Abstract
Background
Retention in care is key to improving HIV outcomes. Our goal was to describe “churn” in patterns of entry, exit, and retention in HIV care in the US and Canada.
Methods
Adults contributing ≥1 CD4 count or HIV-1 RNA (HIV-lab) from 2000–2008 in North American Cohort Collaboration on Research and Design (NA-ACCORD) clinical cohorts were included. Incomplete retention was defined as lack of 2 HIV-labs (≥90 days apart) within 12 months, summarized by calendar year. We used beta-binomial regression models to estimate adjusted odds ratios (OR) and 95% confidence intervals (CI) of factors associated with incomplete retention.
Results
Among 61,438 participants, 15,360 (25%) with incomplete retention significantly differed in univariate analyses (p<0.001) from 46,078 (75%) consistently retained by age, race/ethnicity, HIV risk, CD4, ART use, and country of care (US vs. Canada). From 2000–2004, females (OR=0.82, CI:0.70–0.95), older individuals (OR=0.78, CI:0.74–0.83 per 10 years), and ART users (OR= 0.61, CI:0.54–0.68 vs all others) were less likely to have incomplete retention, while black individuals (OR=1.31, CI:1.16–1.49, vs. white), those with injection drug use (IDU) HIV risk (OR=1.68, CI:1.49–1.89, vs. non-IDU) and those in care longer (OR=1.09, CI:1.07–1.11 per year) were more likely to have incomplete retention. Results from 2005–2008 were similar.
Discussion
From 2000 to 2008, 75% of the NA-ACCORD population was consistently retained in care with 25% experiencing some change in status, or churn. In addition to the programmatic and policy implications, our findings identify patient groups who may benefit from focused retention efforts.
Keywords: retention, churn, HIV clinical care, North America, HRSA HAB, National HIV/AIDS Strategy
Introduction
There are marked disparities in the initial access to, and subsequent retention in, HIV clinical care according to patient demographic and clinical factors [1–8]. Retention in care is inherently related to the epidemiologic concept of “churn”, introduced by Gill and Krentz as a framework for describing the continuous movement of individuals entering, re-entering, and exiting clinical care at any given point in time.[9] While retention may be viewed as an individual’s pattern of clinical care over time and may be summarized across a population, churn may be viewed as an aggregate measure reflecting differing patterns of retention among different segments of a population over time. Analyzing churn in a longitudinal clinical cohort can complement the analysis of clinical retention by revealing the structure of patient movement that underlies the status of patients described as “retained in care” or not in a given period.
Both retention in care and the churn phenomenon have implications for the epidemiology and management of HIV in the United States and Canada, as well as the design, implementation, and evaluation of prevention and treatment strategies. Recent national guidelines for Ryan White funded clinics in the US define longitudinal medical service utilization and provide a standard by which to quantify retention and churn [10,11]. Application of these standards may facilitate the identification of factors associated with a lack of retention, presenting potential targets for intervention and improvement.
In this study, we describe patterns of churn and predictors of incomplete retention in care using data from the North American Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of clinical and interval cohort studies of HIV-infected individuals [12]. Our goal was to provide both a descriptive overview and an analytically sound quantitation of factors related to retention and churn in the clinical HIV population of the US and Canada between 2000 and 2008. Further, we provide methods for tracking the future progress in improving retention in HIV care, consistent with the national HIV/AIDS strategies in both countries [13,14] and recent consensus practice guidelines [15].
Methods
Study design and population
The NA-ACCORD is a multi-site collaboration of interval and clinic-based cohort studies of HIV-infected adults (≥ 18 years old) receiving care in the US and Canada [16]. NA-ACCORD is one of the multi-national cohort studies sponsored by the National Institute of Health‘s International Epidemiological Databases to Evaluate AIDS (IeDEA) consortium. Details on the NA-ACCORD collaboration have been published previously [12]. Briefly, contributing cohorts have standardized cohort-specific methods of data collection. At scheduled intervals, cohorts submit data regarding enrolled participants’ demographic characteristics, vital status, prescribed antiretrovirals, clinical diagnoses, and dates and results of laboratory tests including HIV-1 RNA viral load and CD4+ lymphocyte count (HIV-lab), whether collected during routine outpatient care or from inpatient or trial settings if available. HIV-lab dates are submitted as dates of specimen collection, and among clinical cohorts, only patients with ≥2 clinical visits within 12 months are enrolled into the NA-ACCORD, therefore limiting the NA-ACCORD clinical population to those patients who have established themselves as “in care” upon cohort entry; this enrollment criterion is assessed by sites themselves, and is based on actual clinic encounter data, not on HIV-lab data (on which the rest of our churn analyses are based). All data are transferred securely to the NA-ACCORD’s central Data Management Core, where they undergo quality control for completeness and accuracy per a standardized protocol before they are combined into harmonized data files. Quality control includes measures to reduce the probability that an individual was concurrently participating in more than one clinical cohort. The activities of both the NA-ACCORD centrally and each of the participating cohort studies to participate in NA-ACCORD have been reviewed and approved by the respective local institutional review boards.
