, and all participants provided informed consent.
Measurement of Baseline Characteristics
Self-Reported Data
Age, ethnicity, and physical activity were assessed by self-report. An estimate of usual frequency of vigorous leisure-time physical activity was reported, using a defined response set ranging from “rarely or never” to “5 or more times per week.” The use of antihypertensive, lipid-lowering and antidiabetic medications were also assessed by self-report.
Anthropometric Measurements
Height and weight were measured in duplicate to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight/height squared (kg/m2). WC was measured to nearest 0.1 cm at the level of the iliac crest at the end of normal respiration. The waist to hip ratio (WHR) was measured by dividing WC by hip circumference (measured at the level of the greater trochanters with legs closed). Seated systolic/diastolic blood pressure was measured three times using a mercury manometer, after a five minute rest by centrally trained technicians using identical equipment. The mean of the last two measurements was used to calculate blood pressure.
CT-Derived Measurements
CT imaging for abdominal fat distribution was obtained under a standardized protocol and scans were read centrally (19). Participants received a scout view of the abdomen and pelvis followed by three axial images, all while participants had suspended respiration. Three 10-mm-thick images were obtained through the L2–L3, L4–L5 and T11–T12 disc spaces. If the T11–T12 image did not include liver and spleen, a fourth image was obtained using a scout to determine an appropriate intervertebral disc location. Liver and spleen density were quantified in Hounsfield units (HU) in the entire liver and spleen as visualized in the slice, excluding any visible vasculature. The image obtained at the L4–L5 disc space was used for determination of VAT area and SAT area; bowel fat was excluded. A bimodal histogram of adipose and muscle tissue was generated for each subject image and used to determine the adipose tissue area. Thus each subject is used as its own control to determine the range of adipose tissue HU. Adipose tissue was highlighted and computed with an average attenuation range of −138 to −40 HU for VAT and −154 to −42 HU for SAT. The ratio of liver to spleen density (LSR) was calculated; LSR <1.0 is an accepted cut point for a discrete outcome of NAFLD (20). Unadjusted liver density is also accepted as a continuous outcome (21). All CT images were coded for pathology and image quality; poor-quality studies were excluded from analysis (19). Percent total body fat and lean mass were obtained using dual-energy X-ray absorptiometry (DXA) scan. Liver density and DXA studies were obtained only at follow-up examination five years following the baseline examination (i.e. no baseline measurements available).
Laboratory Measurements
Plasma triglyceride (TG) concentrations, high-density lipoprotein (HDL-C) cholesterol concentrations, alanine transaminase (ALT), aspartate transaminase (AST), and γ-glutamyl transpeptidase (GGT) levels were determined by enzymatic colorimetric assays using a Chemistry Analyzer Model ATAC 8000 (Elan Diagnostics, Smithfield, RI). Plasma glucose was measured using the glucose oxidase technique on an automated autoanalyzer (YSI, Yellow Springs, OH). Insulin was measured by radioimmunoassay (Linco Research, St Charles, MO). HOMA (Homeostasis model assessment) was used to evaluate insulin resistance using the formula: fasting plasma insulin level (microunits per milliliter) × fasting plasma glucose level (millimoles per liter)/22.5. Plasma adiponectin concentration was quantified using radioimmunoassay (Linco Research, St Charles, MO). High-sensitivity C-reactive protein (hs-CRP) was measured using an ultrasensitive enzyme-linked immunosorbent assay (Calbiochem, La Jolla, CA).
Definition of Cardiometabolic Risk Factors
Six possible metabolic abnormalities were used to distinguish between MH and MA phenotypes (7). These included (i) elevated blood pressure, defined by systolic/diastolic blood pressure ≥130/85 mmHg or documented use of antihypertensive drugs; (ii) elevated TG defined by fasting TG level ≥150 mg/dL, (iii) decreased HDL-C level defined by gender-specific criteria (i.e. HDL-C < 40 mg/dL in men and < 50 mg/dL in women) or documented use of a lipid-lowering medication; (iv) elevated glucose level defined by fasting glucose level ≥ 100mg/dL or documented use of antidiabetic medication; (v) insulin resistance, defined by HOMA-IR > 5.13; and (vi) subclinical inflammation, defined by hs-CRP levels ≥3 mg/L.
Body Size Phenotype Definitions
We employed a previously developed and rigorously evaluated definition of MH and MA (7) to overweight (BMI 25.0–29.9 kg/m2) and obese participants (BMI ≥ 30.0 kg/m2). Participants with BMI < 25.0 kg/m2 were defined as normal weight and not further sub-divided as MH or MA. The MH overweight phenotype was defined as BMI 25.0–29.9 kg/m2, and having no or one metabolic abnormality. The MH obese phenotype was defined as BMI ≥ 30.0 kg/m2 and having no or one metabolic abnormality. The MA overweight phenotype was defined as BMI 25.0–29.9 kg/m2, and having 2 or more metabolic abnormalities. The MA obese phenotype was defined as BMI ≥ 30.0 kg/m2 and having 2 or more metabolic abnormalities. We also included a comparison group comprised of normal weight participants defined as BMI ≤ 25.0 kg/m2 with a healthy metabolic profile based on our definition of MH (N=234). Further, we excluded 26.4% (N=84) of the normal weight participants from the analyses who were deemed to be MA based on our definition of metabolic health.
