Abstract
Emerging evidence for differences between individuals with autism spectrum disorder (ASD) and neurotypical (NT) individuals in somatic processing and brain response to touch suggests somatosensory cortex as a promising substrate for elucidating differences in functional brain connectivity between individuals with and without autism. Signals from adjacent digits project to neighboring locations or representations in somatosensory cortex. When a digit is stimulated, i.e. touched, its representation in cortex is directly activated; local intracortical connections indirectly activate non-primary cortical representations corresponding to adjacent digits. The response of the non-primary cortical representations is thus a proxy for connection strength. Local overconnectivity in autism implies that the nonprimary/primary response ratios of the ASD group will be higher than those of the NT group. D1 and D2 of the dominant hand of the participant were individually stimulated while we recorded neural responses using magnetoencephalography (MEG). The cortical representations of D1 and D2 (somatosensory evoked fields) were computed from the ensemble averaged data using (i) dipole model fits, and (ii) singular value decomposition (SVD). Individual adjacent/primary response ratios were measured, and group response ratio data were fitted with straight lines. Local overconnectivity in autism implies steeper ASD versus NT group slopes. Our findings did not support local overconnectivity. Slopes were found to be significantly shallower for the ASD group than the NT group. Our findings support the idea of local underconnectivity in the somatosensory cortex of the brains of individuals with ASD.
Keywords: Connectivity, Somatotopy, Cortical inhibition, Local excitation, Tactile, Homeostasis, Touch, MEG
1.0. Introduction
A number of high-level descriptions of the autism syndrome have been proposed over the years: Autism has been characterized as reduced empathy associated with an extreme form of the male brain (Baron-Cohen, 2002), deficits in executive function (Ozonoff, Pennington, & Rogers, 1991), weak “central coherence” or inability to bind disparate parts into a coherent whole (Happe & Frith, 2006), and impaired theory of mind ability (Baron-Cohen, Leslie, & Frith, 1985). These theories have succeeded in characterizing the behavioral symptoms of autism; concurrently, theories have been proposed for the neural mechanisms underlying the autism syndrome and in this context, abnormal neural connectivity has emerged as an explanatory scaffold for synthesizing behavioral accounts of autism. Within this overarching biological framework, there has been intense interest in the idea that the brains of individuals with autism are characterized by an overabundance of local connections and sparse long-range connections (Belmonte et al., 2004; Just, Cherkassky, Keller, & Minshew, 2004), perhaps because of differences in synapse growth during development. Connections between different areas of the cortex can be reasonably thought of as long-range connections, whereas connections within the same brain area can be thought of as short-range connections. Several experimental studies have examined functional long-range connectivity between areas in the brains of individuals with autism (Anderson et al., 2011; Barttfeld et al., 2011; Braeutigam, Swithenby, & Bailey, 2008; Castelli, Frith, Happe, & Frith, 2002; Horwitz, Rumsey, Grady, & Rapoport, 1988; Just, Cherkassky, Keller, Kana, & Minshew, 2007; Just, et al., 2004; Kana, Keller, Cherkassky, Minshew, & Just, 2006), but the physiological study of functional local connectivity within a brain area has lagged behind (Wilson, Rojas, Reite, Teale, & Rogers, 2007).
The somatosensory pathway is a promising candidate for testing the hypothesis of local neural overconnectivity. Autism spectrum disorders (ASDs) are developmental disorders rather than the result of acquired injury or disease, and their basis is likely to be distributed in neural networks, including those involved in somatosensory processing, rather than in isolated structures of the brain. Furthermore, deficits in sensorimotor function and hypo- or hyper-sensitivity to touch (Baranek, Parham, & Bodfish, 2005; Rogers, Hepburn, & Wehner, 2003) (see also Grandin, 1995, pg. 43) have been commonly observed in individuals with ASD. In fact, tactile sensitivity is a common feature in the stereotyped repetitive interests and behaviors domain used in making a diagnosis of autism. Finally, a number of recent studies have found differences in the somatosensory pathway of individuals with and without autism (Casanova et al., 2006; Coskun et al., 2009; Miyazaki et al., 2007). In summary, the somatosensory pathway is a promising neural substrate for testing current theories of atypical functional connectivity in autism.
The somatosensory pathway from the skin to primary somatosensory cortex is topographically organized (Gardner & Kandel, 2000): Signals from adjacent digits, e.g. D1 (thumb) and D2 (index digit) of the same hand, project to neighboring representations in somatosensory cortex. When the distal tip of a digit (e.g. D1) is stimulated with a gentle pressure stimulus, the mechanoreceptors underneath the stimulus site become active, and neurons in the cortical representation of D1 of primary somatosensory cortex downstream, are activated after a short synaptic delay. The neurons in cortex that are activated from projections from a patch of skin via the thalamus constitute the cortical representation of said patch (e.g. the cortical representation of D1 is the region in cortex directly activated by the tactile stimulation of digit D1). Activity in the cortical representation of D1 spreads and activates, via local, within-area intracortical connections, cortical representations of adjacent digits, henceforth termed non-primary cortical representations, e.g. the cortical representation of D2 is a non-primary cortical representation for D1 stimulation (Suppl. Fig. 0). Indeed, studies in the rat have shown that columns in layer IV – the input layer – of the somatosensory cortex function as independent parallel processors, each of which individually transforms thalamic input from their corresponding primary whisker for subsequent processing by horizontal intracortical connections (Goldreich, Kyriazi, & Simons, 1999). Thus, the cortical columnar response to the stimulation of an adjacent whisker or digit is attributable largely to local intracortical excitatory connections. In other words, the ratio of the response to the tactile stimulation of D1 of the cortical representation of D2 to that of the cortical representation of D1 (primary) is a physiological measure of local intracortical excitatory connection strength. Local overconnectivity in autism implies that the nonprimary/primary response ratios will be higher for the ASD group than the NT group, which means correspondingly that the slopes of the regression lines that fit the nonprimary/primary data will be steeper for the ASD group than the NT group. We tested our prediction using magnetoencephalography (MEG). It is important to note that computations and comparisons of group line slopes are more desirable over simple comparisons of arithmetic means of group response ratios, because the latter is more sensitive to large deviations in values of the ratio, and more susceptible to noise, therefore.