Inclusion criteria
Only participants in clinical cohorts of the NA-ACCORD who had ≥1 HIV-lab any time between January 2000 and December 2008 were included in this analysis. This allowed us to focus on patterns of care retention using definitions compatible with guidelines issued by the US Department of Health and Human Service’s Health Resources and Services Administration (HRSA) HIV/AIDS Bureau (HAB) for HIV clinical care, though not all participants were covered by HRSA HAB programs [10]. The 13 included cohorts have clinical sites in eighteen US states and territories and three Canadian provinces: Alabama, California, Colorado, Florida, Georgia, Illinois, Maryland, Michigan, Missouri, New York, North Carolina, Ohio, Oregon, Pennsylvania, Tennessee, Texas, Washington, Washington, D.C., Alberta, British Columbia, and Quebec. Contributing cohorts were categorized by care management structure to facilitate comparisons between US and Canadian cohorts; US cohorts were classified as centrally managed if all medical services were available through a single organization (Kaiser-Permanente and Veterans Aging Cohort Study) or not (all other contributing clinical sites); the former are more similar to Canadian sites where all patients have access to centrally administered universal healthcare.
Outcome
As in other studies [8,17], HIV laboratory tests were used as a surrogate for access of care and adherence. For each individual, follow-up began the year of their first HIV-lab after enrollment in the participating cohort study; follow-up ended with their last HIV-lab up to December 31, 2008. As illustrated in Figure 1, individuals were categorized as “in care” in each calendar year if they had at least one HIV-lab >90 days and <12 months after the prior HIV-lab, and “out of care” during that calendar year otherwise (regardless of when during the calendar year the prior HIV-lab occurred); this is concordant with the HRSA HAB performance measure for retention in HIV care, though status was determined by elapsed time between HIV-labs and then anchored to calendar time, not determined by HIV-lab collection within a specific quarter of the calendar year as HRSA HAB standards suggest (Figure 1). These definitions also agree with the “core indicators of HIV clinical care” recommended by the Institute of Medicine of the National Academies of the US in March 2012 [18]. Accordingly, incomplete retention in care during the study period was defined as having at least one “out of care” calendar year, and complete retention as always having “in care” calendar years, between the beginning and end of follow-up. Incomplete retention was used as the outcome in regression analyses, allowing identification of factors associated with a risk of falling out of care, and thus possible intervention targets for improving retention.
Figure 1.
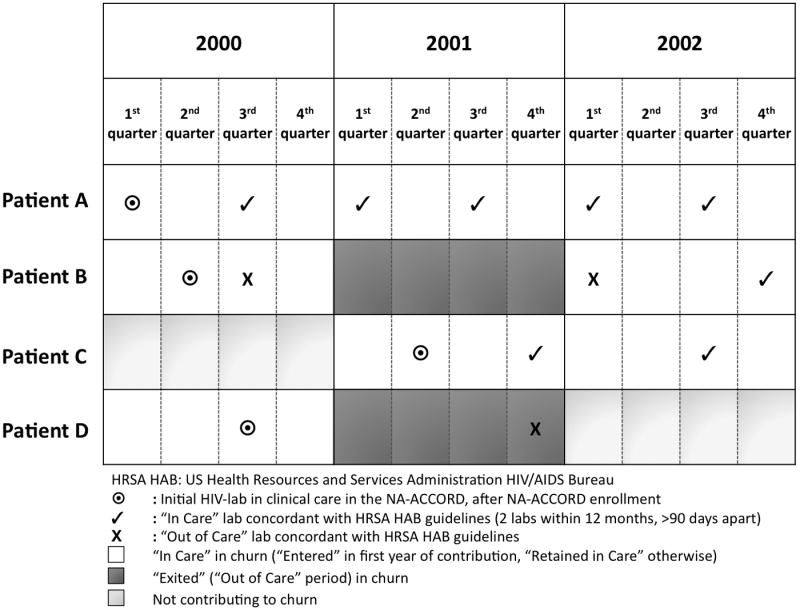
Examples of patterns of retention and churn in HIV clinical care concordant with HRSA HAB guidelines, and consequential categorization of contribution to the analysis. Patient A has 3 years “In Care”. Patient B has 2 years “In Care” and 1 year “Exiting” churn. Patient C has 1 year with no contribution, then 2 years “In Care”. Patient D has 1 year “In Care”, then 1 year “Exiting” churn, and 1 year with no contribution.