Statistical Analysis
Quantitative variables were expressed as means and standard deviations and qualitative variables as number of participants and percentages. The IRAS Family Study consists of correlated data between family members (17). Thus, all family relationships were examined using the generalized estimating equation approach using the SAS (Cary, NC) PROC GENMOD procedure. The models account for familial correlation using a sandwich estimator of variance under exchangeable correlation. The α-level for testing significance of main effects was set a priori at p<0.05. General linear mixed models were used to calculate adjusted beta estimates to examine whether body size phenotype (MH vs. MA) was associated with VAT, SAT, or liver density. Logistic regression was used to calculate odds ratio of NAFLD across body size phenotypes. All analyses were repeated after exclusion of 118 persons with type 2 diabetes (not shown).
RESULTS
Seventy percent (70%) of the Hispanic cohort were overweight (32%) or obese (38%). Forty-one percent of overweight participants (n=138) and 19% of obese participants (n=74) met criteria for MH (Table 1). In addition to expected differences in metabolic factors used to define these groups (Table 1), MH individuals were, on average, younger than MA groups, more physically active, less likely to be on medications, had smaller WC and a lower WHR, and had higher levels of circulating adiponectin. Liver enzymes which may serve as a biochemical marker for NAFLD were not consistently associated with phenotype. AST was lower in MH obese and GGT was lower in MH overweight participants. ALT did not differ in either obese or overweight phenotypes. VAT areas were lower in MH (p<0.0001); however, SAT areas did not differ between MH and MA groups (p=0.3221; p=0.171). Consequently, visceral to subcutaneous tissue area ratio (VSR) was lower in MH compared to MA individuals (p=0.0096; p<0.0001). Liver density was higher in the MH obese group compared to MA obese individuals, indicating lower levels of fat in liver (p=0.0022). Similarly, NAFLD prevalence was reduced in MH groups (22.6% versus 44.1%, in MH and MA obese groups, and 17.9% versus 27.3% in MH and MA overweight groups, respectively). Percent fat and lean mass as measured by DXA did not differ between MH and MA phenotypes.
Table 1.
Baseline Characteristics of IRAS-Family Hispanic Participants.*
| Baseline Characteristics | Obese Phenotype | Overweight Phenotype | Normal Weight** | ||||
|---|---|---|---|---|---|---|---|
| Healthy | Abnormal | P-value | Healthy | Abnormal | P-value | ||
| N | 74 | 323 | 138 | 201 | 234 | ||
| Age (years) | 36.7(12.1) | 44.4(13.4) | <.0001 | 37.0(10.9) | 45.9(14.8) | <.0001 | 35.6(12.4) |
| Female, N (%) | 41(55.4) | 206(63.8) | 0.3623 | 81(58.7) | 97(48.3) | 0.0827 | 150(64.1) |
| Center, N (%) | |||||||
| SA | 44(59.5) | 212(65.6) | 0.8301 | 68(49.3) | 100(49.8) | 0.9490 | 101(43.2) |
| SLV | 30(40.5) | 111(34.4) | 70(50.7) | 101(50.2) | 133(56.8) | ||
| Physical Activity, N (%) | |||||||
| Rarely/never | 16(21.6) | 114(35.5) | 0.0582 | 18(13.0) | 60(29.9) | 0.0014 | 31(13.2) |
| 1–3/mth | 20(27.0) | 82(25.5) | 29(21.0) | 47(23.4) | 50(21.4) | ||
| 1/week | 11(14.9) | 37(11.5) | 25(18.1) | 18(9.0) | 28(12.0) | ||
| 2–4/week | 18(24.3) | 72(22.4) | 49(35.5) | 55(27.4) | 93(39.7) | ||
