Abstract
Despite similar behavioral hypersensitivity, acute and chronic pain have distinct neural bases. Here we used intraplantar injection of Complete Freund’s Adjuvant (CFA) to directly compare activity of pain-modulating neurons in the rostral ventromedial medulla (RVM) in acute versus chronic inflammation.
Heat- and von Frey-evoked withdrawal reflexes and corresponding RVM neuronal activity were recorded in lightly anesthetized animals either during the first hour after CFA injection (acute) or 3–10 days later (chronic). Thermal and modest mechanical hyperalgesia during acute inflammation were associated with increases in the spontaneous activity of pain-facilitating ON-cells and suppression of pain-inhibiting OFF-cells. Acute hyperalgesia was reversed by RVM block, showing that the increased activity of RVM ON-cells is necessary for acute behavioral hypersensitivity. In chronic inflammation, thermal hyperalgesia had resolved, but mechanical hyperalgesia had become pronounced. The spontaneous discharges of ON- and OFF-cells were not different from controls, but the mechanical response thresholds for both cell classes were reduced into the innocuous range. RVM block in the chronic condition worsened mechanical hyperalgesia.
These studies identify distinct contributions of RVM ON- and OFF-cells to acute and chronic inflammatory hyperalgesia. During early immune-mediated inflammation, ON-cell spontaneous activity promotes hyperalgesia. After inflammation is established, the anti-nociceptive influence of OFF-cells is dominant, yet the lowered threshold for the OFF-cell pause allows behavioral responses to stimuli that would normally be considered innocuous. The efficacy of OFF-cells in counteracting sensitization of ascending transmission pathways could therefore be an important determining factor in development of chronic inflammatory pain.
Introduction
Chronic pain is not merely prolonged activation of normal pain pathways, but instead reflects plasticity in both peripheral and central neuronal circuits. The rostral ventromedial medulla (RVM) is the final output relay from a well-studied pain-modulating system [14]. This circuit modulates nociceptive transmission pathways during acute injury, but is also thought to maintain sensitization during chronic pain [26,39,45].
The transition from acute to chronic pain is accompanied by physiological and molecular changes in the RVM. For example, the effectiveness of electrical stimulation in inhibiting nociceptive behaviors fluctuates over the first 24 hours following injection of an inflammatory agent in the hindpaw [17–18,51]. In the days after induction, inflammation also produces changes in NMDA, AMPA, trkB, opioid, and neurokinin-1 receptor expression and function [16–19,28,31,45,49], as well as changes in local glial activation [47]. However, the functional significance of many of these molecular and cellular changes remains unclear. Because the RVM can independently facilitate and inhibit nociception [13,26], enhanced behavioral sensitivity could reflect increased descending facilitation, reduced descending inhibition, or a combination of both.
The RVM inhibits and facilitates nociceptive transmission pathways through the actions of two classes of neurons, “OFF-cells” and “ON-cells”, respectively [14,26], but the specific contributions of the ON- and OFF-cell classes to different chronic pain states are not well understood. In acute neurogenic inflammation, the spontaneous firing of both cell classes is altered, with ON-cell discharge significantly increased and OFF-cell firing depressed. The increase in ON-cell activity is necessary for hyperalgesia [4,30]. By extension, if chronic inflammation were simply a continuation of the acute condition, then the increased spontaneous firing of the ON-cells should be maintained. However, in another model of chronic pain, nerve injury, RVM ON-cells do not display abnormal spontaneous activity [5,38]. Instead, both ON- and OFF-cells become sensitized and display abnormal responsiveness to innocuous tactile stimulation [5]. The dissimilar pattern of RVM activity in acute neurogenic inflammation compared to chronic nerve injury indicates that, despite clear evidence implicating the RVM in behavioral hypersensitivity in both conditions, the underlying RVM processes mediating hyperalgesia are not the same. This difference could relate to the type of injury (inflammatory vs. neuropathic) or to its time-course (acute vs. chronic).
The goal of the present experiments was to record the activity of identified ON- and OFF-cells at acute (1 hour) and chronic (3–10 days) time points during localized inflammation induced by injection of complete Freund’s adjuvant (CFA) in the plantar hindpaw. These time points were chosen based on reports that the RVM contribution to behavioral sensitivity fluctuates over the first 24 hours after injection, but is then generally stable for up to two weeks [20,43,45]. This approach allowed us to compare directly the activity of pain-facilitating and pain-inhibiting RVM neurons under standard conditions during acute and chronic immune-mediated inflammation, and to test the net RVM contribution to thermal and mechanical hypersensitivity at both time points.
Materials and Methods
All experimental procedures followed the guidelines of the National Institutes of Health and of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. Methods used here were approved by the Institutional Animal Care and Use Committee at the Oregon Health & Science University.
Inflammation
For studies of acute inflammation, male Sprague-Dawley rats weighing between 250 and 350 g were anesthetized and stabilized in a lightly anesthetized state (described below). Saline (0.1 ml) or Complete Freund’s Adjuvant (CFA, heat-killed Mycobacterium tuberculosis in mineral oil, 1 mg/ml, 0.1 ml, Sigma-Aldrich) was injected subcutaneously into the plantar surface of the left hindpaw. In a subset of animals, paw thickness and surface temperature were measured at the base of the ankle before and 15 minutes after hindpaw injection.
To induce chronic inflammation, rats weighing less than 250 g were briefly anesthetized with isoflurane (4%, 4–5 minutes). Saline (0.1 ml) or CFA (0.1 ml) was injected subcutaneously into the plantar surface of the left hindpaw. Animals were anesthetized 3–10 days later (mean ± SEM: 6.2 ± 0.5 days) for electrophysiological and behavioral studies, at which point they weighed between 250 and 350 g. This range of days was chosen based on pilot data and as consistent with the known duration of behavioral hypersensitivity following plantar CFA administration, which typically peaks at one day (24 h), but is maintained for two weeks or more [28,43,45].
Experimental animals
A total of 112 animals was used in this work. For electrophysiological studies of RVM neurons in acute inflammation, we used a within-subject design comparing pre- and post-injection neuronal activity and thermal and mechanical nociception. Seventeen animals were treated with saline and 24 with CFA. We recorded 6 NEUTRAL-cells, 5 OFF-cells, and 6 ON-cells in the saline group and 10 NEUTRAL-cells, 7 OFF-cells, and 11 ON-cells in the CFA-treated group. To determine the contribution of the RVM to behavioral hypersensitivity in these animals, RVM neurons were inactivated by lidocaine microinjection in additional animals following plantar saline (n = 8) or CFA (n = 6) injection.
For electrophysiological studies of RVM neurons during chronic inflammation, we used a between-subject design, comparing neuronal activity and mechanical and thermal nociception in CFA-treated animals against that in saline-treated and naïve control groups. Since data from saline-treated and naïve animals were not significantly different, those groups were combined for subsequent analyses. Twenty-three control animals and 22 CFA-injected animals were studied. We recorded 8 NEUTRAL-cells, 10 OFF-cells, and 12 ON-cells in control animals, and 8 NEUTRAL-cells, 11 OFF-cells, and 13 ON-cells in CFA-treated animals. To determine the contribution of the RVM to behavioral hypersensitivity in animals with chronic inflammation, RVM neurons were inactivated by lidocaine microinjection in an additional five controls and seven CFA-treated animals.