Churn, is an aggregate measure of individual-level patterns of retention in care, including patients entering, moving out of, returning, remaining out of, or remaining stable in care (Figure 1). Patients who died during the study period exited in the calendar year of their death and did not contribute beyond that year. Serial cross-sectional studies were nested by calendar year to describe the patterns of churn. The association of risk factors with incomplete retention and the consistency of within-patient behavior were described longitudinally using various regression approaches (described below).
Factors investigated for association with incomplete retention
We investigated year of birth, self-reported race/ethnicity, HIV transmission risk group, sex, and highly active antiretroviral therapy (ART) use as factors associated with incomplete retention in care. Race/ethnicity was categorized as non-Hispanic black, non-Hispanic white, Hispanic, and other/unknown. HIV transmission risk group was categorized as men who have sex with men (MSM), injection drug use (IDU), heterosexual contact, and other/unknown but was dichotomized as IDU vs. non-IDU in regression to avoid collinearity of MSM with male sex. Patients with both sexual and IDU transmission risk were categorized as IDU. ART was defined as a regimen of ≥3 antiretroviral agents from at least two classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) regimen containing abacavir (ABC) or tenofovir (TDF). Patients were classified as using ART if ART was prescribed for ≥2 months in a calendar period; measures of ART adherence were not available. CD4+ lymphocyte count and HIV-1 RNA were excluded from regression analyses because of their potential for time-varying mediation between the exposures and the outcome of incomplete retention.
Statistical analyses
Differences by clinical and demographic characteristics were determined by χ2 test for categorical variables and Kruskal-Wallis test for continuous variables. Trends in discontinuity of care during follow-up were evaluated using a generalized estimating equation (GEE) logistic regression model for proportions remaining in care on calendar year; this trend test accommodated clustering within individuals.
To examine predictors of incomplete retention, multivariable models were constructed to address the likelihood of strong confounding between multiple factors examined and simultaneously obtain appropriately adjusted estimates of effect. Two types of models were fit. First, beta-binomial models were fit separately for those initiating care in 2000 and 2005. These models account for trends in proportions with continuous care in different time frames and minimize potential statistical artifacts of data truncation [19]. Second, mixed effects logistic models were also fit with random intercepts for individuals and random slopes by participating cohort site to allow slightly more flexibility in individual trajectories of incomplete retention, both by individual and cohort [20,21]. Both models adjusted for total time in care to account for differential times at risk for incomplete retention.
Beta-binomial models for 2000–2004 and 2005–2008 appeared to fit the data best; results for these models are reported here and were similar to those from other models available as supplementary data (see Text, Supplemental Digital Content 1, which contains details about the statistical approach, fit diagnostics for the results, and applicable code). Analyses were conducted in Stata v. 11.2 (Stata Corporation, College Station, TX) and R v. 2.13.1 (www.r-project.org).
Results
The subset of patients with ≥1 year ”out of care” was statistically significantly younger (median 41 vs. 42 years) than those “in care” throughout the study. Those with incomplete retention were also over-represented compared to patients consistently retained among non-Hispanic blacks (30% vs. ≤23% for all other racial groups), among those with IDU as HIV risk factor (35% vs. ≤25% for all other risk factors), among those who were ART-naïve at time of first HIV-lab (29% vs. 23%), and among those who received care in the United States (vs. Canada) (26% vs. 18%), respectively. In addition, the subset of patients with ≥1 year ”out of care” had higher CD4 count (median 347 vs. 319 cells/mm3), and was in clinical care for a longer period (maximum of 6.03 vs. 2.31 years) than those only contributing to the stably “in care” portion of churn because of uninterrupted retention in care (Table 1).
Table 1.