| 5+/week | 9(12.2) | 16(5.0) | 17(12.3) | 21(10.4) | 32(13.7) | ||
| BP meds, N(%)˜ | 3(4.1) | 68(21.1) | 3(2.2) | 32(15.9) | 1(0.4) | ||
| Lipid meds, N (%)˜ | 0(0.0) | 23(7.1) | 2(1.5) | 14(7.0) | 1(0.4) | ||
| Diabetes meds, N (%)˜ | 2(2.7) | 49(15.2) | 0(0.0) | 22(11.0) | 0(0.0) | ||
| SBP, mmHg˜ | 114.6(13.6) | 124(16.9) | 112.6(13.3) | 121.7(17.3) | 108.0(10.9) | ||
| DBP, mmHg˜ | 75.1(8.4) | 79.2(10.1) | 74(8.5) | 78.2(9.1) | 70.9(8.0) | ||
| HDL-C, mg/dL˜ | 46.2(9.1) | 38.6(10.9) | 48.6(12.5) | 38.7(12) | 51.1(11.1) | ||
| Triglycerides, mg/dL˜ | 95.4(41) | 149.9(90.5) | 82.2(30.8) | 162.3(103.5) | 69.1(29.4) | ||
| Fasting Glucose, mg/dL˜ | 90.3(6.4) | 113.7(38.1) | 91.1(6.8) | 107.2(38.4) | 88.3(10.1) | ||
| SI | 1.8(1.2) | 0.8(0.8) | <.0001 | 2.8(2) | 1.4(1.3) | <.0001 | 3.6(1.9) |
| HOMA˜ | 3.1(1.4) | 7(5.8) | 2.5(1.2) | 4.1(2.6) | 1.7(0.9) | ||
| Hs-CRP, mg/L˜ | 2.7(3.8) | 5.7(5) | 1.7(1.8) | 4(4.5) | 1.3(2.1) | ||
| Adiponectin, µg/mL | 15.2(11.7) | 11.5(5.9) | 0.0140 | 14.8(6.7) | 11.4(6.6) | 0.0003 | 16.6(6.7) |
| ALT, U/L | 11.4(10.8) | 12.6(10.1) | 0.3164 | 11.0(11.8) | 12.6(11.8) | 0.1903 | 8.1(4.9) |
| AST, U/L | 18.2(7.6) | 21.1(10.4) | 0.0173 | 19.1(10.6) | 19.3(8.8) | 0.8029 | 17.0(7.4) |
| GGT, U/L | 40.1(37.8) | 45.9(44.0) | 0.3294 | 34.2(44.7) | 46.8(47.6) | 0.0234 | 29.2(39.1) |
| BMI, kg/m2˜ | 33.2(2.6) | 35.7(4.7) | 27.4(1.5) | 27.4(1.4) | 22.1(1.9) | ||
| Waist Circumference, cm | 97.5(8.6) | 103.9(11.1) | 0.0007 | 85.3(7.6) | 89.1(7.7) | 0.0003 | 74.2(7.0) |
| WHR | 0.86(0.09) | 0.88(0.08) | 0.0638 | 0.84(0.07) | 0.88(0.08) | <.0001 | 0.8(0.1) |
| VAT, cm2 | 115.3(47.8) | 160.4(60.2) | <.0001 | 88.6(33.6) | 119.3(45.2) | <.0001 | 56.1(31.8) |
| SAT, cm2 | 466.8(119.6) | 487.1(131.3) | 0.3221 | 307.6(80.2) | 293.9(78.6) | 0.1712 | 191.5(75.8) |
| VSR | 0.28(0.17) | 0.36(0.18) | 0.0096 | 0.31(0.15) | 0.44(0.22) | <.0001 | 0.33(0.20) |
| Liver Density+, (HU) | 50.8(10.8) | 44.8(13.2) | 0.0022 | 54.6(10.4) | 52.1(11) | 0.0815 | 59.1(8.5) |
| Liver to Spleen Ratio+ (LSR) | 1.10(0.23) | 1.00(0.30) | 0.0091 | 1.18(0.23) | 1.12(0.23) | 0.0411 | 1.26(0.17) |
| NAFLD (LSR <1) +, N (%) | 12(22.6) | 105(44.1) | 0.0025 | 19(17.9) | 39(27.3) | 0.0476 | 10(5.8) |
| % Fat from DXA+ | 36.4(8.7) | 38.9(7.2) | 0.1025 | 32.2(7.3) | 32.4(7.5) | 0.8583 | 28.8(8.3) |
| % Lean from DXA+ | 61(8.4) | 58.8(7.0) | 0.1167 | 64.9(7.1) | 64.8(7.3) | 0.8919 | 68(8.0) |
N (%) or mean (SD); p<0.05 indicates significant differences between metabolically healthy (MH) and metabolically abnormal (MA) obese or overweight adjusted for familial correlation.
Normal Weight metabolically healthy (MH) participants only.
For abbreviations :ALT indicates alanine transaminase, AST aspartate transaminase, BMI body mass index, BP blood pressure, DBP diastolic blood pressure, DXA dual X-ray absorptiometry, GGT gamma-glutamyl transpeptidase, HDL-C high-density lipoprotein cholesterol, HOMA, homeostasis model assessment, Hs-CRP high-sensitivity C-reactive protein, HU Hounsfield units, LSR liver to spleen ratio, NAFLD non-alcoholic fatty liver disease, SI fasting insulin, SA San Antonio, SAT subcutaneous adipose tissue, SBP systolic blood pressure, SLV San Luis Valley, WHR waist-hip ratio, VAT visceral adipose tissue, and VSR visceral to subcutaneous tissue area ratio.
