Abstract
Eating behaviors and obesity are complex phenotypes influenced by genes and access to foods in the environment, but few studies have investigated the interaction of these two variables. The purpose of this study was to use a gene-environment interaction model to test for differences in children's food acceptance and body weights. Inherited ability to taste 6-n-propylthiouracil (PROP) was assessed as a marker of oral taste responsiveness. Food environment was classified as “healthy” or “unhealthy” based on proximity to outlets that sell fruits/vegetables and fast foods using Geographic Information Systems (GIS). The cohort consisted of 120 children, ages 4–6 years, recruited from New York City over 2005–2010. Home address and other demographic variables were reported by parents and PROP status, food acceptance, and anthropometrics were assessed in the laboratory. Based on a screening test, children were classified as PROP tasters or non-tasters. Hierarchical linear models analysis of variance was performed to examine differences in food acceptance and body mass index (BMI) z-scores as a function of PROP status, the food environment (“healthy” vs. “unhealthy”), and their interaction. Results showed an interaction between taster status and the food environment on BMI z-score and food acceptance. Non-taster children living in healthy food environments had greater acceptance of vegetables than taster children living in healthy food environments (p≤0.005). Moreover, non-tasters from unhealthy food environments had higher BMI z-scores than all other groups (p≤0.005). Incorporating genetic markers of taste into studies that assess the built environment may improve the ability of these measures to predict risk for obesity and eating behaviors.
INTRODUCTION
Childhood obesity is one of the most urgent threats to public health. The increased prevalence of this disease over the past three decades can be attributed to an environment that promotes excessive food intake and sedentary behavior (1). Increasing fruit and vegetable intake has been emphasized in obesity prevention efforts because overweight children's diets tend to be high in energy dense foods and low in essential nutrients, fiber, and whole grains (2). However, interventions that use a “one size fits all” approach to increasing fruit and vegetable intake and decreasing energy intake may undermine inherent food acceptance predispositions. Because children primarily make food choices based on taste (3), it is essential to consider the role of food acceptance on the development of childhood obesity.
Food acceptance patterns are influenced by parental feeding practices, the home food environment (4), and food availability (5). However, genes also influence food acceptance, the most well-known of which is the inherited ability to taste bitter thiourea compounds, such as PROP and phenylthiocarbamide (PTC) (6, 7). Approximately 70% of the U.S. Caucasian population is sensitive to the bitter taste of these compounds (“tasters”), while 30% are considered non-tasters (8). Tasters have been shown to have a greater number of fungiform papillae and taste buds on these papillae, (9) making them more sensitive to basic tastes (6), and textures, including fat (10–12). Tasters may experience greater perceived intensity that contributes not only to their ability to detect bitter flavors in foods, but possibly to their dislike of foods with bitter taste qualities, such as black coffee, grapefruit, and certain vegetables (7). In contrast, non-tasters tend to show heightened acceptance for certain high-fat foods (12, 13). In addition, several studies have demonstrated higher BMIs in non-tasters as compared to their taster counterparts, but in children, these relationships have only been noted in males (14, 15), while in adults the relationship has been strongest in females after controlling for dietary restraint (16, 17). Furthermore, several studies in female breast cancer patients have shown no relationship between PROP status and body weight (18). The discrepancy in findings suggests there may be environmental covariates that interact with PROP status that have not been adequately assessed.
Several recent studies suggest that aspects of the built environment, such as proximity to supermarkets and healthy food outlets are associated with higher fruit and vegetable intake (19, 20) and lower BMIs (21). Similarly, unhealthful lifestyles and higher BMIs are more prevalent among individuals living in environments that have a high density of fast food outlets (21). Exposure to food in one's environment cannot be used as a direct proxy for food intake, but may play a role in the development of food acceptance patterns since parents are more likely to purchase food from outlets that are near their homes (19). Elements of the built environment may influence the relationship between PROP taster status, food acceptance and BMI and help explain some of the discrepancies between earlier studies that do not control for environmental variables.