Comparison of persons with complete retention in HIV care to those with ≥1 “out of care” year in HIV care, among 61,438 individuals with ≥1 HIV-lab in the NA-ACCORD, 2000–2008
| Characteristic | Retention in Care: Complete | Retention in Care: Out ≥1 Time | P-valuea | ||
|---|---|---|---|---|---|
| Total | 46,078 | (75) | 15,360 | (25) | <0.001 |
| Ageb (years) | 42 | (36, 50) | 41 | (35, 48) | <0.001 |
| Sex | 0.52 | ||||
| Male | 38,478 | (75) | 12,792 | (25) | |
| Female | 7,600 | (75) | 2,568 | (25) | |
| Race/Ethnicity | <0.001 | ||||
| Non-Hispanic White | 18,048 | (77) | 5,264 | (23) | |
| Non-Hispanic Black | 15,174 | (70) | 6,416 | (30) | |
| Hispanic | 4,635 | (80) | 1,179 | (20) | |
| Other/Unkown | 8,221 | (77) | 2,501 | (23) | |
| HIV Risk | <0.001 | ||||
| MSM | 16,528 | (81) | 3,949 | (19) | |
| IDU | 7,584 | (65) | 4,171 | (35) | |
| Hetero | 9,116 | (76) | 2,890 | (24) | |
| Other/Unknown | 12,850 | (75) | 4,350 | (25) | |
| CD4+ Countb (cells/mm3) | 319 | (150, 515) | 347 | (178, 553) | <0.001 |
| HIV-1 RNAb (log10 copies/mL) | 3.80 | (2.32, 4.82) | 3.79 | (2.60, 4.71) | 0.15 |
| ARTb | <0.001 | ||||
| Naïve | 14,548 | (71) | 5,844 | (29) | |
| Experienced | 31,530 | (77) | 9,516 | (23) | |
| Country | <0.001 | ||||
| US | 39,799 | (74) | 13,962 | (26) | |
| Canada | 6,279 | (82) | 1,398 | (18) | |
| Maximum Time in Care (years) | 2.31 | (0.57, 5.29) | 6.03 | (3.75, 8.43) | <0.001 |
χ2 for categorical, Kruskal-Wallis for continuous variables
At 1st year of data contribution
Numbers are “Number (%)” if categorical, “Median (Inter-Quartile Range)” if continuous
HIV-lab: CD4+ lymphocyte count or HIV-1 RNA viral load
MSM: sexual contact between men; IDU: injection drug use; Hetero: heterosexual contact; ART: antiretroviral therapy
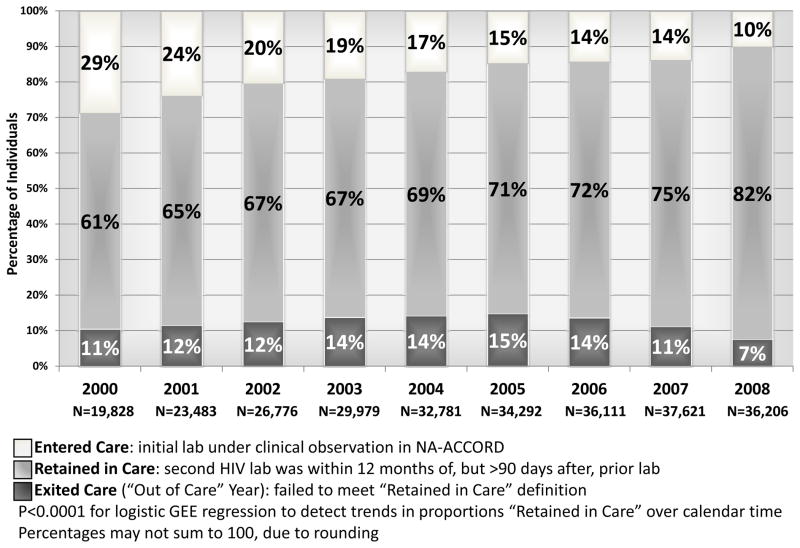
There was an improvement in retention in HIV clinical care in North America over calendar time from 2000 to 2008. Of the 61,438 patients meeting inclusion criteria, 25% had one or more ”out of care” episodes, and thus incomplete retention, over the entire study period. We observed a significant decrease in proportions of those with incomplete retention (from 39% to 18%), comprised of patients entering and exiting the churn, per calendar year over follow-up (Figure 2). These trends in the percentages of the population not stably retained, which the churn decomposes further into categories describing the nature of patient movements through care, were sustained even when stratifying contributing cohorts by care management structure: US Centralized, US Decentralized, and Canadian (see Table, Supplemental Digital Content 2, which shows the percentages of patients in various categories of care over calendar time). However, participants in Canadian cohorts were “in care” in higher proportions from 2000 through 2007 compared to their American counterparts.