Statistical testing not performed because these variables define the MH and MA phenotype.
Measures obtained at follow-up, sample sizes reduced by approximately 20%
With adjustment for age, gender, geographic location, family relationships, liver enzymes, and BMI, MH obese participants still had lower VAT (p=0.0005), VSR (0.02), and higher liver density (p=0.0002), than MA obese participants, and MH overweight participants had lower VAT (p=0.008) and VSR (p=0.004) than MA overweight participants (Table 2 and Figures 1–3). Odds of NAFLD were reduced in MH (OR= 0.34, P=0.0007 for obese and OR=0.60, P=0.1031 for overweight) compared to MA (not shown). As in unadjusted comparisons, no differences were observed for SAT, percent fat and percent lean mass. When we excluded participants with diabetes from these analyses, despite a reduction in precision, the interpretation did not change (not shown).
Table 2.
Measures of Fat distribution Among Metabolically Healthy and Metabolically Abnormal Hispanics in the IRAS Family Study.*
| Obese Phenotype | Overweight Phenotype | Normal Weight** (n=234) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolically healthy (n=74) |
Metabolically abnormal (n=323) |
1P-value | Metabolically healthy (n=138) |
Metabolically abnormal (n=201) |
2P-value | 3P-value | 4P-value | ||
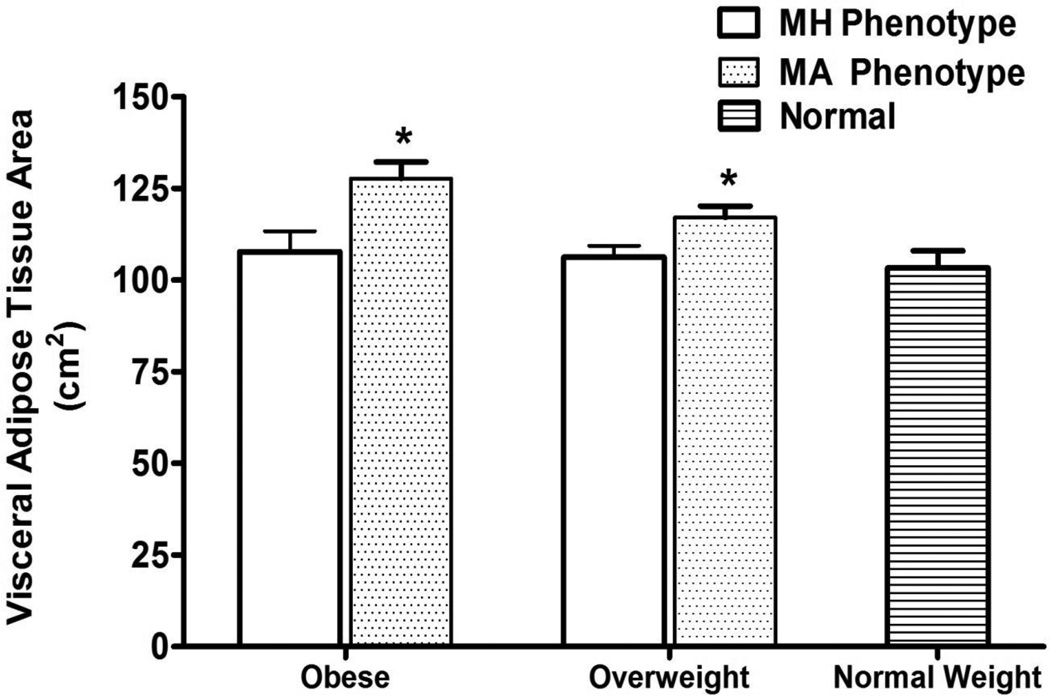
| VAT, cm2 | 107.7 (5.9) | 127.7 (4.7) | 0.0005 | 106.3 (3.1) | 117.1 (3.1) | 0.0083 | 103.3 (4.7) | 0.6078 | 0.4160 |
| SAT∫, cm2 | 458.9 (13.4) | 476.0 (7.0) | 0.2546 | 293.7 (6.7) | 302.3 (5.4) | 0.3186 | 171.8 (4.7) | <0.0001 | <0.0001 |
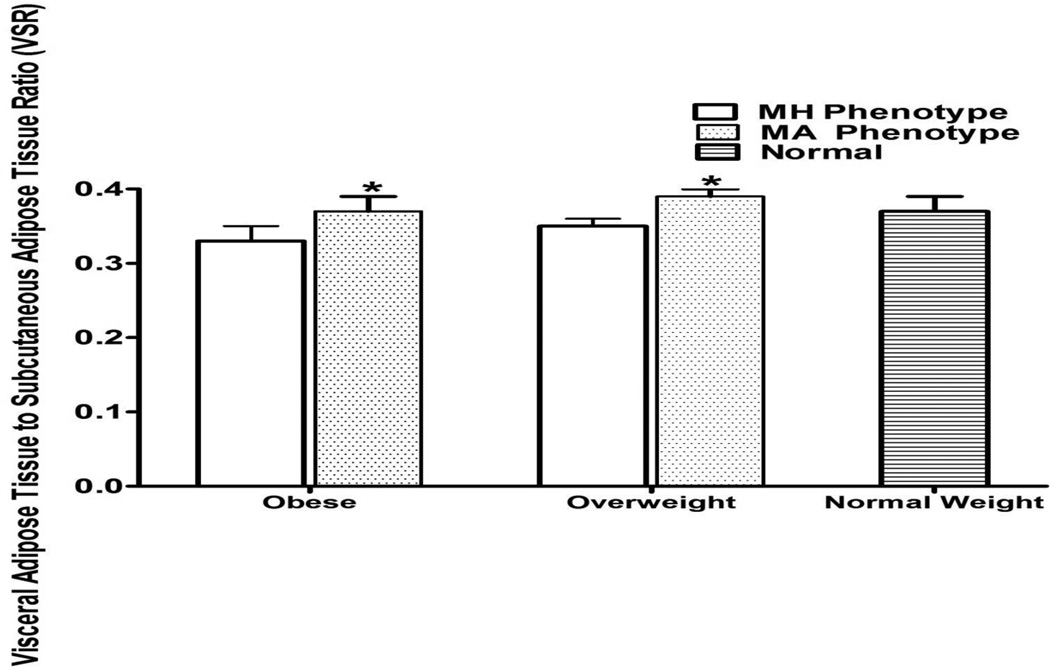
| VSR | 0.33 (0.02) | 0.37 (0.02) | 0.0205 | 0.35 (0.01) | 0.39 (0.01) | 0.0044 | 0.37 (0.02) | 0.0830 | 0.0843 |
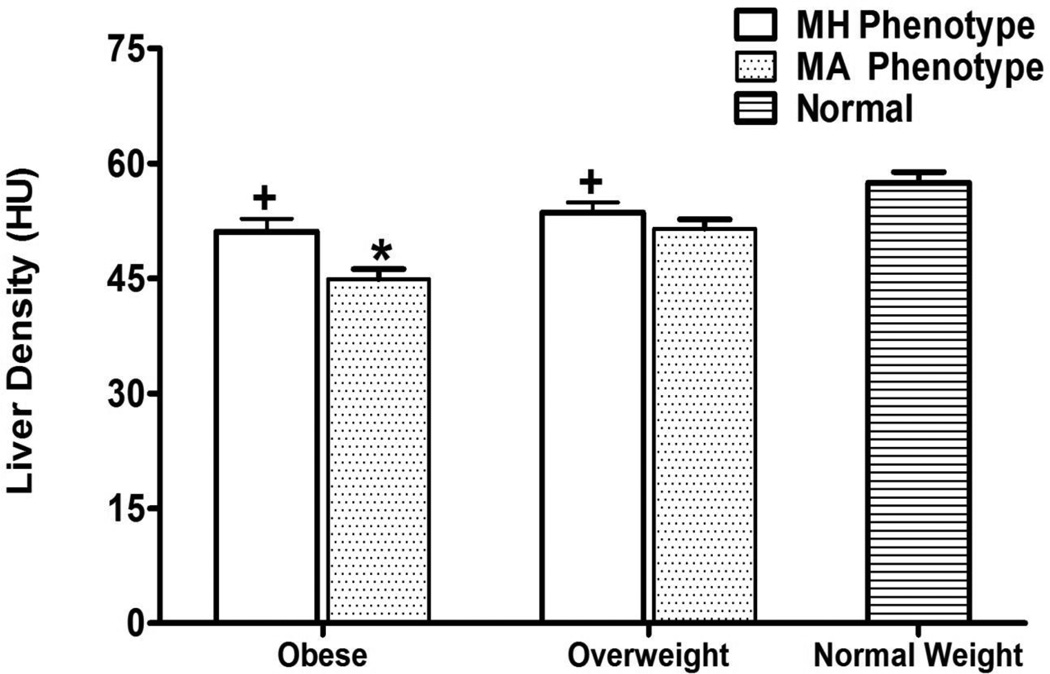
| Liver Density+ (HU) | 51.1 (1.7) | 44.9 (1.4) | 0.0002 | 53.6 (1.3) | 51.5 (1.2) | 0.1642 | 57.5 (1.4) | 0.0132 | 0.0145 |
| LSR + | 1.10 (0.03) | 0.98 (0.03) | 0.0008 | 1.17 (0.03) | 1.10 (0.02) | 0.0383 | 1.24 (0.03) | 0.0087 | 0.0534 |
| % Fat from DXA+ | 33.3 (0.7) | 33.6 (0.4) | 0.6445 | 32.7 (0.5) | 32.9 (0.4) | 0.6988 | 30.6 (0.6) | 0.0057 | 0.0008 |
| % Lean from DXA+ | 64.1 (0.7) | 63.8 (0.3) | 0.6898 | 64.5 (0.5) | 64.3 (0.3) | 0.7097 | 66.4 (0.5) | 0.0104 | 0.0016 |
Adjusted for age, gender, and clinic, liver enzymes, and BMI (Adjusted mean (SE) values with p-values indicating obese and overweight group differences; p <0.05 indicates significant differences between MH and MA groups).