Lightly Anesthetized Preparation
Electrophysiological recordings and RVM drug injections in lightly anesthetized animals were performed as described previously [5,30,32]. Animals were initially deeply anesthetized with either sodium pentobarbital (60 mg/kg, i.p.) or isoflurane (4% in oxygen). A catheter was placed in an external jugular vein for subsequent infusion of methohexital and the animal placed in a stereotaxic frame. While the animal was still deeply anesthetized, a craniotomy was drilled for access to the RVM.
After the surgical preparation was completed, the methohexital infusion was adjusted so that animals displayed no spontaneous movement or vocalization, but withdrew briskly from noxious heat or mechanical stimulation applied to an untreated hindpaw (15–30 mg/kg/hr). In animals initially induced using pentobarbital, we waited at least 2.5 hours before starting electrophysiological recording, since even minimal levels of pentobarbital mask mechanical hyperalgesia in lightly anesthetized rats [5]. Room temperature was kept around 25 °C, and body temperature maintained between 36 and 37 °C using a heating pad. Heart rate was monitored using EKG, and respiratory rate was monitored by recording changes in air pressure at the nares, as described in detail elsewhere [7]. The experimental protocol was started once heart rate, core temperature, and respiratory rate were stable and methohexital flow rate not adjusted for a minimum of 45 min.
Electrophysiological Recording
Stainless-steel microelectrodes (Microprobe, Gaithersburg, MD) with gold- and platinum-plated tips were used for all recordings. Signals were amplified 10,000-fold, sampled at 20 kHz, bandpass filtered (150 Hz to 15 kHz), and displayed on an oscilloscope. The signal was simultaneously digitized and stored for off-line analysis.
RVM neurons were isolated and characterized as ON-cells, OFF-cells, or NEUTRAL-cells. This mutually exclusive and exhaustive classification is based on firing patterns relative to nocifensor withdrawals [15]. At the withdrawal, “ON-cells” become active (if not already active), “OFF-cells” stop firing (if active), and NEUTRAL-cells do not change firing rate. Both OFF-cells and ON-cells can fire steadily or cycle between periods of activity and inactivity. Because an ON-cell with high basal firing can be easily misclassified as a NEUTRAL-cell [2], possible NEUTRAL-cells with continuous spontaneous activity were verified as such prior to starting the protocol by giving a brief bolus of anesthetic to the point that the withdrawal reflex was abolished. Firing of spontaneously active ON-cells slows or stops with this manipulation, which unmasks reflex-related responses.
Behavioral Testing
For thermal nociceptive testing, a Peltier device (Yale Instrumentation, New Haven, CT) was lightly applied to the plantar surface of the hindpaw, heated at a constant rate of 1.5 °C/s from 35 °C to a maximum of 53 °C. To avoid damage to the paw, the Peltier device was removed when the animal withdrew. Trials were initiated at 4–5 min intervals, with an attempt to capture a period when the cell under study was active (OFF-cells) or inactive (ON-cells), which allowed us to measure the withdrawal-related pause and burst that characterize these neurons. NEUTRAL-cells were tested at 4–5 min intervals irrespective of cell activity.
For mechanical testing, von Frey Fibers (4, 8, 15, 26, 60, and 100 g) were applied in ascending order to the interdigital webbing for a period of either 8 s or until a withdrawal was elicited, with three trials at each tested force. Three testing sites were rotated. Withdrawals were recorded via electromyograph (EMG) electrodes placed 1 cm apart in the muscles of the calf. Individual trials were initiated at intervals of at least 1 min, with longer interstimulus intervals used when necessary to capture a period when OFF-cells were active or ON-cells inactive.
Experimental Protocols
In electrophysiological studies of acute inflammation, the experimental protocol was initiated after isolation and characterization of one (or occasionally, two) well distinguished neuron (or neurons) as an ON-, OFF- or NEUTRAL-cell. The first 10–15 minutes of neuronal activity was recorded without any nociceptive testing; this period was used to measure baseline spontaneous activity in the absence of stimulation. Three thermal trials with the Peltier device were then performed on the experimental (left) hindpaw. To minimize the potential confound of secondary hyperalgesia from the recent noxious thermal trials, mechanical testing using von Frey fibers was initiated at least 5 min after the last thermal trial. Saline or CFA was then injected into the plantar surface of the left hindpaw. Spontaneous firing rate was determined (10-minute sample beginning 1–2 min after completing the hindpaw injection), followed by three thermal trials and a second round of mechanical testing of the treated paw. One hour after hindpaw injection, this was repeated, with a third 10-minute sample of spontaneous firing, followed by three additional thermal trials and repeated mechanical testing. For all experiments, only one protocol was performed in each animal.
In behavioral studies of the role of the RVM in acute CFA-evoked hypersensitivity, we used a within-subject design. Baseline thermal and mechanical sensitivity were determined, and CFA or saline injected into the left hindpaw. Thermal and mechanical testing were repeated 5 – 10 min after injection for the experimental paw, followed by lidocaine (4%, 200 nl) injection into the RVM to block all neuronal activity. Thermal and mechanical testing were again repeated in the 10 – 30 min after lidocaine injection.
In electrophysiological studies of chronic inflammation, activity of RVM neurons in controls was compared with that in animals injected with CFA 3–10 d previously. Following isolation and characterization of an RVM neuron or neurons, spontaneous firing rate was determined as above. Three thermal trials were then initiated for each hindpaw in alternating succession. Following a 5–10 min recovery period, mechanical threshold testing was performed for the two hindpaws. In some cases, the von Frey fiber thresholds had been determined prior to the initiation of the protocol, and mechanical testing then commenced at one level below that threshold. Otherwise, von Frey fibers between 4 and 100 g were applied.
In behavioral studies of the role of the RVM in chronic CFA-evoked hypersensitivity, thermal and mechanical responses for the treated hindpaw were measured before and after injection of lidocaine in the RVM (4%, 200 nl) in controls or animals injected 3–10 d previously with CFA in the left hindpaw.