Figure 2.
“Churn” in the NA-ACCORD by calendar year, 2000–2008
Because we sought to identify possible intervention targets to improve clinical retention in line with national HIV/AIDS strategies, our modeling strategy addressed factors associated with incomplete retention as the outcome, highlighting risk characteristics for falling out of care. In beta-binomial regression, among patients entering care in 2000 and 2005, age per 10-year increase and ART use at time of first HIV-lab were associated with increased likelihood of complete retention. In contrast, black patients (vs. whites), those with IDU risk (vs. non-IDU), and those with increased time from enrollment in care (per year) had increased likelihood of incomplete retention (Table 2). Female sex was significantly associated with reduced likelihood of incomplete retention among those entering care in 2000 (OR=0.82, CI: 0.70–0.95), but not among those entering in 2005 (OR=1.11, CI: 0.91–1.36). There was high within-individual correlation (ρ=0.24, CI: 0.22–0.26; ρ=0.20, CI: 0.17–0.23), denoting consistency of retention and churn patterns within individuals over time (Table 2).
Table 2.
Characteristics associated with incomplete retention among individuals contributing ≥1 HIV-lab between 2000 and 2008. Odds ratios (OR) from beta-binomial regression identify factors associated with increased (OR>1) or decreased (OR<1) likelihood of incomplete retention.
| Factor | Beta-binomial 2000–2004a | Beta-binomial 2005–2008b | ||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Female Sex | 0.82 | (0.70, 0.95) | 1.11 | (0.91, 1.36) |
| Age (per 10 y) | 0.78 | (0.74, 0.83) | 0.80 | (0.74, 0.86) |
| Non-Hispanic Black (vs. Non-Hispanic White) | 1.31 | (1.16, 1.49) | 1.36 | (1.15, 1.62) |
| IDU Risk (vs. non-IDU) | 1.68 | (1.49, 1.89) | 1.34 | (1.11, 1.61) |
| ART (at baseline) | 0.61 | (0.54, 0.68) | 0.73 | (0.63, 0.84) |
| Time Since Enrollment (per year) | 1.09 | (1.07, 1.11) | 1.22 | (1.16, 1.29) |
|
| ||||
| ρ | (95% CI) | ρ | (95% CI) | |
|
| ||||
| Intra-class Correlation Coefficient | 0.24 | (0.22, 0.26) | 0.20 | (0.17, 0.23) |
Model includes individuals entering care in 2000 contributing data 2000–2004, N=5,718
Model includes individuals entering care in 2005 contributing data 2005–2008, N=5,071
Time since enrollment: years under clinical observation in NA-ACCORD
Non-overlapping periods of 5 & 4 years used to eliminate artifacts of truncation in separate beta-binomial models
All models adjusted for contributing cohort and additional race/ethnicity categories of “Hispanic” and “Other/Unknown”
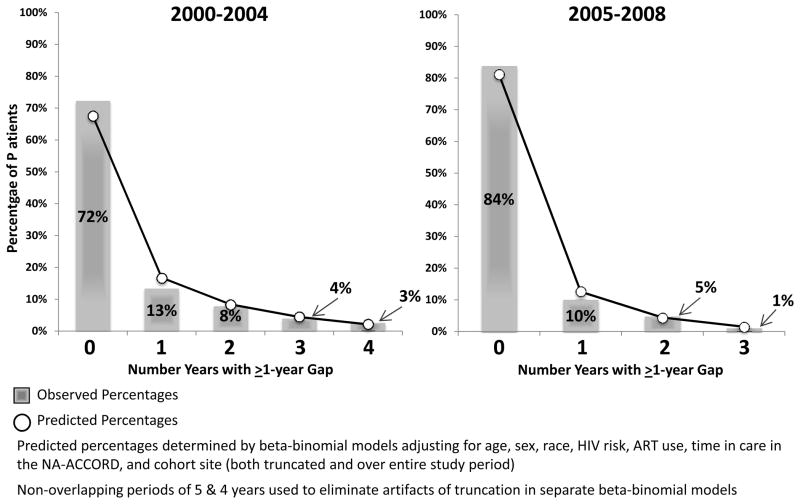
Predicted patterns of retention and churn (i.e., proportions of the cohort with 0 “out of care” years, 1 “out of care” year, etc.) derived from the beta-binomial models closely matched observed patterns, indicating the power of these models to account for within-individual correlation of observations over time and overdispersion from the binomial distribution (Figure 3).