Normal Weight metabolically healthy (MH) participants only.
Measures obtained at follow-up, sample sizes reduced by approximately 20%
SAT not adjusted for BMI
p-values comparing metabolically healthy obese to metabolically abnormal obese
p-values comparing metabolically healthy overweight to metabolically abnormal overweight
p-values comparing normal weight to metabolically healthy obese
p-values comparing normal weight to metabolically healthy overweight
Figure 1.
Visceral Adipose Tissue Areas for Obese, Overweight, and Normal Weight Hispanic Americans in the IRAS Family Study∫.
* p-values <0.05 comparing VAT between MH phenotypes to MA phenotypes.
∫VAT was not different between normal weight phenotype and MH obese or MH overweight phenotypes. Adjusted for age, gender, and clinic site. MA indicates metabolically abnormal and MH metabolically healthy.
Figure 3.
Visceral Adipose Tissue to Subcutaneous Adipose Tissue Ratio for Obese, Overweight, and Normal Weight Hispanic Americans in the IRAS Family Study∫.
* p-values <0.05 comparing metabolically healthy phenotypes to metabolically abnormal phenotypes. Adjusted for age, gender, and clinic site.
∫VSR was not different between normal weight phenotype and MH obese or MH overweight phenotypes.
Adjusted for age, gender, and clinic site.
The normal weight comparison group was compared to both MH obese and overweight groups (Table 2) adjusted for age, gender, geographic location, family relationships, liver enzymes, and BMI. VAT areas and VSR did not differ between normal weight and MH obese or overweight participants. LSR did not differ between normal weight and MH overweight participants. In contrast, both MH obese and overweight participants had lower liver density, higher SAT, higher percent fat, and lower percent lean mass than normal weight participants. .
DISCUSSION
In this cohort of Hispanic individuals, MH obese and overweight participants had lower CT-determined measures of VAT, VSR, liver density, and decreased odds of NAFLD compared to MA groups, despite similar body size based on BMI. These findings persisted with adjustment for age, gender, geographic location, family relationships, liver enzymes, and BMI. Second, VAT areas did not differ between normal weight and MH obese or overweight individuals, despite differences in BMI. Similarly, LSR did not differ between normal weight and MH overweight individuals, despite differences in BMI. Finally, VSR did not differ in MH obese and overweight compared to normal weight individuals. Taken together, these findings suggest that the term "metabolically healthy" may be useful to identify obese and overweight individuals who, despite their higher BMI, may not be at any greater risk of type 2 diabetes and CVD compared to individuals with a normal BMI.
This is the first study in a large Hispanic cohort that has evaluated abdominal fat distribution in different body size phenotypes. Several small studies have shown the MH obese phenotype, compared to the MA obese phenotype, is associated with lower VAT despite similar amounts of SAT (8–11; 22–24). For example, in a study of 113 obese, sedentary postmenopausal women, body composition was measured by dual-energy x-ray absorptiometry and body fat distribution was measured by computed tomography scan (8). When comparing MH obese to MA obese groups, no differences were observed for subcutaneous adipose tissue. However, MH obese individuals had significantly less visceral adipose tissue than MA obese individuals (p<0.05) (8). In a separate study of 43 obese, sedentary postmenopausal women, subjects were classified as MH obese or MA obese and body composition (fat mass and lean body mass) and body fat distribution (abdominal visceral and subcutaneous adipose tissue areas, mid-thigh subcutaneous adipose tissue and muscle attenuation) were measured (22). Despite comparable total body fatness between groups (45.2 +/− 5.3% vs. 44.8 +/− 6.6%; P>0.05), MH obese individuals had 49% less visceral adipose tissue than MA obese subjects (141 +/− 53 vs. 211 +/− 85 cm(2); P: <0.01). No difference was noted between groups for abdominal subcutaneous adipose tissue (453 +/− 126 vs. 442 +/− 144 cm (2); P: = NS), total fat mass (38.1 +/− 10.6 vs. 40.0 +/− 11.8 kg), and muscle attenuation (42.2 +/− 2.6 vs. 43.6 +/− 4.8 HU) (22).