2.0. Methods
Participants
Magnetoencephalography (MEG) signals from 13 individuals with a clinical diagnosis of autism spectrum disorder, or ASD (18.7 ± 1.0 years old; 4 female) and 17 neurotypical, or NT, individuals (19.2 ± 1.2 years old; 4 female) were recorded. The groups were matched for age (p = 0.83, two-tailed t-test) and gender (p = 0.69, Fisher’s exact test). Five individuals in the autism group had clinical diagnoses of pervasive developmental disorder-not otherwise specified, one of Asperger syndrome, and the remaining seven of Autistic Disorder. All individuals in the autism group met our research criteria for an ASD, as determined by a finding of Autism Spectrum Disorder using the Autism Diagnostic Observation Schedule (Lord, Rutter, DiLavore, & Risi, 1999) and the Autism Diagnostic Interview, Revised (Rutter, Le Couteur, & Lord, 2003) administered by clinicians trained to research reliability. Potential participants were excluded when there was evidence of brain injury, seizure disorder, or neurotropic infection or disease, or if they had a history of identified severe psychopathology such as bipolar disorder, schizophrenia, or behavior problems severe enough to make accurate and reliable testing difficult. All participants were right handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). All individuals with autism had strong verbal skills, and were without intellectual disability: full scale IQs and verbal IQs derived from the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) were greater than 85 (full scale IQ: 103.7 ± 4.5; verbal IQ: 101.9 ± 4.9; performance IQ: 103.1 ± 4.5). NT participants were volunteers without a history of ASD or other major developmental or psychiatric illness. Their IQs were above-average (full scale IQ: 118 ± 3.0; verbal IQ: 113.0 ± 4.0; performance IQ: 118.0 ± 2.0) and were significantly higher than those of the ASD group (full scale IQ: p = 0.02; verbal IQ: p = 0.017; performance IQ: p = 0.012). It is important to note that participants did not have to perform any cognitive task at all, and therefore, differences in underlying activity between the groups are not likely to be based on differences in IQ (see Supplementary materials for correlations between the extracted MEG signals and IQ measures). Prior informed consent was obtained from all participants, or participants and their parents, under a protocol approved by the University of Texas Health Science Center-Houston and the University of Houston.
Stimuli
Pneumatically driven mechanical taps (25 pounds per square inch, or 25 psi) of 40 ms duration (20 ms rise time) were delivered individually to the distal tips of the thumb (D1), and index finger (D2) of the dominant hand of participants in separate blocks of epochs. This is a benign tactile stimulus that elicits a mild sensation on the skin; none of the participants indicated any discomfort with this procedure, but the stimulus amplitude (25 psi) is nonetheless clearly above the sensory detection threshold of 17 psi. Each digit had its own dedicated pressure transducer. Participants were told that a pressure pulse will be delivered and that all they had to do was to close their eyes, relax, and stay still. As mentioned above, there was no task to perform and therefore, no demand on participants’ cognition. A training block containing five epochs before the experimental recordings helped familiarize participants with the stimuli.
Procedure
Participants lay supine on a comfortable bed and kept their eyes closed. Fiducial markers were placed on their forehead and in the ears. The locations of the fiducial markers were recorded into the computer by means of a digitizer (stylus pen). The digitizer was slowly rolled over the participant’s scalp and the shape of his or her head was thus recorded. Using the digitization points and the fiducial marker locations, a single sphere head model was created that best fit each participant’s head (Fieldtrip toolbox, MATLAB). Every effort was made to keep the participant comfortable, and all completed the procedure without difficulty.
MEG recordings
All MEG recordings used a whole-head neuromagnetometer containing an array of 248 gradiometers (Magnes WH3600, 4D Neuroimaging Inc., San Diego, California, USA). The instruments were placed in a magnetically shielded and sound attenuated room (Vacuumschmelze Gmbh & Co., KG, Hanau, Germany). In separate blocks, we ran 2000 epochs of stimulation of the index digit (D2), and 700 epochs of stimulation of the thumb (D1). The additional epochs on D2 stimulation were for investigating the effects of continual stimulation on neural response and, as such, are for an altogether different study than the present. A single epoch lasted 575 ms and included a 120 ms pre-stimulus baseline. Data were acquired with a 1.0-Hz high-pass cutoff at a sampling rate of 290 Hz. Portions of the signal that were correlated to sensors placed far away from the head were likely to be noise and were subtracted out. Epochs remaining were used for analysis.
Analysis
Prior to analysis, epochs containing exaggerated moments such as eye blinks (peak to peak deflections > 2pT) were discarded. The criteria caused us to discard 8.2% ± 1.4% and 6.2% ± 1.7% of D1 stimulation epochs from the NT and ASD groups respectively, and 7.2% ± 1.8% and 9.1% ± 1.7% of D2 stimulation epochs from the NT and ASD groups respectively. Statistical tests on arcsin transformed percent values yielded no significant differences in the percentage of epochs discarded as a function of group (D1: p = 0.376; D2: p = 0.513). Remaining epochs were used for further analysis. We used two different approaches to analyze the response to stimulation, with the express purpose of knowing if our findings were sensitive to the use of analysis technique. The two approaches are described below.