The purpose of this study was to assess the interaction between PROP taster status and the food environment on children's reported food acceptance and body weight. Food environment was assessed by both the quantity and quality of neighborhood food outlets. PROP taster status was used as a biomarker of bitter taste response with potential implications for food acceptance and obesity risk. We hypothesized that taster status would interact with the environment to differentially influence food acceptance and BMI z-score. More specifically, non-taster children who lived in food environments categorized as “unhealthy” would have greater acceptance of high-fat foods and higher body mass indexes (BMIs) compared to non-taster children who lived in “healthy” food environments, or taster children who lived in either food environment. This is the first study to look at the interaction between PROP taster status and the built environment with respect to food.
METHODS AND PROCEDURES
Participant Characteristics
A total of 120 children, 4–6 years-old (mean ± SD = 5.2 ± 0.8) were part of this study. The participants came from all five boroughs of New York City and were ethnically diverse: 33.9% Hispanic/Latino, 33.1% African American, 14% white, 3.3% Asian/East Asian, 14.9% self-identified as “other” which typically signified more than one racial background. Children varied in weight status with 2.5% underweight (<5th percentile BMI-for-age), 57.5% normal weight (5th – 85th percentile BMI-for-age), 20% overweight (85th – 95th percentile BMI-for-age) and 20% obese (≥95th percentile BMI-for-age) (22). Mean BMI z-score of the entire cohort was 1.1 ± 1.0, which translates to the 75th percentile BMI-for-age. Approximately 26% of parents reported that their annual income was ≤ $20,000.
Families were recruited by placing advertisements on a popular internet website and in and around the hospital community. Interested parents contacted research staff and were screened over the phone. Children were eligible to participate if they were healthy and not on any medications, had been to school, and had no learning disabilities or food allergies. Families received modest compensation for participating in the study, and children received a small toy following each session. Parents, the majority (~85%) of who were mothers, provided written consent for their children to participate. This study was approved by the Institutional Review Board of St. Luke's Roosevelt Hospital.
Experimental Design
Data for the present cross-sectional study were extracted from two datasets of children who participated in studies from 2005–2010 done at the Child Taste and Eating Laboratory of St. Luke's Roosevelt Hospital. The purpose of both studies was similar, to determine the role of PROP taster status in children's food acceptance, eating behaviors, and obesity risk. For each study, children and their parents attended 4 laboratory visits conducted during dinner time, from 4:30 – 6:30 pm. Parents completed a series of demographic, food inventory, and parental feeding practice questionnaires on behalf of their children, and children worked one-on-one with researchers to assess food acceptance, PROP status, and anthropometrics (see additional details below). Data from these cohorts have also been published elsewhere (15).
Food Acceptance
Food acceptance (likes and dislikes) was assessed for 36 common foods that were shown to children as pictures. Three categories of foods relevant to the present study were created: 1) unhealthy foods, 2) fruits and fruit juices, and 3) vegetables. Unhealthy foods included 14 highly palatable, snacks and sweets: ice cream, doughnuts, chocolate chip cookies, pie, milkshakes, chocolate milk, chocolate pudding, soda, hard candy, chips, French fries, pizza, macaroni and cheese, and hot dogs. Fruits and fruit juices consisted of 4 foods: strawberries, bananas, grapes, and apple juice. Vegetables consisted of 8 foods: spinach, broccoli, Brussels sprouts, celery, carrots, green peppers, string beans, and tomatoes. All foods were presented to children as color 8 * 10 inch clip art photos in a laminated binder. Presentation order was not randomized across children. To assess food acceptance, children were presented with the picture, asked to identify the food in the picture, and then asked to report liking of the food on an age appropriate, monadic scale (23).