In addition to the observation that MH obese individuals have lower VAT levels compared to their MA obese counterparts, several studies have found that MH obese individuals have lower levels of ectopic liver fat and potentially a decreased risk of NAFLD (1, 12–16). In a study of 314 obese Germans, MH obese participants had significantly less ectopic fat (i.e. liver) compared to MA obese participants (1). In a study of 82 Italian women screened for MH obesity, MA individuals had significantly greater evidence of fatty liver (i.e. hepatic steatosis) and thus higher concentrations of hepatic enzymes (i.e. AST, ALT, and GGT) than their MH counterparts (12). Finally, in a study of 104 obese postmenopausal women, those with the MH obesity phenotype had a lower fatty liver index compared to the MA obese subjects. (13). However, given the limited data available, it has yet to be elucidated whether an increase in liver fat is an independent predictor of metabolic health in obese individuals apart from the other observed body distribution differences. The present study extends these earlier findings to a large cohort of Hispanic individuals at significant risk for overweight, obesity, and NAFLD. Our data suggest that a less fatty liver and smaller VAT areas may be important defining features of cardiometabolic health in obese and overweight individuals.
The primary strength of the present study is that data were derived from a large sample of comprehensively phenotyped Hispanics using direct measures of insulin resistance, inflammatory markers, and CT-derived adipose tissue distribution (19). To our knowledge, this is the first report to describe similarities in fat depot location between normal weight and the MH obese or overweight phenotype, and this is only the second report characterizing MH obesity in a Hispanic cohort (7). Finally, the IRAS Family data in this study also possesses unique, precise information regarding NAFLD.
Our study has several limitations. First, although the concept of describing obese and overweight individuals based on cardiometabolic risk is becoming more recognized in the scientific community, the definition of body size phenotype has not been standardized. We chose our criteria based on procedural rigor for selecting cut-points (7, 25). Second, our study population came from two distinct regions of the US, which may limit generalizability of our findings to other Hispanics (26). Third, 5 years elapsed between collection of baseline measurements (including cardiometabolic risk factors defining MH and MA), liver density measurement, NAFLD assessment, and total body fat measurements. However, despite the time difference between measures, we found clear evidence of decreased VAT, decreased liver density, and reduced odds of NAFLD in MH overweight or obese individuals compared to MA participants. Finally, we are unable to evaluate the association between the MH phenotype and CVD risk given the limitations of our data collection from IRAS-Family. Several recent studies have examined the association between MH obesity and subclinical CVD with surrogate endpoints such as carotid artery intima media thickness, aortic pulse wave velocity, coronary calcification, and heart rate variability with conflicting results (25, 27–28).
This study is noteworthy because it provides specific and sensitive markers describing fat distribution in visceral, subcutaneous, and ectopic (i.e. liver) adipose tissue. CT-derived measures in our study indicated that visceral and liver fat depots are defining features of MH obesity and overweight: these groups tend to have similar visceral and liver fat depots to normal weight counterparts, but lower visceral and liver fat depots compared to MA participants. Several intervention trials have indicated that weight loss in MH obese individuals may be ineffective or paradoxically harmful regarding cardiometabolic risk factors, whereas other trials have shown significant improvement in these risk factors in MH obese participants(29–33).These discrepancies likely reflect inconsistent criteria for MH versus MA obesity, and as this study demonstrates, if distinctions between body size phenotypes are to be adopted more widely, they must be validated in a range of settings and different patient populations.
In conclusion, the present study indicates that the MH overweight and obesity phenotypes are relatively common in our Hispanic participants, who live in San Antonio, TX and San Luis Valley, CO. Previously, MH or MA obesity has been distinguished by specific cardiometabolic risk factors such as blood pressure. However, we found that fat stored in visceral and liver depots can also be used to differentiate the presence of the MH and MA phenotype. These findings are significant as they suggest that obesity as defined by BMI may not have the same physiologic importance for every individual. As the obesity crisis has reached global epidemic proportions, the necessity for alternative approaches to primary and secondary prevention and perhaps policy implementation is paramount. From a clinical as well as public health standpoint, distinguishing MH and MA obesity could help identify which individuals are at increased risk and would benefit the most from intensive weight loss specific intervention (34). Future research on body size phenotypes (including subsequent risk of CVD and type 2 diabetes) will assist in developing approaches for the detection, treatment, and prevention of disease that are more tailored to individual patients (34).
Figure 2.
Liver Density Areas for Obese, Overweight, and Normal Weight Hispanic Americans in the IRAS Family Study.
* p-values <0.05 comparing VAT between MH phenotypes and MA phenotypes. Adjusted for age, gender, and clinic site.
+p-values <0.05 comparing VAT between MH phenotypes to normal weight phenotype. Adjusted for age, gender, and clinic site.
ACKNOWLEDGMENTS
Dr. Samaropoulos was supported by the T32 training grant on quality of Care and Outcomes Research in Cardiovascular Disease and Stroke Grant No T32HL087730. IRAS Family Study funding was obtained through grants R01HL060944, R01061019, R01HL060919, and R01HL060894. We thank Karen Klein for her editorial assistance in the preparation of this manuscript.