Source modeling approach
For each participant and digit separately, all the artifact free epochs were ensemble averaged. Then, for a given body part (e.g. D1), the ensemble averaged MEG data (D1data248x122) and the participant’s single sphere head model were combined between 30 and 100 ms post-stimulus onset to obtain a best fitting dipole model, utilizing the Fieldtrip toolbox in MATLAB. The best fitting dipole is the one that has the least squared error between modeled and actual data. Dipole coordinates and orientations were computed for the best fitting dipole. Next, for the dipole, a forward solution, termed a lead field (D1lf1×248), was computed which contains the field distributions of the MEG sensors. Finally, the time courses of the dipoles (or the source waveforms) of D1 and D2 in response to D1 stimulation were obtained by projecting the lead field on to the ensemble averaged data (D1dip_wf1x122=D1lf1×248•D1data248×122; D2dip_wf1x122=D2lf1×248•D1data248×122). Analogous computations were performed for the case when D2 was stimulated.
Goodness of fits of the resulting D1 and D2 dipole sources were computed and we generally found reasonably high values for both: On average, D1 dipole goodness of fits were 81.6% ± 2.0% (ASD group mean = 81.5%, NT group mean = 81.7%), and D2 dipole goodness of fits were 86.5% ± 1.9% (ASD group mean = 87.7%, NT group mean = 85.6%). We further measured the degree of correlation between the modeled data obtained from dipole source modeling on the one hand and actual MEG data on the other, and the correlation coefficients were 0.77 ± 0.02 (ASD group r2 = 0.77, NT group r2 = 0.77) and 0.83 ± 0.02 (ASD group r2 = 0.84, NT group r2 = 0.82) for D1 and D2 data comparisons respectively. The somewhat superior goodness of fits and correlation coefficients of D2 data as compared to D1 data owes to the higher signal to noise ratio of the acquired D2 signal, which is due to the fact that there were more epochs of D2 versus D1 (2000 vs. 700 epochs) stimulation. Combined, the high goodness of fits and moderately high correlations between actual data and dipole modeled data indicate that the acquired MEG signals and source localization were of reasonably high quality.
Virtual sensor (singular value decomposition) approach
A virtual sensor was created that utilized signals from all sensors using a technique called singular value decomposition (SVD), which has been used before in MEG studies (van Ede, Jensen, & Maris, 2010). SVD provides a linear combination of MEG sensor data, and thus utilizes signals from all 248 MEG sensors but does not explicitly model the spatial coordinates of the underlying source of activity. In general, the purpose of SVD is to reduce a dataset containing a large number of values (248 time series, in the present case) to a dataset containing significantly fewer values, but which still contains a large fraction of the variability present in the original data. SVD analysis results in a more compact representation of the correlations present in the multi-sensor MEG data and can provide insight into spatiotemporal variations underlying the MEG signal. For the present purposes, the first SVD component, which accounts for the largest degree of variance, was used to form the virtual sensor, and it is a weighted sum of signals from all 248 sensors. The approach is described below in more detail.
For each participant and digit separately (D1, and D2), we ensemble averaged all artifact-free epochs. For a given body part (e.g. D1), we isolated these data 30–100ms following stimulus onset (this corresponds to 22 time points at a 290 Hz sampling rate), and obtained a 248 X 22 matrix (D1A248x22). Next, we decomposed the matrix A using SVD (). The columns of U form a set of orthonormal output basis vector directions for A. The first column of U (D1U248x1), which accounts for the largest proportion of the variance in the underlying data, is a vector of weights assigned to the signal recorded from each of the 248 sensors, and as such, is the virtual sensor corresponding to the cortical representation of D1. The above procedure was repeated to obtain the virtual sensor corresponding to the cortical representation of D2. The time courses of the D1 and D2 virtual sensors (from −120 to 200 ms relative to stimulus onset) in response to D1 stimulation, namely the somatosensory evoked fields or SSEFs, were then obtained (respectively, ) and used for further computations.
One way of determining the quality of the SVD virtual sensor is to quantify the proportion of variance accounted for by the SVD analysis. The formula for the proportion of variance captured by the first component is given by
where the evis are the non-zero singular values of the matrix Σ (where M = UΣV*), and are the square roots of the non-zero eigenvalues of M*M or MM *, and ev1 is the largest non-singular value in the matrix Σ.
In general, the first component of SVD of the multi-sensor MEG data accounted for a large proportion of the variance in our signal. The first component of the signal in response to the tactile stimulation of D1, which corresponds to the D1 representation in cortex, accounted for 60.5 ± 4.1% (mean ± s.e.m.) in the ASD group and 63.4 ± 3.0% in the NT group. The first component of the signal in response to D2 stimulation accounted for 71.0 ± 3.0% in the ASD group and 73.8 ± 3.4% in the NT group. The better SVD fits of D2 data can be attributed to the enhanced signal to noise ratio of the acquired signal owing to the larger number of epochs of D2 versus D1 stimulation. In summary, the first component of the variance captures a large proportion (60–75%) of the overall variance in the signal, and therefore provides a high quality signal for analysis.