Acceptance scores for each of the foods were assessed using different response scales in the two studies. In the first cohort, children responded using a forced choice procedure to report that they like, dislike, or had never tried the food. In the second study, children reported liking on a 5-pt. hedonic facial scale anchored with the following adjectives: Super Bad, Bad, Maybe Good or Bad, Good, Super Good (23). In order to standardize the liking ratings across the two studies, responses were recoded into one of three categories, 1) neutral/like, 2) dislike, and 3) never tried. For ratings assessed by the 5-point scale, Super Bad and Bad were both recoded as dislike and Maybe Good or Bad, Good, and Super Good were recoded as neutral/like. Recoding the data in this manner allowed us to combine children across the two studies into one dataset for the present analyses. In both studies, children also had the option to report that they had never tried a food, although this category was used infrequently (< 5% of the time). Once the data were recoded, we tallied across the foods to determine the total number of unhealthy foods, fruits, and vegetables children reportedly liked, and the number children reportedly disliked. Exploratory analyses were also done to analyze the unhealthy foods according to predominant taste characteristics, sweet/sweet-fats (i.e. ice cream, doughnuts, chocolate chip cookies, pie, milkshakes, chocolate milk, chocolate pudding, soda, hard candy) and savory-fats (i.e. chips, French fries, pizza, macaroni and cheese, and hot dogs).
PROP Status Measurement
Children were classified as “tasters” or “non-tasters” by having them “sip and spit” a solution containing 0.56 mmol/l PROP (6-propyl-2-thiouracil; Aldrich Chemical, Milwaukee, WI) in distilled water and perform a forced choice procedure according to methods from Mennella and colleagues (24). In this procedure, if the drink is tasteless or tastes like “water” or “nothing,” children are instructed to give it to a Big Bird puppet. If the drink is “bitter, sour, or yucky,” children give it to Oscar the Grouch so he can dispose of it in his trash can. In research studies done on similar aged children, this procedure has shown high test-retest reliability (Spearman's rho = 0.92) (14).
Anthropometric Measures
Weight and height were taken by trained research assistants on a standard balance scale and stadiometer, respectively. Children were measured in stocking feet and light clothing. Height and weight were used to determine BMI (kg/m2) and BMI z-scores using the Centers for Disease Control and Prevention growth charts conversion program for SAS version 9.0 (SAS, Cary, NC) (25).
Characterization of the Neighborhood Food Environment
Children's zip codes were extracted and the area around each child's home was geocoded using GIS (Geographic Information System) Version 9.0 (ESRI, Redlands, California). The ArcView buffer tool was used to create a boundary with a half-mile radius around each zip code. The upper limit for neighborhood environment assessment used in previous studies is one mile (26), but many urban planners consider a half-mile a more reasonable walkable distance (27), particularly for children.
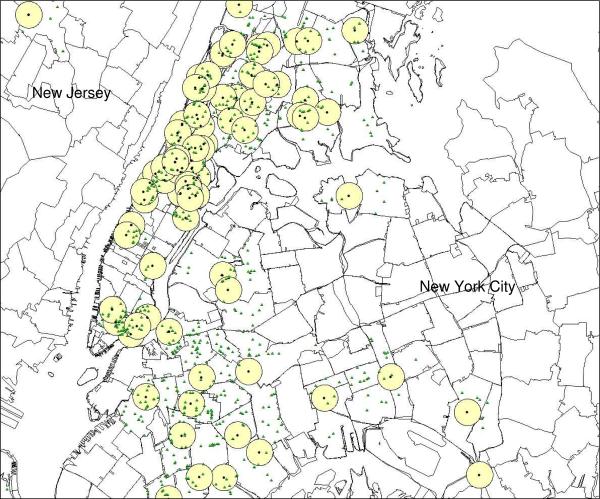
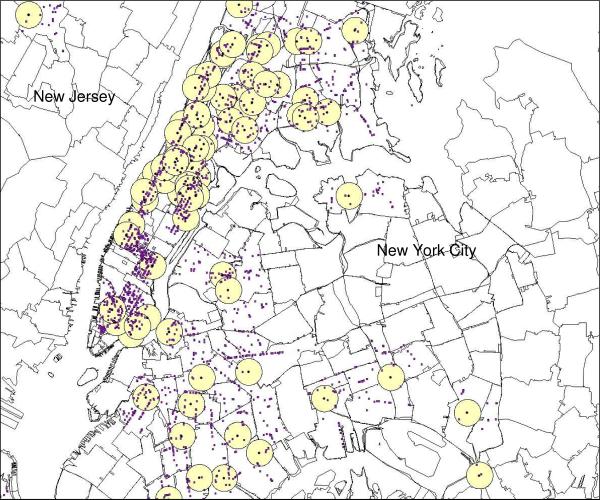
Additional data on retail food stores contained within the half-mile radius around children's zip codes were obtained from ReferenceUSA (28), a commercial provider of databases containing location and business classification using a Standard Industrial Code (SIC). The United States Securities and Exchange Commission assigns all businesses a primary SIC code for descriptive and statistical purposes. These codes identify the type of merchandise sold at each outlet and were used in this study to differentiate between healthy and unhealthy food stores (http://www.osha.gov/pls/imis/sic_manual.html). For the purposes of the present study, we defined food outlets as either “healthy” or “unhealthy” depending on several criteria. Healthy food outlets were those most likely to sell fruits and vegetables--fruit and vegetable markets, health food stores, grocery stores and supermarkets. Unhealthy food outlets were those that offered primarily energy dense, low-nutrient foods, such as chain fast food restaurants, local fast food outlets, and stores selling items such as fried food, candies, doughnuts, ice cream, and other confections. Corner stores, also known as “bodegas” in New York City, were also classified as unhealthy because they tend to offer snack and convenience food items compared to larger grocery stores and supermarkets (29). Restaurants that were not fast food establishments were excluded from the present analysis since their effect on weight status is unclear in the literature (30). Representation of the fruit and vegetable (healthy) and unhealthy food outlets around each child's homes are presented in Figures 1 and 2. Only children from New York City (Manhattan, Bronx, Brooklyn, Staten Island, and Queens) were included in the final analyses.
Figure 1.
Healthy food outlets (small triangles) within each half-mile radius (circles) around each child's residence (black dots). Only children from New York City (n=120) were included in final analyses.
Figure 2.
Unhealthy food outlets (small dots) within each half-mile radius (circles) around each child's residence (black dots). Only children from New York City (n=120) were included in final analyses.
Creating an Index of Children's Food Environments
In order to classify children's food environments as predominantly “healthy” or “unhealthy” for the purpose of data analyses, an index was calculated by dividing the number of healthy food outlets by the number of unhealthy food outlets within a half-mile radius of their home. Higher scores indicated an unhealthier food environment, while lower scores indicated a healthier food environment. This index was categorized for data analysis using a median split to determine if the food environment was “healthy” (< 50th percentile of unhealthy food outlets/healthy food outlets) or “unhealthy” (≥ 50th percentile of unhealthy food outlets/healthy food outlets).
Statistical Analysis
Statistical analyses were performed using SPSS, version 18.0 (SPSS, Chicago, IL) for Windows XP and SAS version 9.2 (SAS Institute Inc., Cary, NC) was used to carry out post-hoc tests on interactions. Descriptive statistics were used to analyze continuous (means, standard deviations) and categorical (frequencies) variables. To adjust for clustering of children within the food environment, hierarchical linear mixed models were created. All interactions of PROP status (tasters vs. nontasters) and the food environment (healthy vs. unhealthy) on BMI z-score and food acceptance (dependent variables) were tested in these models. Where appropriate, Scheffé's post-hoc tests were done to test for significant differences. A cut-off of p≤0.10 for the relationship with the dependent variable was used to determine which covariates of those tested to include in the models. Covariates included were: family income, child race, and population of the area where the child lives. All categorical covariates were dummy coded before including them in the models. Race was recoded as either Hispanic/African-American or neither. This categorization was used because our data indicated that both food environment index and BMI z-score were associated with report of African-American or Hispanic race. Moreover, in New York City in particular, recent reports indicate racial and ethnic minorities not only carry a heavier disease burden, they also are more likely to live in food deserts or environments with low access to fruits and vegetables (31). All hypotheses were two-tailed and a cut-off of p<0.05 was used for significance. All data in the results section are presented as means ± standard deviation (SD) and figures show data as means ± standard errors (SE).