Figure 3.
Characterizing individuals with different numbers of “out of care” years (or absences from care) over follow-up. Bars reflect observed percentages of individuals with different numbers of absent years; points reflect fitted values from multivariable beta-binomial models
Discussion
There were significant increases in both the total proportion and the demographic heterogeneity of the population stably retained in HIV clinical care in the US and Canada between 2000 and 2008. A concerning level of patients over the study period (25%), however, experienced incomplete retention, highlighting a challenge in delivery of optimal HIV care and the necessity of accounting for patterns of retention and churn when describing the epidemiology of HIV/AIDS in North America.
Population-level trends in Figure 2 suggest that some, but incomplete, progress has been made over the past decade or so in increasing regional stability of retention in care across the US and Canada. Further analysis is needed to discern possible reasons for this slow but steady improvement. The US National HIV/AIDS Strategy, released in 2010, proposes to both “establish a seamless system to immediately link people to continuous and coordinated quality care when they are diagnosed with HIV” and to “increase the proportion of Ryan White HIV/AIDS Program clients who are in continuous care (at least 2 visits for routine HIV medical care in 12 months at least 3 months apart) from 73 percent to 80 percent” by 2015 [13]. The Canadian Public Health Association’s national goals outlined in 2005 also note the importance of sustained clinical care, though it does not specify a concrete threshold for improvement [14].
As noted elsewhere, changes in the constitutive HIV population of a region over time can alter the prevalence and severity of disease among different at-risk groups, necessarily altering ideal targets for intervention, resource allocation, and assessment of prevention efficacy [9]. These issues, as well as the obvious health benefits of clinical care for patients, remain important considerations even as national HIV/AIDS strategies set forth ambitious goals for patient engagement and retention in care, and the CDC notes the impact of suboptimal retention on prevention of HIV transmission [7–8, 13–14, 18].
Differences in incomplete retention identified by sex, race/ethnicity, ART use, and HIV risk, present clear targets for interventional efforts to improve retention in HIV clinical care and advance these goals. The disappearance of sex as a significant factor in multivariable analyses restricted to the last 4 years of the study period suggests that disparities in retention by sex may be narrowing, though it is possible that unmeasured confounding factors affected this observation. Additionally, associations of increased “time in care” with increased likelihood of incomplete retention may imply a need to employ rigorous methods to encourage routine follow-up and re-engagement even among care-experienced patients. Differences in proportions with incomplete retention between the US and Canada throughout the study period may also deserve further scrutiny [22].
Disconnecting from regular HIV care has been shown to have major clinical consequences with regard to immune health and virologic failure [2–5]. However, the observed CD4 count/HIV-1 RNA viral load patterns in this analysis appear to differ from observations in the literature, with higher CD4 counts among those with interruptions in care and no difference in HIV-1 RNA viral load between those who were consistently retained and those who weren’t. This is likely due to incomplete retention being an outcome here, not an exposure. Prior studies treated retention in care as the exposure and CD4 count/HIV-1 RNA viral load as outcomes, and so observed that patients with ”out of care” periods during follow-up often returned to care with lower CD4 count and higher HIV-1 RNA viral load, as one might expect [1,3]. Such markers of disease progression were not included as time-varying covariates in this analysis because retention in care likely influences health status, and health status likely influences retention in care. Quantification of potential differences in clinical outcomes by churn and retention status may be a logical next step to these analyses but will require more sophisticated methods that can account for time-updated mediation and causally interrelated factors such as these.