M40, M80 component computation
For the M40 and the M80 components of the SSEF, the points in the time series where the signal deviated from pre-stimulus baseline values were obtained. The component’s half maximum value is defined as the signal whose amplitude is halfway between the signal at the base of the component and the component’s peak. The time series was linearly interpolated by a factor of 1000 in order to obtain a more precise estimate of the locations of the half maxima (one on either side of the component peak) in the time series. The amplitude of the given component was defined as the area of the signal (in femtoTesla * milliseconds) under the waveform that lay between the locations of the half maxima. The area measure has been used extensively in electroencephalography (EEG) studies (Hillyard, Squires, Bauer, & Lindsay, 1971; Picton & Hillyard, 1988) and is generally chosen to reduce the variability inherent in determining a single peak in a given component. Moreover, the area measure naturally utilizes more of the signal, i.e. averages over a wider range of time durations, than an amplitude peak measure, thereby providing a higher signal to noise ratio or SNR (an analogous argument holds in the temporal domain for utilizing signals from all sensors in obtaining a measure rather than selecting a single one on the basis of some criterion).
As mentioned above (see Methods: MEG Recordings), owing to time constraints, the number of epochs of D1 stimulation and D2 stimulation differed in our study. In spite of this difference, mean (± s.e.m.) M40 responses of the cortical representations of D1 and D2 to their respective stimuli were statistically indistinguishable under the dipole source modeling (D1 response – 192.5 ± 29.9 fT.ms vs. D2 response – 170.7 ± 38.5 fT.ms, t(29) = 0.497, p = 0.623; two-tailed paired t-test) as well as SVD (D1 response – 157.5 ± 26.4 fT.ms vs. D2 response – 139.3 ± 29.3 fT.ms, t(29) = 0.735, p = 0.468) approaches. Combining data from both groups, a small but statistically significant difference in M80 amplitudes of D1 and D2 response was observed with dipole source modeling (t(29) = 2.089, p = 0.046); although there were fewer epochs of D1 stimulation (700 epochs) than D2 stimulation (2000 epochs), the response of the D1 representation in cortex to D1 stimulation (2220.3 ± 241.9 fT.ms) was larger than the response of the D2 representation in cortex to D2 stimulation (1739.8 ± 206.1 fT.ms). In contrast, the M80 amplitudes obtained using SVD were not statistically distinguishable (D1 response – 2043.8 ± 247.1 fT.ms vs. D2 response – 1899.1 ± 230.0 fT.ms, t(29) = 0.662, p = 0.513). Overall, the D1 and D2 response means differed slightly, if at all, and the number of epochs of tactile stimulation did not predict relative response amplitudes. Of note, the relevant measure to the question at hand is a between-group comparison of cortical response to D1 (or D2) stimulation, and not response to D1 versus D2 stimulation, which has little bearing on the question in this study.
For each group (ASD, NT), the area measure response of the non-primary cortical representation was plotted (ordinate) with respect to the area measure response of the primary cortical representation (e.g. D2/D1 response to the stimulation of D1). The non-primary/primary cortical response ratios for a given group (NT or ASD) were linearly regressed under a least squares criterion. The line slopes of the NT and ASD groups were compared.
Statistics