Histology
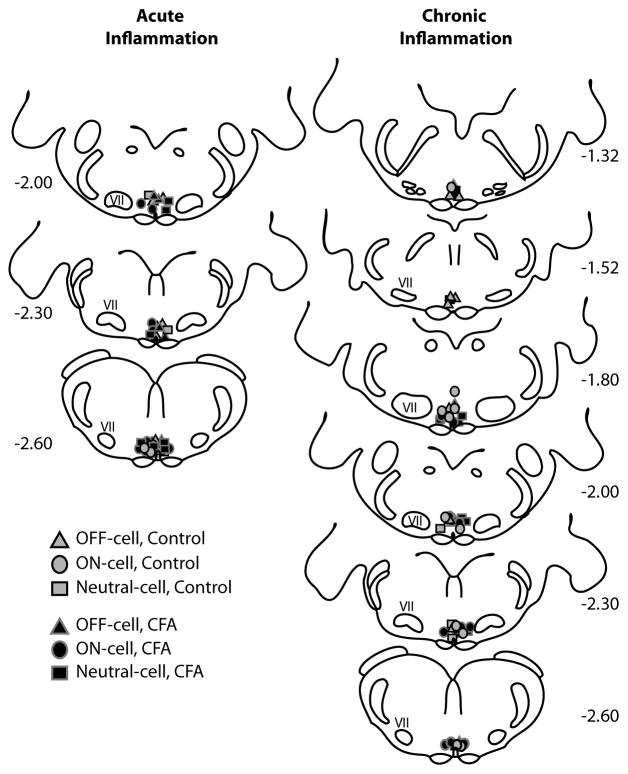
At the conclusion of the protocol, recording sites were marked with an electrolytic lesion. RVM microinjection sites were marked by green fluorescent beads (Invitrogen, Eugene, OR), either mixed in with the lidocaine or injected separately at the completion of the experiment. Animals were overdosed with methohexital and perfused transcardially with saline followed by 10% formalin. The brainstem was blocked, cut into 60 μm thick sections, and the recording or injection site visualized on a BX51 Olympus microscope. Sites were mapped in accordance with the atlas of Paxinos and Watson [37]. The RVM was defined as the area of the raphe magnus and adjacent reticular formation at the level of the facial nucleus (−1.04 to −2.6 mm relative to interaural line, ± 0.6 mm lateral, and 9–10 mm ventral to the brain surface). Locations of neurons recorded in CFA-treated and control groups are plotted in Fig. 1. Neurons outside of the RVM boundaries were excluded from analysis.
Fig. 1.
Locations of recordings sites within the RVM. ON-cells, OFF-cells, and NEUTRAL-cells were distributed between sections at −1.04 mm and −2.60 mm relative to the interaural line, with the majority of the cells recorded between −1.80 mm and −2.60 mm.
Data Analysis
Neuronal and EMG activity, heart rate, respiratory rate, paw heat-stimulus temperature, and von Frey application timing were recorded using Spike2 (Cambridge Electronic Design, Cambridge, England). Action potentials were sorted using template matching on waveforms, and discriminated on an individual spike basis.
The behavioral threshold was defined as the lowest von Frey fiber force at which the animal withdrew its paw from stimulation in at least two of three trials. Latency to withdraw was the time difference between the onset of the heat or von Frey stimulus and the first point of positive inflection on the EMG. EMG magnitude was quantified by subtracting the baseline and then smoothing, rectifying, and integrating the resultant signal [5]. These data are therefore expressed as arbitrary units.
Reflex-related changes in activity for both ON- and OFF-cells were quantified by comparing firing rates at the time of the withdrawal (3 s interval beginning 0.5 s prior to the withdrawal) to that in the 10 s before stimulus onset. With mechanical stimulation, where withdrawal was not always evident, mean firing rate was measured over the entire 8 s period after the onset of the stimulus, regardless of when the stimulus was withdrawn, and compared to the 10 s period prior to stimulus onset.
Statistics
Data are presented as mean ± SEM, with p values of less than 0.05 considered statistically significant.
Acute effects of CFA administration on thermal withdrawal threshold and EMG magnitude were analyzed with ANOVA followed by Dunnett’s test for comparison with baseline. Changes in mechanical withdrawal threshold from baseline were determined using either a Wilcoxon’s signed-rank test or a Friedman’s test with Dunn’s post-hoc test. Effects of acute CFA treatment or RVM lidocaine injection on withdrawal magnitude and latency of response from mechanical stimuli were determined using a two-way ANOVA and Bonferroni post-hoc tests, with time (before/after hindpaw injection) as a repeated measure. Spontaneous activity after acute CFA injection was compared with baseline using a Friedman’s test with Dunn’s post hoc test. Changes in evoked activity (ON-cell increase and OFF-cell inhibition) were analyzed with a Wilcoxon’s signed-rank test. Effects of lidocaine on thermal withdrawal thresholds were determined using two-way ANOVA, with time and treatment as factors, followed by Bonferroni post-hoc tests.
For the chronic CFA-treated and control groups, anesthetic requirements and heart rate were compared using t-tests for independent means. The thresholds for responses to von Frey probes were compared using a Kruskal-Wallis ANOVA followed by Dunn’s post-hoc test. Thermal withdrawal thresholds for ON- and OFF-cell responses, areas under the curve for stimulus-responses, withdrawal latencies, and EMG magnitudes were compared using one-way ANOVA followed by Tukey’s post-hoc tests. Spontaneous firing rates of the different groups were compared for each cell class using a Mann-Whitney U. For lidocaine injection experiments, magnitude and latency of response were compared using a two-way mixed-design ANOVA and Bonferroni post-hoc test, with force as one factor and pre- vs. post-lidocaine injection as the repeated factor. Correlations were calculated using Spearman’s rho.
Results
Acute CFA injection produces thermal hyperalgesia and slight but measurable mechanical hypersensitivity in lightly anesthetized animals
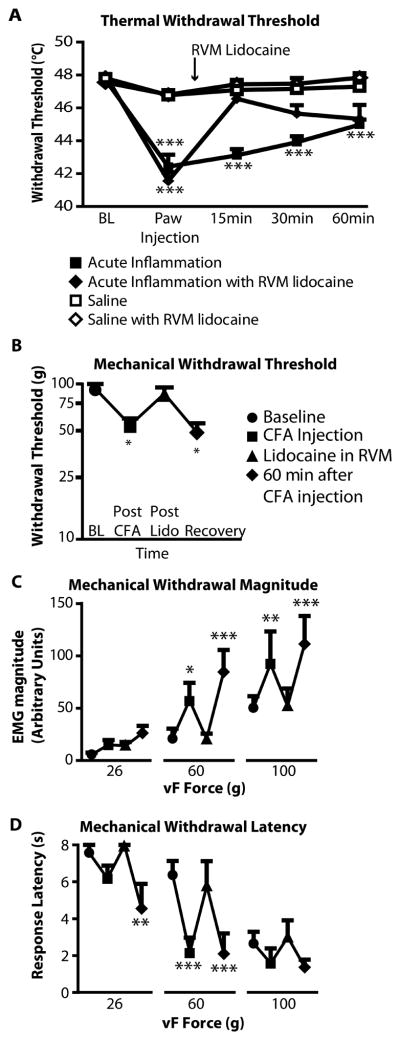
In lightly anesthetized animals, plantar injections of CFA produced localized erythema, edema, and small spontaneous twitches of the injected hindpaw. Paw thickness was increased from 5.42 ± 0.06 mm to 6.26 ± 0.11 mm by 10 min after CFA injection (p < 0.0001). Paw temperature was increased from 29.5 ± 0.6 to 33.8 ± 0.3 °C (p < 0.0001), comparable to previous reports in awake behaving animals after CFA hindpaw injection [28]. Control injections of saline produced a smaller but statistically significant increase in paw thickness (from 5.36 ± 0.12 to 5.71 ± 0.08 mm, p = 0.02). Paw temperature was not altered following saline injection (from 30.0 ± 0.7 to 30.6 ± 0.7 °C, p = 0.2). Heart rate and breathing frequency were both significantly increased following plantar injection of CFA (heart rate: 361.7 ± 4.5 to 369.8 ± 5.1 beats/min, p = 0.007; breathing rate: 1.61 ± 0.05 breaths/s to 1.65 ± 0.05 breaths/s, p = 0.023) but not saline (heart rate: 369.4 ± 7.2 to 366.1 ± 7.4 beats/min, p = 0.29; breathing rate: 1.67 ± 0.05 breaths/s to post-injection: 1.63 ± 0.05 breaths/s, p = 0.41).