This study has structural and statistical limitations. Because visit data are not currently available throughout NA-ACCORD, HIV-labs were used as surrogates for accessing clinical care. Though this may have resulted in slight over- or under-estimates of true healthcare access, and absence of incomplete retention defined using laboratory access may not imply retention in clinical care, HIV-labs are themselves markers of care subject to clinical guidelines for annual frequency and have been used previously in similar analyses [8, 15, 18]. The group with incomplete retention comprised, by definition, those that re-engaged in care subsequent to being “out of care” but before the end of the study. This pattern may differ from the longitudinal view of visit contributions to follow-up in a time-to-event analysis; contributions to churn could be viewed more appropriately as successive cross-sectional slices of care patterns in a region over time, contrasted with longitudinal analyses where individuals can be “lost to follow-up” for the remainder of the study after exiting care. Considering these distinctions and the characteristics of entrants to the NA-ACCORD (enrollment with ≥2 visits in a 12 month period), observed patterns in these analyses may represent overestimates of proportions retained in care over the study period. Furthermore, NA-ACCORD cannot track the movement of individual patients between clinical cohorts, so it is possible that patients who “exited” the churn in a given period (or did not return to care and were subsequently not counted), actually accessed care within another clinic, private practice, public health department, or elsewhere in the US or Canada. Due to the de-identified nature of the merged clinical data available for analysis, this issue may not be resolvable, though large geographic dispersion of sites within the NA-ACCORD may mitigate it. Finally, even though models capable of accounting for overdispersion from a binomial distribution and within-individual clustering of observations were used, they entailed fitting predictions to an observed distribution of “out of care” period counts that were highly right-skewed and flat; even so, deviations of predicted counts and probabilities from observed proportions with discontinuities in retention were not dramatic.
Despite these limitations, the analysis undertaken was an important first step toward quantifying churn and patterns of retention among large segments of the clinical HIV population in North America; in particular, the methods used were able to capture within-person tracking of care access over time and discern groups at higher risk of gaps in clinical care such as young persons, black individuals, and those with IDU HIV transmission risk or not accessing ART, groups which may benefit from enhanced retention efforts. Additional analyses indicate patients in US clinical cohorts within NA-ACCORD have similar demographics to HIV cases captured in the National HIV surveillance system [Keri Althoff, personal communication], supporting the notion that our findings may reflect trends in the larger adult population living with HIV.
As implied by the significant portion of this study population exiting or entering in the churn in each calendar year, and the demographic and clinical differences between patients retained in care and those who are not across the study period, cross-sectional analyses attempting to address policy questions by quantifying disease burden or other health metrics may suffer from fundamental measurement issues, and may therefore not offer an accurate picture of the clinical HIV-infected population at any one time. Therefore, the churn phenomenon will continue to play an important role in the epidemiology of HIV/AIDS in the US and Canada, and in light of renewed focus on uninterrupted care for the improvement of patient health outcomes and the interruption of transmission, will continue to serve as a benchmark indicator of progress toward greater stability in clinical retention among the HIV population of North America. This topic therefore merits further analysis with this and other study populations and similarly sophisticated epidemiologic methods capable of rendering valid estimates under the conditions of a dynamic population that is in churn.
Supplementary Material
Acknowledgments
We would like to thank all individuals involved with the NA-ACCORD collaboration, including staff, investigators, and patients, for their invaluable contributions to this work.
Sources of Support:
This work was supported by grants from the National Institutes of Health [U01-AI069918, U10-AA013566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI42590, U01-AI68634, U01-AI68636, U01-HD32632, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, R01-DA04334, R01-DA12568, R01-MH54907, R24-AI067039, Z01-CP010176, N02-CP55504, R01-DA11602, AI-69432, K01-AI071754, R01-AA16893, K24-DA00432, K23-AI610320, K01-AI071725, R01-AI069434] and the Agency for Healthcare Research and Quality [HS 290-01-0012].
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Conflicts of Interest:
P. F. Rebeiro: No Conflicts
K. N. Althoff: No Conflicts
K. Buchacz: No Conflicts
M. J. Gill: No Conflicts
M. Horberg: No Conflicts
H. Krentz: No Conflicts
R. Moore: No Conflicts
T. Sterling: No Conflicts
J. T. Brooks: No Conflicts
K. A. Gebo: No Conflicts
R. Hogg: No Conflicts
M. Klein: No Conflicts
J. Martin: No Conflicts
M. Mugavero: No Conflicts
S. Rourke: No Conflicts
M. Silverberg: No Conflicts
J. Thorne: No Conflicts
S. J. Gange: No Conflicts
- XIX International AIDS Conference, Washington, DC, July 2012.
- 45th Annual Society for Epidemiologic Research Meeting, Minneapolis, MN, June 2012.
- 16th International Workshop on HIV Observational Databases, Athens, Greece, March 2012.
- 15th International Workshop on HIV Observational Databases, Prague, Czech Republic, March 2011.
Contributor Information
Peter Rebeiro, Dept. of Epidemiology, Johns Hopkins University, Baltimore, Maryland, USA.
Keri N. Althoff, Dept. of Epidemiology, Johns Hopkins University, Baltimore, Maryland, USA.