SPSS (version 11.5) was used for statistical analyses (SPSS Inc., Chicago, Illinois, USA). Student’s t-test (two-tailed) examined the validity of the following null hypothesis: slopes of the least squares linear regressors of the response ratios of the ASD and NT groups do not differ.
3.0 Results
M40
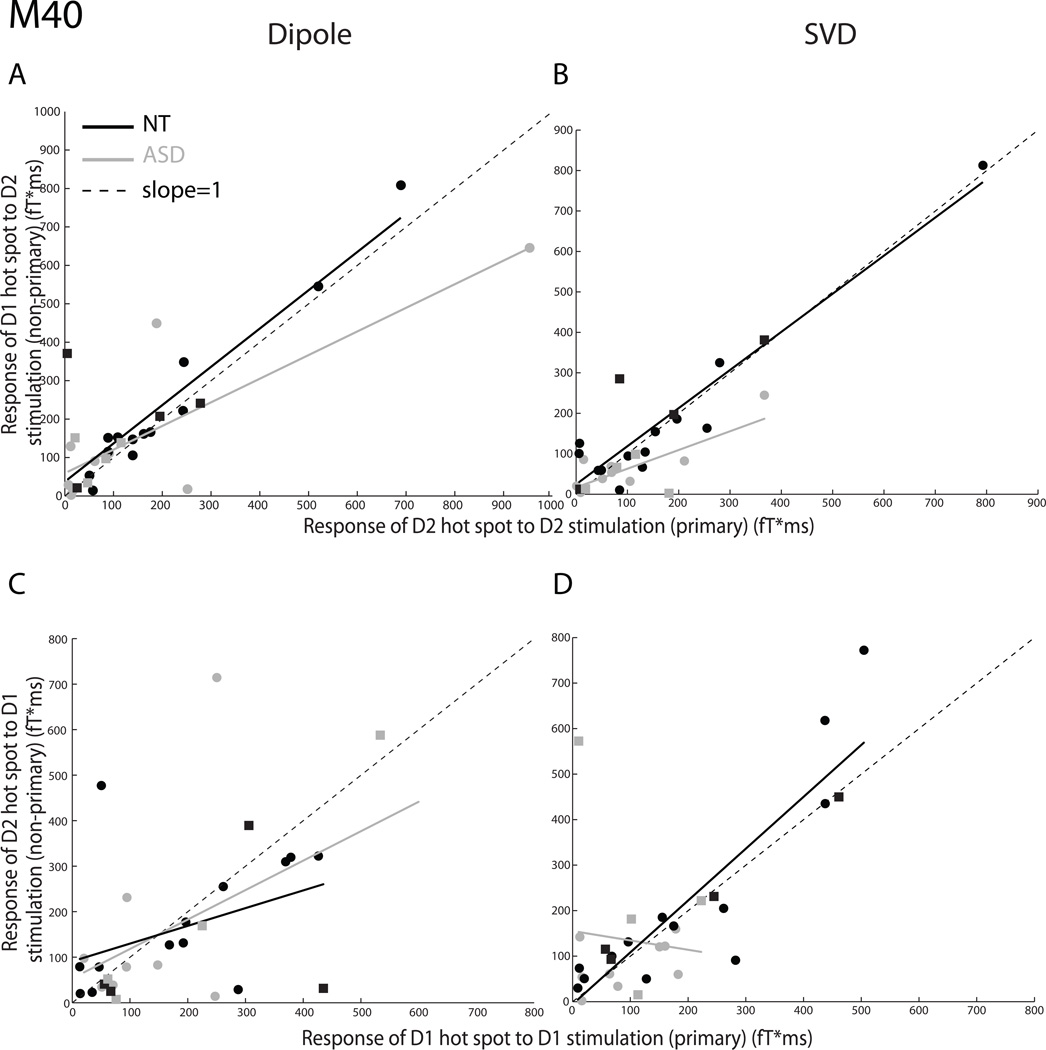
Fig. 1 shows data for the short-latency M40 component of the SSEF, analyzed using the dipole source modeling (left panel) and SVD (right panel) approaches. Figs. 1A and B illustrate response to the tactile stimulation of digit D2. Fig. 1A plots the ratio of the response of the D1 dipole to the response of the D2 dipole to the tactile stimulation of digit D2, and the least-squares straight line fits of ASD and NT group response ratios. The slope of the D1/D2 response ratios of the ASD group (slope = 0.62 ± 0.13), as compared to the slope for the NT group (slope = 0.99 ± 0.14), was shallower, and the difference was marginally significant (t(26) = 2.02, p = 0.054, two-tailed), indicating weaker response in ASD of the cortical neurons representing a particular digit to the tactile stimulation of an adjacent digit. Fig. 1B, which plots the ratios and regression lines using the SVD approach, confirms the results illustrated in Fig. 1A. In fact, the slope of the D1/D2 SVD response ratios of the ASD group (slope = 0.46 ± 0.12) is significantly shallower than that of the NT group (slope = 0.94 ± 0.10; t(26) = 2.50, p = 0.019, two-tailed), which suggests weaker spread of cortical activity via local intracortical connections in the ASD group as compared to control. Figs. 1C and D illustrate response to the tactile stimulation of digit D1. Fig. 1C plots the results of the dipole modeling; the slopes of the regression lines of the D2/D1 response ratios of the ASD (0.65 ± 0.31) and NT (0.39 ± 0.23) groups were statistically indistinguishable (t(26) = −0.67, p = 0.511, two-tailed). Fig. 1D plots the results of the SVD analysis. The slope of the D2/D1 response ratios of the ASD group (−0.21 ± 0.60) was shallower than the corresponding slope for the NT group (1.14 ± 0.14), and the difference in slopes was significant (t(26) = 2.19, p = 0.038, two-tailed), again indicating weaker local propagation of activity in the somatosensory cortex of individuals with autism as compared to control. Overall, our analyses suggest that the early activity of the cortical representation of a digit, when an adjacent digit is mechanically stimulated, is not stronger and sometimes, even significantly weaker, in the brains of individuals with ASD.
Fig. 1.
The short-latency M40 cortical response of the autism spectrum disorder (ASD) and neurotypical (NT) groups to adjacent digit stimulation. All somatosensory evoked fields (SSEFs) were computed using either dipole source modeling (Fig. 1A and 1C on the left) or singular value decomposition (Fig. 1B and 1D on the right). SSEF magnitude corresponding to the cortical representation of the adjacent, non-primary digit is plotted (ordinate) with respect to SSEF magnitude corresponding to the cortical representation of the stimulated, primary digit (abscissa). Each point represents a single participant (ASD: gray; NT: black; males: circles; females: squares). Non-primary/primary SSEF response ratios were plotted and linearly fitted (ASD group: solid gray lines; NT group: solid black lines); the resulting slopes for the ASD and NT groups were compared to a slope of 1.0 (dotted line) and to each other. A, B. D1/D2 SSEF ratios in response to the tactile stimulation of D2 are shown. C, D. D2/D1 SSEF ratios in response to the tactile stimulation of D1 are shown.