Within 15 min of CFA injection, there was significant heat hyperalgesia, with the withdrawal threshold decreased by approximately 5 °C. This hyperalgesia was maintained for the entire 60-minute observation period (Fig. 2A). Control injections of saline produced a slight (< 1 °C), but statistically significant, reduction in thermal withdrawal threshold (Fig. 2A). Withdrawal magnitude was not significantly increased in either group (Fig. 2B).
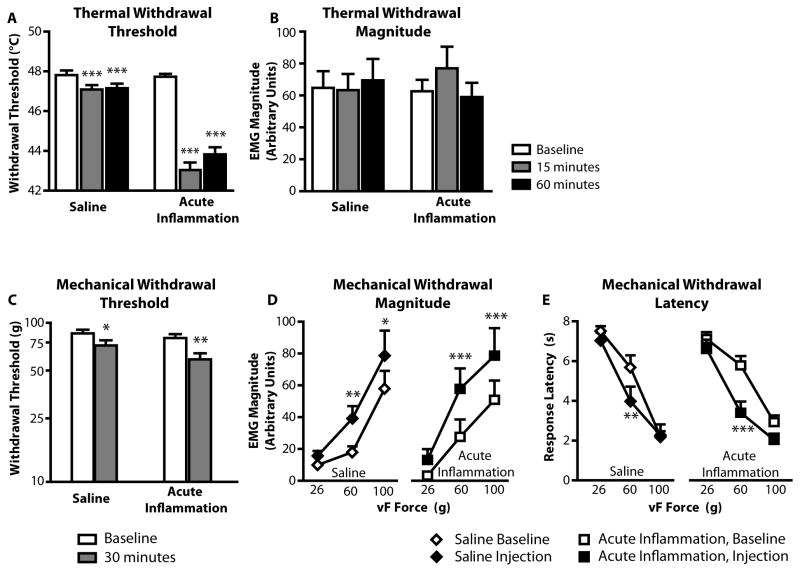
Fig. 2. Thermal and mechanical hyperalgesia within the first hour following CFA injection.
A) Threshold for heat evoked withdrawal was substantially reduced in CFA-treated animals (F2,46 =102.0, p < 0.0001, n = 24). Reduced threshold in saline-treated animals was small but statistically significant (F2,32 = 12.0, p < 0.0001, n = 17). The two groups had comparable thresholds in baseline (unpaired t-test, p = 0.85).
B) Magnitude of withdrawal from thermal stimuli (given as arbitrary units, AU) was not affected by injection of CFA or saline (saline: F2,32 = 0.96, p = 0.39; CFA: F2,46 = 1.55, p = 0.22). Baseline response magnitudes were not different (unpaired t-test, p = 0.68).
C) Mechanical withdrawal thresholds were also reduced, although only two animals, both in the CFA-treated group, responded to fibers normally considered innocuous (Wilcoxon signed ranks test, p = 0.003 and p = 0.048 for CFA and saline injections, respectively)
D,E) Withdrawal magnitude was increased (two-way ANOVA, F1,44 = 17.3, p < 0.0001 for saline; F1,66 = 33.3, p < 0.0001 for CFA-injected) and response latency reduced (two-way ANOVA, F1,44 = 13.0, p < 0.001 for saline; F1,66 = 21.0, p < 0.0001 for CFA-injected). *p < 0.05, **p < 0.01, ***p < 0.001 compared to pre-injection baseline at the same force.
Mechanical sensitivity was modestly enhanced in both CFA- and saline-treated animals. The threshold for withdrawal from von Frey fibers was lowered (Fig. 2C), and the magnitude of withdrawals evoked by 60 and 100 g fibers was increased (Fig. 2D). The latency to withdraw to the 60 g fiber was also slightly reduced for both groups (Fig. 2E).
Lightly anesthetized animals thus develop potent thermal hyperalgesia within the first hour following injection of CFA. There is also a modest but measurable mechanical hypersensitivity in both CFA-treated and control animals.
Spontaneous and reflex-related activity of RVM neurons in acute inflammation
The behavioral hypersensitivity seen in the first hour following CFA treatment was associated with changes in the activity of RVM ON- and OFF-cells, but not NEUTRAL-cells. As shown in the examples in Fig. 3A, the spontaneous firing of ON-cells was increased within minutes of the injection, while that of OFF-cells was reduced, although not completely inhibited. These changes were not maintained however, and activity was no longer significantly different from baseline at 60 min post-injection (Fig. 3B). The reflex-related activation of ON-cells was unchanged following CFA, as was activity evoked by von Frey stimulation (Fig. 3C). Thus, the characteristic activation at the time of withdrawal was unaffected by acute inflammation, although the heat-evoked withdrawal occurred at a lower temperature. The OFF-cell pause was modestly enhanced following CFA, but only for heat-evoked withdrawal; suppression of firing during von Frey probing was not different from baseline (Fig. 3C). Saline injection had no effect on the spontaneous or reflex-related firing of ON-cells or OFF-cells.
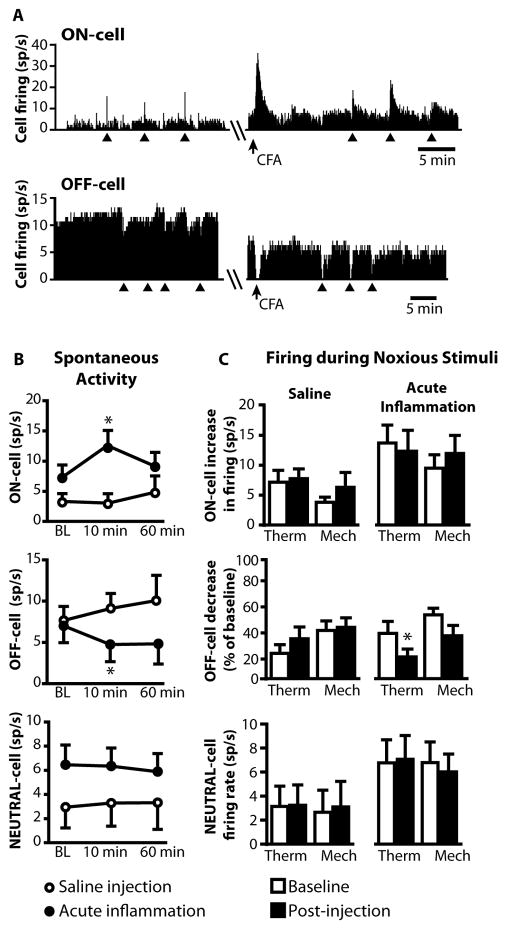
Fig. 3. Spontaneous and withdrawal-related activity of ON-, OFF-, and NEUTRAL cells during acute inflammation.
A) Ratemeters showing spontaneous and thermal withdrawal-related activity of a typical ON-cell and OFF-cell before and after CFA injection.