Footnotes
DISCLOSURES
The authors declared no conflict of interest.
REFERENCES
- 1.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1282. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP. Healthy obesity. Curr Opin Clin Nutr Metab Care. 2009;12:438–443. doi: 10.1097/MCO.0b013e32832c6db7. [DOI] [PubMed] [Google Scholar]
- 4.Muller MJ, Bosy-Westphal A, Heller M. 'Functional' body composition: differentiating between benign and non-benign obesity. Biology Report. 2009;1:75. doi: 10.3410/B1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velho S, Paccaud F, Waeber G, Vollenweider P, Marques-Vidal P. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr. 2010;64:1043–1051. doi: 10.1038/ejcn.2010.114. [DOI] [PubMed] [Google Scholar]
- 6.Bluher M. The distinction of metabolically 'healthy' from 'unhealthy' obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 7.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering; prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2009;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 8.Messier V, Karelis AD, Prud’homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity. 2010;18(5):911–917. doi: 10.1038/oby.2009.364. [DOI] [PubMed] [Google Scholar]
- 9.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 10.Hayes L, Pearce MS, Firbank MJ, Walker M, Taylor R, Unwin NC. Do obese but metabolically normal women differ in intra-abdominal fat and physical activity levels from those with the expected metabolic abnormalities? A cross-sectional study. BMC Public Health. 2010;10:1–9. doi: 10.1186/1471-2458-10-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CS, Massaro JM, Hoffman U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 12.Tarantino G, Pizza G, Colao A, Pasanisi F, Conca P, Colicchio P, Finelli C, et al. Hepatic steatosis in overweight/obese females: new screening method for those at risk. World J Gastroenterol. 2009;15(45):5693–5699. doi: 10.3748/wjg.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messier V, Karelis AD, Robillard ME, Bellefeuile P, Brochu M, Lavoie JM, et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism. 2010;59:20–24. doi: 10.1016/j.metabol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity. 2010;18:1510–1515. doi: 10.1038/oby.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Adamo E, Cali Am, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henkin L, Bergman RN, Bowden DW, et al. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann Epidemiol. 2003;13(4):211–217. doi: 10.1016/s1047-2797(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 18.Wagenknecht LE, Mayer E, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS): objective, design, and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 19.Wagenknecht LE, Scherzinger AL, Stamm ER, et al. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity. 2009;17(6):1240–1246. doi: 10.1038/oby.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo R, Ricci C, Masutti F, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28(4):297–302. [PubMed] [Google Scholar]
- 21.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR AM J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 22.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 23.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 24.Jennings CL, Lambert EV, Collins M, Joffe Y, Levitt NS, Goedecke JH. Determinants of insulin-resistant phenotypes in normal-weight and obese Black African women. Obesity. 2008;16:1602–1609. doi: 10.1038/oby.2008.233. [DOI] [PubMed] [Google Scholar]
- 25.Khan UI, Wang D, Thurston RC, et al. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The Study of Women’s Health Across the Nation (SWAN) Atherosclerosis. 2011:1–8. doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young KA, Fingerlin TE, Langefeld CD, et al. Exploring Correlates of Adiposity in Two U.S. Hispanic Populations Using Social, Behavioral and Genetic Markers: The IRAS Family Study". Ethn Dis. 2011 In press. [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Kim SH, Cho G-Y, et al. Obesity phenotype and cardiovascular changes. J Hypertens. 2011;29(9):1765–1772. doi: 10.1097/HJH.0b013e32834a50f3. [DOI] [PubMed] [Google Scholar]
- 28.Robillard M-E, Bellefeuille P, Comtois AS, Aubertin-Leheudre M, Karelis AD. The metabolically healthy but obese postmenopausal woman presents a favourable heart rate variability profile. Scand Cardiovasc J. 2011;45(5):316–320. doi: 10.3109/14017431.2011.591818. [DOI] [PubMed] [Google Scholar]
- 29.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care. 2010;33(9):1957–1959. doi: 10.2337/dc10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752–1754. doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- 31.Kantarzis K, Machann J, Schick F, et al. Effects of a lifestyle intervention in metabolically benign and malignant obesity. Diabetologia. 2011;54:864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]
- 32.Bouchard DR, Langlois MF, Brochu M, Dionne IJ, Ballargeon JP. Metabolically healthy obese women and functional capacity. Metab Syndr Relat Disord. 2011;10:1–5. doi: 10.1089/met.2010.0101. [DOI] [PubMed] [Google Scholar]
- 33.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT study. J Gerontol A Biol Sci Med Sci. 2010;65:519–525. doi: 10.1093/gerona/glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Primeau V, Coderre L, Karelis AD. Characterizing the profile of obese patients who are metabolically health. Int J Obes. 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]