M80
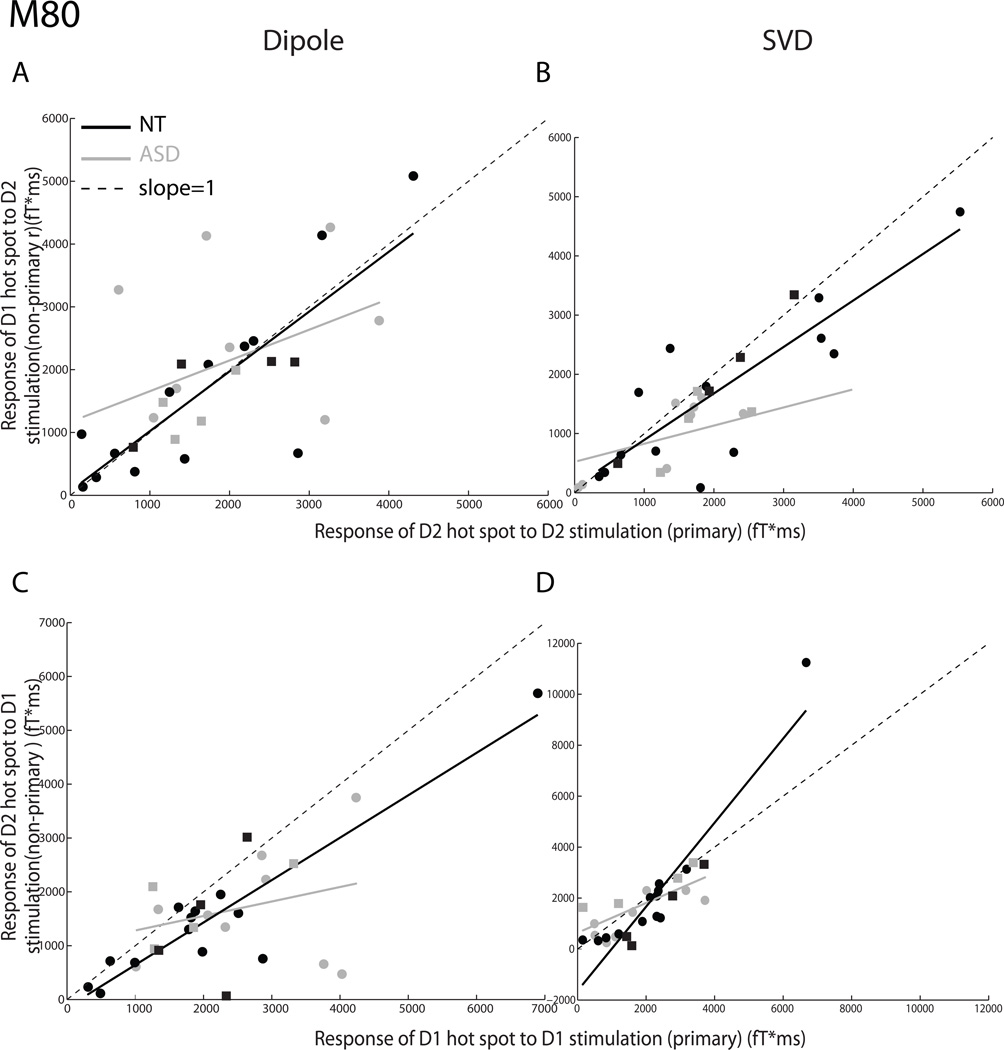
Fig. 2 shows data for the mid-latency M80 component of the SSEF, analyzed using the dipole source modeling (left panel) and SVD (right panel) approaches. Figs. 2A and B illustrate responses to the tactile stimulation of digit D2. Fig. 2A plots the ratio of the responses of the D1 to D2 dipoles to the tactile stimulation of digit D2, and the least-squares linear regressions of D1/D2 response ratios. As Fig. 2A shows, the slope of the linear regressor of D1/D2 response ratios of the ASD group (slope = 0.49 ± 0.32) was shallower than the corresponding slope for NT data (slope = 0.95 ± 0.17), although the difference in slopes did not reach significance (t(26) = 1.40, p = 0.175, two-tailed). Along the same lines but more dramatically, the slope of the linear regressor of D1/D2 response ratios of the ASD group (0.31 ± 0.12) extracted using SVD (Fig. 2B) was shallower than the corresponding slope for the NT group (0.79 ± 0.10; t(26) = 2.24, p = 0.034, two-tailed). Fig. 2C plots D2/D1 response ratios, obtained from dipole source modeling, of ASD and NT individuals to the tactile stimulation of D1. Again, the slope for the ASD group’s data (0.27 ± 0.24) was shallower than that for the NT group’s (0.79 ± 0.12), and the difference was marginally significant (t(26) = −0.67, p = 0.065, two-tailed). Fig. 2D plots the D2/D1 response ratios, obtained from SVD analysis. The results were in the same direction as those obtained from dipole modeling. In fact, the slope of the linear regressor of ASD data (0.58 ± 0.15) was significantly shallower than that of the NT group (1.65 ± 0.16; t(26) = 4.36, p = 0.0002, two-tailed). Combined, our analyses suggest that the mid-latency activation level of the cortical representation of a digit, when an adjacent digit is mechanically stimulated, is weaker, and often significantly so, in the brains of individuals with ASD.
Fig. 2.
The mid-latency M80 cortical response of the autism spectrum disorder (ASD) and neurotypical (NT) groups to adjacent digit stimulation. As in Fig. 1, dipole source modeling (Figs. 2A, C) or SVD (Figs. 2B, D) was used to compute SSEFs. A, B. D1/D2 SSEF ratios in response to the tactile stimulation of D2 are shown. C, D. D2/D1 SSEF ratios in response to the tactile stimulation of D1 are shown.
4.0 Discussion
The present study was designed to physiologically test recent ideas of atypical connectivity in the brains of individuals with autism spectrum disorder, specifically the hypothesis that local neural connectivity is more profuse in the brains of individuals with ASD as compared to control. Mechanical stimulation of a digit (e.g. D1) causes the spatial representation or neurons in its corresponding topographically organized representation in cortex (termed the primary cortical representation) to become active. Local intracortical projections from the primary representation spreads activity to adjoining regions of the cortex including to neighboring representations of adjacent digits (e.g. D2), termed non-primary representations. The spread of activity is a measure of local connection strength and can be thought of as a response gain, i.e. the ratio of the non-primary/primary cortical responses. Using high-resolution, whole-head magnetoencephalography (MEG), and analyzing non-primary/primary cortical response ratios to tactile stimulation in individuals with and without autism, our study showed that contrary to current theory, local, intracortical connections are not stronger in the somatosensory cortex of individuals with autism, and could even be weaker. In the remainder of the discussion, we will explain the relative merits and limitations of the analysis techniques used and of our thinking here, place our findings in the context of related experimental and theoretical studies of autism, and offer directions for future research on neural connectivity in the autism syndrome.