B) CFA but not saline injection increased spontaneous activity of ON-cells (Wilcoxon’s signed ranks test, CFA: p = 0.019, n = 11; Saline: p = 0.96, n = 6) and decreased that of OFF-cells (CFA: p = 0.016, n = 7; Saline: p = 0.37, n = 5). NEUTRAL cell spontaneous activity was unaffected by either treatment (CFA: p = 0.37, n = 10; Saline: p = 0.99, n = 6). BL: baseline.
C) Firing associated with noxious stimulation trials was generally unaffected by CFA. However, the reflex-related OFF-cell inhibition from thermal stimulation was significantly enhanced after CFA injection.
Some NEUTRAL-cells have been reported to change firing properties to be more like ON- or OFF-cells during the development of inflammation following local administration of CFA [34]. We therefore examined NEUTRAL-cells in the present experiments. NEUTRAL cells characterized using our protocol showed no change in spontaneous activity following CFA injection (Fig. 3B). Further, none developed ON- or OFF-like changes in firing.
RVM blockade reverses both thermal and mechanical hyperalgesia during acute inflammation
To determine whether RVM activity contributes to the behavioral hypersensitivity during acute inflammation in lightly anesthetized animals, we blocked RVM activity by microinjecting lidocaine into the RVM in a subset of animals without cell recording. After baseline testing, an injection was made into the hindpaw and thresholds determined. Lidocaine was then microinjected into the RVM. Blockade of RVM activity by lidocaine reversed CFA-induced thermal hyperalgesia, but had no effect in saline-injected controls (Fig. 4A).
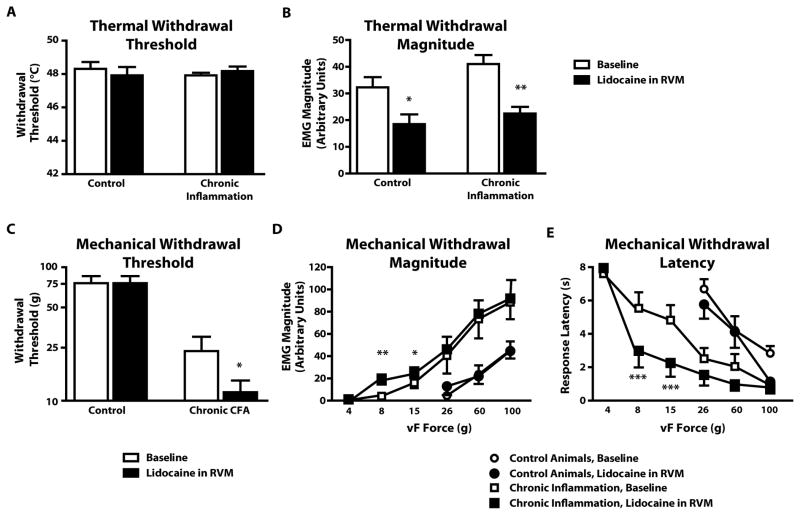
Fig. 4. RVM blockade attenuates both thermal and mechanical hyperalgesia in acute inflammation.
A) CFA injection produced immediate thermal hyperalgesia that partially recovered over the subsequent hour. This effect was reversed by RVM lidocaine. X-axis gives time since hindpaw injection. Two-way repeated-measures ANOVA, with significant effects of both time (F4, 204 = 21.2, p < 0.0001) and treatment on threshold (F3,51 = 38.35, p < 0.0001). *** p < 0.001 post-hoc Bonferroni comparison against hindpaw saline/RVM lidocaine group.
B–D) RVM lidocaine reversibly eliminated the decrease in withdrawal threshold (B), increase in withdrawal magnitude (C), and reduction in withdrawal latency (D) produced by CFA. Hypersensitivity returned approx. 40 min after lidocaine administration (60 min after CFA).
For mechanical threshold, Friedman’s test was followed by Dunn’s post hoc test (p < 0.001, n = 6). ANOVA was used to analyze magnitude (F3,45 = 19.1, p < 0.0001) and latency (F3,45 = 14.9, p < 0.0001). *p < 0.05, **p < 0.01, ***p < 0.001 compared to pre-CFA baseline.
Lidocaine also reversed CFA-induced decreases in mechanical withdrawal threshold (Fig. 4B), increases in response magnitude (Fig. 4C), and decreases in response latency (Fig. 4D). These data show that facilitatory output from the RVM is necessary for both thermal and mechanical hypersensitivity during acute localized inflammation induced by CFA.
Chronic inflammation following CFA injection produces mechanical hypersensitivity but not thermal hyperalgesia in lightly anesthetized animals
The second set of experiments used animals treated with CFA or saline 3 to 10 days prior to the recording session. Anesthetic requirements in the CFA-treated and control groups were similar (21.3 ± 1.2 mg/hr vs. 21.0 ± 1.3 mg/hr, respectively, p = 0.95). Heart rates were also comparable in the two groups (413.8 ± 10.3 vs. 393.3 ± 8.2 beats per min in CFA-treated and controls respectively, p = 0.12). Respiratory rates were not measured in these experiments.
CFA-treated animals did not show long-lasting thermal hypersensitivity. In the chronic condition, heat-evoked withdrawal thresholds of the CFA-treated paw were not different from those on the contralateral side or from the saline-treated control group (Fig. 5A). There was also no difference between sides or groups in response magnitude (EMG, data not shown).
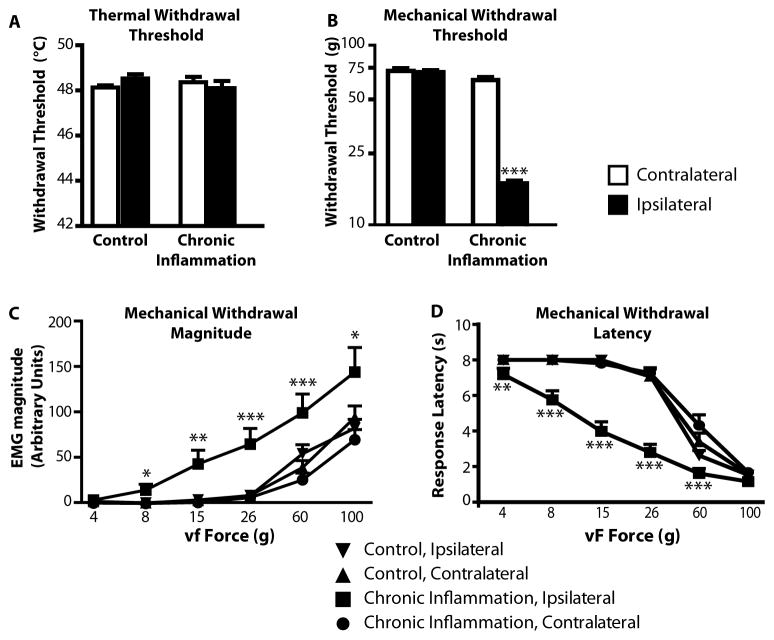
Fig. 5. Mechanical but not thermal hyperalgesia in chronic inflammation.
A) In lightly anesthetized animals at 3–10 days after initial CFA injection, thermal hyperalgesia was not detectable (F3,86 = 0.84, p = 0.47).