Kate Buchacz, Epidemiology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
M. John Gill, Dept. of Medicine, University of Calgary, Calgary, Alberta, Canada.
Michael Horberg, Mid-Atlantic Permanente Research Institute, Kaiser Permanente Mid-Atlantic, Rockville, Maryland, USA.
Hartmut Krentz, Dept. of Anthropology, University of Calgary, Calgary, Alberta, Canada.
Richard Moore, Dept. of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Timothy R. Sterling, Dept. of Medicine, Vanderbilt University, Nashville, Tennessee, USA.
John T. Brooks, Epidemiology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kelly A. Gebo, Dept. of Medicine, Johns Hopkins University, Baltimore, Maryland, USA.
Robert Hogg, Dept. of Health Sciences, British Columbia Centre for Excellence and HIV/AIDS & Simon Fraser University, Vancouver, British Columbia, Canada.
Marina Klein, Dept. of Medicine, McGill University, Montreal, Quebec, Canada.
Jeffrey Martin, Dept. of Epidemiology & Biostatistics, University of California-San Francisco, San Francisco, California, USA.
Michael Mugavero, Dept. of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Sean Rourke, Dept. of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
Michael J. Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland, California, USA.
Jennifer Thorne, Dept. of Ophthalmology, Johns Hopkins University, Baltimore, Maryland, USA.
Stephen J. Gange, Dept. of Epidemiology, Johns Hopkins University, Baltimore, Maryland, USA.
References
- 1.Stone VE. HIV/AIDS in Women and Racial/Ethnic Minorities in the U.S. Curr Infect Dis Rep. 2011 Dec 3; [Google Scholar]
- 2.Berg MB, Safren SA, Mimiaga MJ, et al. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care. 2005 Oct;17(7):902–7. doi: 10.1080/09540120500101658. [DOI] [PubMed] [Google Scholar]
- 3.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007 Jun 1;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 4.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):100–8. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009 Jan 15;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009 Jan;23(1):41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Vital Signs: HIV Prevention Through Care and Treatment — United States. MMWR. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- 8.Hall HI, Gray KM, Tang T, et al. Retention in Care of Adults and Adolescents living with HIV in 13 U.S. Areas. J Acquir Immune Defic Syndr. 2012 Jan 19; doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 9.Gill MJ, Krentz HB. Unappreciated epidemiology: The churn effect in a regional HIV care programme. Int J STD AIDS. 2009 Aug;20(8):540–4. doi: 10.1258/ijsa.2008.008422. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services, Health Resources and Services Administration HIV/AIDS Bureau. HAB HIV performance measures: Medical case management. Nov 23, 2009. [Google Scholar]
- 11.Dombrowski JC, Kent JB, Buskin SE, et al. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS. 2012 Jan 2;26(1):77–86. doi: 10.1097/QAD.0b013e32834dcee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: The North American AIDS cohort collaboration on research and design (NA-ACCORD) Int J Epidemiol. 2007 Apr;36(2):294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The White House Office of National AIDS Policy. The national HIV/AIDS strategy. 2010. Jul, [Google Scholar]
- 14.The Canadian Public Health Association. Leading Together: Canada Takes Action on HIV/AIDS (2005 – 2010) 2005. Oct, [Google Scholar]
- 15.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012 Jun 5;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: Epidemiological issues. AIDS Res Hum Retroviruses. 2007 Jun;23(6):769–76. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 17.Mugavero MJ, Davila JA, Nevin CR, et al. From access to engagement: Measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010 Oct;24(10):607–13. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine of the National Academies. Table 1: ”Core Indicators of HIV Clinical Care”. 2012. Mar, Monitoring HIV Care in the United States, Indicators and Data Systems. [PubMed] [Google Scholar]
- 19.Gange SJ, Muñoz A, Saez M, et al. Use of the beta-binomial distribution to model the effect of policy changes on appropriateness of hospital stays. J R Stat Soc Ser C Appl Stat. 1996;45(3):371–82. [Google Scholar]
- 20.Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev. 2010 Jan;19(1):159–69. doi: 10.1158/1055-9965.EPI-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrondal A, Rabe-Hesketh S. Some applications of generalized linear latent and mixed models in epidemiology: Repeated measures, measurement error and multilevel modeling. Nor Epidemiol. 2003;13(2):265–78. [Google Scholar]
- 22.Krentz HB, Siemieniuk RA, Gill MJ. Similar challenges with retention in care issues. Clin Infect Dis. 2007 Dec 1;45(11):1527. doi: 10.1086/523010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.