Limitations and convergence of analysis techniques
The small sample size, the lack of IQ matching between our autism and neurotypical groups and the relatively high intellectual functioning of our autism sample potentially limit the generalizability of our findings. Despite these limitations, the consistency of findings across two methods of analysis tends to support the validity of our results. SVD performs a linear combination of signals from all 248 sensors and extracts a solution that accounts for much of the variance in the signal across the sensor array, but does not explicitly model the spatial coordinates of the underlying source of activity. Dipole source modeling, on the other hand, uses the world coordinates of the sensors (and of multiple points on the head) to model the MEG recorded activity with a single equivalent current source and thus estimates the activity of the modeled source from the observed sensor data. Given the small sample sizes and inherent sensitivity of response ratios to variations in response to non-primary primary digit stimulation, some divergence in results from both approaches is to be expected. Nevertheless, both approaches identified a weaker normalized response of the non-adjacent digit’s representation in cortex in the ASD group, and the qualitative convergence of both approaches strengthens our belief in the robustness of the basic finding: weaker cortical response of individuals with ASD to the mechanical stimulation of an adjacent digit, and therefore, local underconnectivity in the somatosensory cortex of individuals with ASD.
Local connectivity
Our finding of a smaller non-primary/primary cortical response ratio slope in autism can be interpreted as a difference in synaptic connectivity. It is likely to represent a reduction in local excitatory connectivity, i.e. excitatory connections between neighboring columns in cortex, but the results are also consistent with increase in local inhibition, i.e. suppressive interaction between neighboring columns in somatosensory cortex.
Although there is consensus that differences in neural connectivity underlie autism, there is far less agreement about which particular aspect of local connectivity (i.e. whether excitatory or inhibitory, increase or decrease) in autism is deviant. One of the few studies that has probed local circuitry in autism from a physiological perspective found reduced 40 Hz gamma power from 200–500 ms after sound onset in the left hemisphere of children and adolescents with autism, as compared to NT children (Wilson, et al., 2007). Current theory (Traub, Jefferys, & Whittington, 1997) and strong empirical evidence (Cardin et al., 2009) argue that gamma oscillations are generated by synchronous activity of fast-spiking inhibitory interneurons. Thus, Wilson et al’s (2007) findings imply reduced local neural inhibition in autism. If reduced gamma power is found in several brain areas and occurs as early as infancy (Wilson et al. (2007) studied children and adolescents), aberrant local inhibition would become a viable candidate for the genesis of the profound reshaping in autism of excitatory and inhibitory neural circuitry. Cardin et al. (2009) further showed that the synchronous activity of excitatory pyramidal neurons in cortex generate lower frequency oscillations. Unfortunately, Wilson et al. (2007) only reported on 40 Hz oscillations. A more complete study over a wider range of frequencies (0–80 Hz) in the brains of at-risk infants is, therefore, in order.
On the other hand, recent studies have found evidence for local underconnectivity, in accord with the present finding. Discriminant function analysis of EEG spectral coherence on 1304 subjects with autism with ages ranging from 1 to 18 years old found reduced short-distance coherences indicating poor local network function in autism (Duffy & Als, 2012). A recent review of studies on structural and functional connectivity in autism concluded there was no evidence for local underconnectivity of the frontal cortex (Vissers, Cohen, & Geurts, 2012). In sum, local underconnectivity of the cortex in autism
Physiological investigations of related but otherwise separate theories of connectivity in humans have yielded conflicting findings. For instance, a noisy network has been proposed to underlie autism. Not only did the first empirical test of this hypothesis fail to support the idea but instead its findings weakly argued against a noisier network in autism (Coskun et al, 2009b); in contrast, two subsequent studies, using similar analyses methods as those used in the original study, found weak evidence for noisy synapses in autism (Milne, 2011; Dinstein et al., 2012).
While there have not been many functional studies of local connectivity to date, studies examining aspects of perception known to rely on local neural connectivity exist. Here too, the studies diverge. A study examining tactile perception in individuals with autism found that temporal order judgments of stimuli presented at a skin site, under the influence of synchronized conditioning stimuli on a near-adjacent skin site, deteriorated 3–4 fold in control subjects, whereas those of individuals with autism were unaffected (Tommerdahl, Tannan, Holden, & Baranek, 2008). The authors reasoned that the lack of local spatial interaction at the level of perception indicated reduced local connections between adjacent neuronal ensembles in the primary somatosensory cortex of individuals with autism. Our physiological finding of local underconnectivity in the somatosensory pathway of autism is in accord with these behavioral findings. In a study of visual crowding – an effect in which the perception of a visual target is reduced in the presence of flankers and lateral inhibitory connections are believed to underlie it – it was found that the crowding effect observed in controls was reduced in the autism group (Keita, Mottron, & Bertone, 2010), arguing for a decrease in local inhibitory connectivity in the visual cortex of individuals with autism.
Mouse models of Rett’s syndrome and Fragile-X – disorders that share behavioral symptoms with autism – have yielded dissimilar findings on connectivity as well. On the one hand, Gibson et al. (2008) have observed a decrease in excitatory drive to fast-spiking inhibitory neurons and concomitant increase in neuronal excitability in a mouse model of Fragile-X; on the other hand, animal models of autism such as Rett’s syndrome (Dani et al., 2005; Dani & Nelson, 2009) and neuroglin 3 mutation (Chubykin et al., 2007) mouse models have shown a clear increase in local inhibition, decrease in neural excitability, and reduction in excitatory synaptic connectivity.