B–D) Mechanical hyperalgesia was fully developed, with lowered threshold (B), a left and upward shift in the stimulus-response magnitude relationship (C), and a reduction in response latency evident throughout the stimulus range tested (D). For C and D, when an overall ANOVA was significant, a Tukey’s post-hoc test was used to compare groups at each fiber force. *p < 0.05, **p < 0.01, ***p < 0.001 compared to contralateral paw, which was not different from ipsilateral or contralateral controls at any force.
In contrast with the absence of thermal hypersensitivity, mechanical hypersensitivity in the CFA-treated paw was substantial. The threshold for withdrawal to von Frey fiber probing of the treated paw was significantly reduced (Fig. 5B) in the chronic group, and response magnitude was increased across the range of tested fibers (Fig. 5C), and response latency was shorter (Fig. 5D) compared to the contralateral side and controls.
Animals subjected to prolonged localized inflammation thus display profound mechanical hypersensitivity in the lightly anesthetized state, with thresholds significantly lower than that seen in the acute condition. Notably, only 8% of the animals that had been tested in the first hour following CFA administration showed thresholds in the innocuous range (< 26 g). By contrast, 85% of animals tested in the chronic condition exhibited thresholds below 26 g (χ2 = 26.1, p < 0.0001). No saline-treated animal, in the acute or chronic condition, responded to fibers in the innocuous range. The increased sensitivity in the chronic condition can also be seen in comparisons of response magnitude. The response to probing with the 100 g fiber was significantly enhanced in the chronic compared to the acute condition (compare Figs. 2D and 5C, p = 0.035).
Activity of RVM neurons during chronic inflammation
The pattern of activity of RVM neurons in animals subjected to chronic inflammation was distinct from that seen in the first hour after CFA treatment. Unlike RVM neurons recorded during acute inflammation, ON- and OFF-cells showed comparable rates of spontaneous firing in the chronic/CFA-treated and chronic/control groups (Fig. 6A).
Fig. 6. Spontaneous and withdrawal-related activity of ON-, OFF-, and NEUTRAL-cells during acute inflammation.
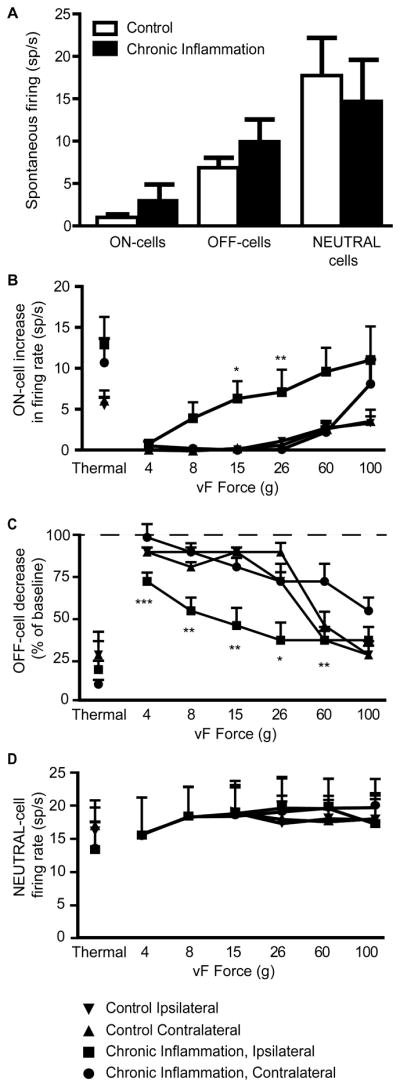
A) Spontaneous firing rates of the different cell classes were not significantly different in CFA-treated and control animals. (Wilcoxon’s test: ON-cells, p = 0.16; OFF-cells, p = 0.35, NEUTRAL-cells, p = 0.54, n = 8 – 12 group).
B–D) Evoked responses of ON-cells (B), OFF-cells (C) and NEUTRAL-cells (D). Activity during thermal and mechanical trials is plotted on the same graph for each class. Both ON- and OFF-cells recorded in CFA-treated animals showed significant shifts in the mechanical force vs. response relationship for stimulation of the treated paw compared to the contralateral side or in controls. There was no effect of group or side on responses during heat trials. NEUTRAL-cells did not respond to thermal or mechanical stimulation.
When an overall ANOVA was significant, a Tukey’s post-hoc test was used to compare groups at each fiber force. *p < 0.05, **p < 0.01, ***p < 0.001 compared to contralateral paw, which was not different from ipsilateral or contralateral controls at any force.
In contrast to spontaneous firing, the mechanically evoked ON- and OFF-cell changes in activity were enhanced in the CFA-treated animals, and paralleled the shift in behavioral sensitivity. First, the threshold for evoking a change in cell activity was reduced (ipsilateral hindpaw: 14.2 ± 3.8 g; contralateral hindpaw: 74.1 ± 7.1 g, p < 0.0001). Not surprisingly, since the responses of these neurons are associated with behavior rather than with noxious stimulation per se, a high correlation existed between behavioral and neural thresholds (Spearman’s rho = 0.84). Both cell classes also exhibited a leftward shift in the stimulus response curve (Fig. 6B,C). Finally, the response-related activation of ON-cells (Fig. 6B) was correlated with withdrawal magnitude (magnitude shown in Fig. 5C; rho = 0.24, p < 0.0001).
For thermal stimulation, there was no effect of CFA treatment on the response-related changes in activity for either ON- or OFF-cells (Fig. 6B and C; for ON-cells: p = 0.25, for OFF-cells: p = 0.75).
Firing of NEUTRAL-cells was comparable in chronic CFA-treated and control groups, with no difference in spontaneous firing rates (Fig. 6A). NEUTRAL-cells also did not respond to von Frey probing of any force, whether applied to inflamed or non-inflamed hindpaws (Fig. 6D), and no difference was observed among groups during thermal stimulation (Fig. 6D, p = 0.45).
RVM blockade potentiates mechanical hypersensitivity in animals subjected to chronic inflammation
We next blocked activity of the RVM in order to determine whether this region contributes to mechanical hypersensitivity after chronic inflammation, as it does to thermal hyperalgesia in acute inflammation. The threshold for heat-evoked withdrawal was not changed by lidocaine injection into the RVM in either chronic/CFA-treated or control groups (Fig. 7A). However, the response magnitude was significantly reduced after RVM block in both groups (Fig. 7B).
Fig. 7. Effects of RVM lidocaine on behavioral responses to heat (A, B) and von Frey stimulation (C, D, E) in animals subjected to chronic inflammation.
A) Thermal withdrawal thresholds were not significantly altered by lidocaine in the RVM in control (n = 5) or CFA-treated (n = 7) animals.
B) RVM blockade significantly reduced the magnitude of the heat-evoked reflex in both groups (paired t-test, p = 0.024 for control animals, p = 0.005 for CFA-injected animals).
C) RVM block potentiated mechanical hypersensitivity in CFA-treated animals (Wilcoxon’s test, p = 0.03, n = 7), with no effect in controls (p = 0.99, n = 5).
D) RVM block resulted in a small but statistically significant increase in the magnitude of withdrawals evoked by innocuous stimulation of the treated paw, with no effect on the response to noxious stimuli.
E) RVM block resulted in a significant decrease in the latency of the response evoked by innocuous stimulation of the treated paw, with no effect on the response to noxious stimuli.
For D and E, *p < 0.05, **p < 0.01, ***p < 0.001, compared to post RVM lidocaine injection, paired t-tests at innocuous forces.
Mechanical hypersensitivity in the treated paw was potentiated by RVM block. Mechanical threshold, already lower than controls in baseline, was further reduced following RVM lidocaine in animals subjected to chronic hindpaw inflammation. RVM block had no effect on withdrawal threshold in control animals (Fig. 7C). There was a small, but statistically significant increase in the magnitude of the EMG evoked by innocuous stimuli at the lowest levels of stimulation (Fig. 7D), and the responses to innocuous stimulation occurred with a shorter latency (Fig. 7E). Therefore, the net influence of the RVM on mechanical sensitivity in the chronic inflammation group was not facilitatory, but rather inhibitory.
Discussion
Molecular, pharmacological, and behavioral evidence demonstrate that plasticity in the central nervous system maintains and reinforces chronic pain. The present experiments considered the physiology of individual RVM neurons as another area of potential plasticity in the transition from acute to chronic pain. Single-unit recording from identified RVM ON-, OFF-, and NEUTRAL-cells was combined with behavioral measures of thermal and mechanical sensitivity to test the role of RVM neurons in acute, compared to chronic, immune-mediated inflammation. Firing of RVM neurons in the first hour of inflammation following administration of CFA strongly resembled that seen in other acute inflammatory conditions [4,30,48,58]. However, cell activity recorded 3–10 days later was similar to that seen in a different persistent pain state, chronic nerve injury [5]. Thus, while acute and chronic pain conditions appear similar at the behavioral level, the underlying mechanisms of descending control are distinct, and differentiate acute from chronic hypersensitivity.
Activity of physiologically identified RVM ON- and OFF-cells in acute vs. chronic immune-mediated inflammation
In the first hour following local administration of CFA, RVM ON- and OFF-cells exhibited clear changes in spontaneous activity: overall firing of ON-cells was increased, while that of OFF-cells was suppressed. This pattern of altered spontaneous firing of ON- and OFF-cells is similar to what has been reported previously during acute neurogenic inflammation [4,30,48,58]. These changes were relatively short-lived however, with recovery towards baseline by the end of the first hour after CFA injection. The restoration of spontaneous activity of the ON- and OFF-cells may represent a compensatory process, and is consistent with the report of Miki et al. [34], who found little alteration in the spontaneous firing of ON- and OFF-cells sampled at various intervals over the first day following CFA injection. Reflex-related changes in the activity of these two classes were not markedly altered from baseline.
Recordings at later time points (3–10 days post-treatment) revealed a different pattern of cell activity. For both cell classes, the spontaneous discharges in the CFA-treated and control groups were similar. However, in the CFA group, both ON- and OFF-cells were more sensitive to mechanical stimulation of the treated paw, exhibiting both lowered thresholds and increased response magnitudes. This finding is similar to the observations of Montagne-Clavel [35], who recorded activity of RVM neurons in awake, behaving animals several weeks after induction of polyarthritis produced by injecting large doses of CFA in the tail. As in the present studies, they reported little change in the spontaneous discharges of the recorded neurons, but increased sensitivity to light touch.
These data show that changes in ON- and OFF-cell firing seen in the first hour following CFA injection are not maintained in later stages of inflammation. Instead, ON- and OFF-cell activity in chronic inflammation closely resembles that seen following nerve injury [5]. Spontaneous firing rates return to control levels, but both cell classes become responsive to innocuous tactile stimulation of the affected limb. The similar adaptation of the RVM output in two chronic pain states, one neuropathic and the other inflammatory, suggests that time-course, and not mode of injury, is the important factor underlying altered activity of RVM pain-modulating neurons in persistent pain states. The parallel changes with the two different types of insult also argue that the RVM is not simply mirroring ongoing or abnormal peripheral input.
NEUTRAL-cells in acute and chronic inflammation
All RVM neurons that do not exhibit the reflex-related changes in firing that define ON- and OFF-cells are classified as NEUTRAL-cells, a separation that is confirmed by pharmacological differences among cell classes [21,23–25,40]. The role of NEUTRAL-cells in the genesis and modulation of acute and chronic pain remains controversial. Miki et al. [34] reported that some NEUTRAL-cells developed ON- or OFF-cell properties in the early phases of CFA-induced inflammation. However, these authors did not have pre-inflammation baseline data for the recorded neurons, which makes direct comparison with our findings difficult. In the present studies, NEUTRAL-cell firing was unchanged during the acute phase of CFA-induced inflammation, and we found no evidence of NEUTRAL-cells developing ON- or OFF-like properties. Similar stability of NEUTRAL-cell properties has also been reported in acute neurogenic inflammation [4,30].
Contribution of the RVM to behavioral hypersensitivity in acute vs. chronic immune-mediated inflammation
Descending control from the RVM is generally agreed to be altered in chronic inflammation, but there has been considerably less consensus as to when and whether descending inhibition and/or descending facilitation are recruited [45,51,53]. Hypersensitivity measured behaviorally could reflect increased descending facilitation, decreased or insufficient inhibition, or both. In our paradigm, changes in behavioral sensitivity were monitored in parallel with RVM cell activity, which allowed us to pinpoint the contributions of the pain-facilitating and pain-inhibiting outputs from this region at different time points.
In the first hour following CFA injection, the treated paw showed RVM-dependent thermal and mechanical hypersensitivity. Thermal hyperalgesia was much more pronounced, and the injection per se appeared to have contributed to the modest mechanical hyperalgesia. Since CFA injection resulted in a reduction in OFF-cell firing and did not increase the evoked responses of ON-cells, the reversal of thermal hyperalgesia by RVM block implies that behavioral hyperalgesia was driven by the increased spontaneous firing of the ON-cells. This has been shown previously for acute neurogenic inflammation [30,48,58].
Three to 10 days after CFA treatment, spontaneous activity was restored to control levels, but ON- and OFF-cells showed novel responsiveness to innocuous mechanical stimuli. In these animals, prominent mechanical hypersensitivity was potentiated, not reversed, by inactivation of the RVM. This finding discounts a critical role for ON-cells in mechanical hyperalgesia after CFA, and is consistent with evidence that descending inhibition from the RVM increases as immune-mediated inflammation develops [8,17–18,42,44,51]. It further implies that OFF-cell output to some extent holds sensitized dorsal horn transmission in check. However, this compensation is not complete, since the animals exhibit behavioral hypersensitivity. Moreover, the lowered mechanical threshold for the OFF-cell pause would permit (i.e., disinhibit) behavioral responses to innocuous tactile stimuli. Therefore, mechanical hypersensitivity in the chronic condition reflects not an overall shift towards ON-cell output, as seen in acute inflammation [3–4,22,30,32,41,48,57], but a lowered response threshold or “tipping point” at which descending inhibition is removed. Although descending inhibition is the dominant output, the lowered threshold for ON-cell activation may still contribute to increased sensitivity, as reported for inflammation of visceral and deep tissues [9–10,50,54].