Nevertheless, all the studies discussed above – animal model studies of autism, studies of sensory perception of individuals with autism, and physiological studies of autism – converge in one fundamental manner, namely in indicating an imbalance of excitation and inhibition in the autistic brain that is manifested as a change in local synaptic connectivity. It has been noted before that an imbalance of excitation and inhibition in either direction is likely to lead to profound differences in network dynamics, neural synchrony, and even behavior (Gibson, et al., 2008). We further contend that whereas a global imbalance in excitation and inhibition across the entire brain can be offset by the brain’s homeostatic mechanisms (e.g. a long-term decrease in neuronal excitability to counteract a decrease in inhibition), imbalances of excitation to inhibition ratios but in opposing directions in different brain regions is much harder to offset on a global scale. In summary, the apparently disparate reports may reflect a common basis after all: an imbalance in neural connectivity in any direction, as has been shown in this and other studies, is likely to be related to significant, uncompensated alterations in brain functioning and behavior observed in the autism syndrome.
Future Directions
A problem that plagues most functional studies of connectivity is that the findings are restricted to a limited brain region. Topography is a near-universal property of early sensory cortical functional organization; using a similar paradigm and logic as those used in the present study on somatosensory cortex, studies of local connectivity in other sensory areas will help understand if reduced local connectivity is general or specific to the somatosensory pathway. As discussed above, response imbalances in different directions in different brain regions can be as detrimental to normal brain function and behavior as a global, large-scale homogeneous imbalance. A second important issue is whether aberrant functional connectivity is a cause or effect. One way to investigate this is to extend the present study to younger populations, including perhaps infancy. One of the strengths (and weaknesses) of our paradigm is that there is no task, and no attentional or cognitive demand placed on the participant, which means our paradigm can be usefully applied to less developed populations (e.g. low-functioning individuals with ASD, infants). Finally, establishing a relationship between brain and behavior, i.e. correlating the non-primary/primary response ratio with sensory deficits across development, can address the extent to which a particular abnormality in brain function can cause a departure of a particular behavior from the norm.
5.0 Conclusions
The present findings lend support to the hypothesis of local underconnectivity in (the somatosensory cortex of) individuals with autism. Of note, we do not explicitly measure connectivity using one of several boutique, controversial and not universally agreed upon, measures found in the literature, but rather an incontrovertibly measurable functional consequence of altered brain connectivity on physiological response using an approach grounded in established knowledge of brain function and cortical organization.
Supplementary Material
Acknowledgments
The research was supported by a grant from the National Alliance for Autism Research—Autism Speaks (BRS). MAC was supported in part by a Presidential fellowship from the University of Houston. KAL and DAP were supported by the NIH: P01 HD035471 (KAL) and R01 MH072263 (DAP).
Footnotes
Financial Disclosures The authors report no competing interests.
References
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT, Parham LD, Bodfish JW. Sensory and Motor Features in Autism: Assessment and Intervention. In: Volkmar RPF, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders. Third ed. Vol. II. Hoboken, NJ: Wiley; 2005. pp. 831–857. [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a "theory of mind"? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49(2):254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeutigam S, Swithenby SJ, Bailey AJ. Contextual integration the unusual way: a magnetoencephalographic study of responses to semantic violation in individuals with autism spectrum disorders. Eur J Neurosci. 2008;27(4):1026–1036. doi: 10.1111/j.1460-9568.2008.06064.x. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, et al. How somatic cortical maps differ in autistic and typical brains. Neuroreport. 2009a;20(2):175–179. doi: 10.1097/WNR.0b013e32831f47d1. [DOI] [PubMed] [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, et al. Increased response variability in autistic brains? Neuroreport. 2009b;20(17):1543–1548. doi: 10.1097/WNR.0b013e32833246b5. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102(35):12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29(36):11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 2012 doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Kandel ER. Touch. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th ed. New York, NY: McGraw-Hill; 2000. pp. 451–471. [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100(5):2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldreich D, Kyriazi HT, Simons DJ. Functional independence of layer IV barrels in rodent somatosensory cortex. J Neurophysiol. 1999;82(3):1311–1316. doi: 10.1152/jn.1999.82.3.1311. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Squires KC, Bauer JW, Lindsay PH. Evoked potential correlates of auditory signal detection. Science. 1971;172:1357–1360. doi: 10.1126/science.172.3990.1357. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45(7):749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita L, Mottron L, Bertone A. Far visual acuity is unremarkable in autism: do we need to focus on crowding? Autism Res. 2010;3(6):333–341. doi: 10.1002/aur.164. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Manual. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Milne E. Increased intra-participant variability in children with autism spectrum disorders: evidence from single-trial analysis of evoked EEG. Front Psychol. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Fujii E, Saijo T, Mori K, Hashimoto T, Kagami S, et al. Short-latency somatosensory evoked potentials in infantile autism: evidence of hyperactivity in the right primary somatosensory area. Dev Med Child Neurol. 2007;49(1):13–17. doi: 10.1017/s0012162207000059.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsycholologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in highfunctioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA. Endogenous event-related potentials. In: Picton TW, editor. Human Event-Related Potentials EEG Handbook (revised series) Vol. 3. Elsevier; 1988. pp. 361–426. [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview—Revised Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT. Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local underconnectivity? Behav Brain Funct. 2008;4:19. doi: 10.1186/1744-9081-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci. 1997;4(2):141–150. doi: 10.1023/a:1008839312043. [DOI] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E. Tactile expectation modulates pre-stimulus betaband oscillations in human sensorimotor cortex. Neuroimage. 2010;51(2):867–876. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts MH .Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36(1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;62(3):192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.