Our finding of prolonged mechanical hypersensitivity in lightly anesthetized animals following plantar injection of CFA accords with previous work in awake behaving rats [43]. In these experiments mechanical hyperalgesia was maintained but thermal hyperalgesia had resolved by the later time points (three to ten days after CFA). This latter finding is consistent with the observation that the spontaneous activity of RVM ON- and OFF-cells had also returned to normal (since, as noted above, thermal hyperalgesia is closely tied to an increase in the spontaneous activity of ON-cells). However, others have reported that heat thresholds are lowered for a week or more after plantar CFA injection, although the effect is reported to be maximal within the first 24 hours [18,28,36,46,55]. A number of differences could account for this discrepancy. Our animals were anesthetized, which could suppress activating inputs to ON-cells from higher centers. We also used contact, rather than radiant heat, and a holding temperature (35 °C) that was above skin temperature of both normal and inflamed paws. Paw temperature is not controlled in most studies, and the fact that the inflamed paw is substantially warmer could contribute to the reduced withdrawal latencies reported elsewhere [6,12,27]. In addition, the slow linear heat-ramp used here is thought to activate C-fibers selectively, even in inflamed tissue. By contrast, thermal stimuli used in studies in awake animals typically have a logarithmic rise, which would more likely activate A-fibers [33,59]. Since increased sensitivity of dorsal horn neurons in chronic inflammatory hyperalgesia has been tied to A-fiber afferents [1,52,56], it is possible that our thermal stimulus did not activate inputs relevant to thermal hyperalgesia.
Although RVM inactivation during chronic inflammation did not change thermal withdrawal thresholds, the magnitude of the heat-evoked EMG response was reduced in both CFA-treated and control animals. This finding is consistent with the suggestion of Jinks and colleagues [29] that the ON-cell reflex-related burst contributes to the magnitude of the heat-evoked withdrawal, which may be more related to perception of suprathreshold stimuli rather than threshold.
Conclusion
The RVM influences nociceptive behaviors through descending projections, but changes in this system can be brought about by “bottom-up” processes, including tissue injury and inflammation. During acute inflammation, the normal equilibrium between ON- and OFF-cell outputs is disturbed. Increased ON-cell spontaneous firing acts as a multiplier of dorsal horn activity, and contributes to substantial thermal hyperalgesia. Under chronic conditions, although spontaneous firing has returned to normal, both cell classes show lowered response thresholds, with novel responses to innocuous stimuli. The lowered threshold for the OFF-cell pause allows withdrawal responses to stimuli that would normally be considered innocuous. Variations in how well OFF-cells counteract sensitization of ascending transmission pathways is likely an important factor in determining whether chronic pain develops [11].
Acknowledgments
This work was supported by grants from NINDS: NS066159, NS070374; DRC was supported by a Neurobiology of Disease Fellowship from OHSU Brain Institute.
Footnotes
The authors report no conflict of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates A fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: Reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- 3.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 4.Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin–1 receptors. J Neurophysiol. 2012;107:1210–1221. doi: 10.1152/jn.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrive P, Churyukanov M, Le Bars D. A reassessment of stress-induced “analgesia” In the rat using an unbiased method. Pain. 2011;152:676–686. doi: 10.1016/j.pain.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Cleary DR, Phillips RS, Wallisch M, Heinricher MM. A novel, non-invasive method of respiratory monitoring for use with stereotactic procedures. J Neurosci Meth. 2012;209:337–343. doi: 10.1016/j.jneumeth.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva LF, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duggan AW, Griersmith BT, Headley PM, Maher JB. The need to control skin temperature when using radiant heat in tests of analgesia. Exp Neurol. 1978;61:471–478. doi: 10.1016/0014-4886(78)90262-5. [DOI] [PubMed] [Google Scholar]
- 13.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 14.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. London: Elsevier; 2006. pp. 125–142. [Google Scholar]
- 15.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans of the R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 16.Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366:201–205. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 17.Guan Y, Guo W, Zou S-P, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104:401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 18.Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: A novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harasawa I, Fields HL, Meng ID. Delta opioid receptor mediated actions in the rostral ventromedial medulla on tail flick latency and nociceptive modulatory neurons. Pain. 2000;85:255–262. doi: 10.1016/s0304-3959(99)00280-8. [DOI] [PubMed] [Google Scholar]
- 22.Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004;110:419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 24.Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 25.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 26.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hole K, Tjølsen A. The tail-flick and formalin tests in rodents: Changes in skin temperature as a confounding factor. Pain. 1993;53:247–254. doi: 10.1016/0304-3959(93)90220-J. [DOI] [PubMed] [Google Scholar]
- 28.Hurley RW, Hammond DL. The analgesic effects of supraspinal and opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary ON and OFF neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- 31.LaGraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMullan S, Simpson DAA, Lumb BM. A reliable method for the preferential activation of c- or a-fibre heat nociceptors. J Neurosci Meth. 2004;138:133–139. doi: 10.1016/j.jneumeth.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 35.Montagne-Clavel J, Oliveras JL. Are ventromedial medulla neuronal properties modified by chronic peripheral inflammation? A single-unit study in the awake, freely moving polyarthritic rat. Brain Res. 1994;657:92–104. doi: 10.1016/0006-8993(94)90957-1. [DOI] [PubMed] [Google Scholar]
- 36.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- 38.Pertovaara A, Keski-vakkuri U, Kalmari J, Wei H, Panula P. Response properties of neurons in the rostroventromedial medulla of neuropathic rats: Attempted modulation of responses by [1DMe]NPYF, a neuropeptide FF analogue. Neuroscience. 2001;2001:457–468. doi: 10.1016/s0306-4522(01)00187-7. [DOI] [PubMed] [Google Scholar]
- 39.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 40.Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez F, Vanegas H. Tooth pulp stimulation advances both medullary off-cell pause and tail flick. Neurosci Lett. 1989;100:153–156. doi: 10.1016/0304-3940(89)90676-9. [DOI] [PubMed] [Google Scholar]
- 42.Randich A, Mebane H, Deberry JJ, Ness TJ. Rostral ventral medulla modulation of the visceromotor reflex evoked by urinary bladder distension in female rats. J Pain. 2008 doi: 10.1016/j.jpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiology & Behavior. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 44.Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- 45.Ren K, Dubner R. Descending modulation in persistent pain: An update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 46.Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, mk-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- 47.Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–241. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain. 2010;14:120–129. doi: 10.1016/j.ejpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schepers RJ, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2008;136:320–330. doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- 51.Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- 52.Torsney C. Inflammatory pain unmasks heterosynaptic facilitation in lamina i neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2011;31:5158–5168. doi: 10.1523/JNEUROSCI.6241-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanegas H, Schaible H-G. Descending control of persistent pain: Inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Wei F, Dubner R, Ren K. Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain. 1999;80:127–141. doi: 10.1016/s0304-3959(98)00212-7. [DOI] [PubMed] [Google Scholar]
- 56.Woolf CJ, Shortland P, Sivilotti LG. Sensitization of high mechanothreshold superficial dorsal horn and flexor motor neurones following chemosensitive primary afferent activation. Pain. 1994;58:141–155. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 57.Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–262. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: Behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]