RESULTS
A total of 70.8% of children were classified as tasters (n=85) and 29.2% as non-tasters (n=35), a breakdown that is similar to that seen by other investigators (32). Baseline characteristics of tasters and non-tasters are presented in Table 1. Chi-square analysis showed a difference in the expected frequency of males and females in the PROP taster groups. There was a higher frequency of female non-tasters than expected by chance (p=0.04). There were no differences as a function of PROP status for the other baseline variables.
Table 1.
Characteristics of taster and non-taster children enrolled in study
| Characteristic | Tasters (n = 85) | Non-tasters (n = 35) |
|---|---|---|
| Continuous Variables | mean ± SD | mean ± SD |
| Age (years) | 5.16 ± 0.77 | 5.31 ± 0.76 |
| Categorical variables | (%) | (%) |
| Sex a | ||
| Male | 49.4 | 31.4 |
| Female | 50.6 | 68.6 |
| Race | ||
| Asian | 3.5 | 2.9 |
| African-American/Black | 31.8 | 37.1 |
| Caucasian | 12.9 | 17.1 |
| Hispanic/Latino | 36.5 | 28.6 |
| Other | 15.3 | 14.3 |
| Income | ||
| ≤$20,000/year | 23.9 | 30 |
| ≥$20,000/year | 76.1 | 70 |
| Body Weight Status | ||
| Underweight | 1.2 | 2.9 |
| Normal Weight | 51.8 | 57.1 |
| Overweight | 23.5 | 11.4 |
| Obese | 23.5 | 28.6 |
| Food Environment Index | ||
| “Unhealthy” Food Environment | 55.3 | 51.4 |
|
| ||
| “Healthy Food Environment” | 44.7 | 48.6 |
No significant differences were found between the population characteristics of tasters (n = 85) and non-tasters (n = 35).
Chi-square test showed a significant difference in sex breakdown by PROP taster group (p=0.04)
On average, children reportedly liked 3.4 ± 0.8 out of 4 total fruits/fruit juices, 3.2 ± 2.0 out of 8 vegetables, and 11.2 ± 1.5 out of 14 total unhealthy foods. When the unhealthy foods category was sub-divided, children reportedly liked 7.5 ± 1.2 out of 9 sweet/sweet-fat foods and 4.5 ± 0.7 out of 5 savory fat foods. In contrast, children reportedly disliked 0.5 ± 0.8 total fruits/fruit juices, 3.4 ± 2.2 vegetables, and 1.5 ± 1.5 unhealthy foods. When the unhealthy foods category was sub-divided, children reportedly disliked 1.0 ± 1.1 sweet/sweet fat foods and 0.5 ± 0.7 savory-fat foods.
Associations between Food Acceptance and BMI z-score
Correlations were done to determine if the outcome variables, food acceptance and BMI z-score, were associated with one another. The number of fruits/fruit juices children reportedly liked was positively associated with BMI z-score (rho=0.25; p<0.01). In contrast, the number of fruits/fruit juices children reported disliking was negatively associated with BMI z-score (rho=−0.29; p<0.01). There was a trend for reported liking of sweet/sweet-fat foods to be positively associated with BMI z-score (rho=0.18; p=0.05). Children's reported acceptance of vegetables, unhealthy foods as a group, and savory fats was not associated with BMI z-score (p-values ranging from 0.20 – 0.98).
Main Effects of PROP Taster Status on Food Acceptance and BMI z-score
There were no main effects of PROP taster status on liking for fruits (p=0.92), vegetables (p=0.28), or unhealthy foods treated as a group (p=0.68), or broken up into sweet/sweet fats (p=0.34) and savory fats (p=0.87). There were also no main effects of PROP taster status on reported disliking of fruits (p=.99), vegetables (p=0.38), or unhealthy foods treated as a group (p=0.68), or broken up into sweet/sweet fats (p=0.91) and savory fats (p=0.95). BMI z-score did not differ by PROP status (p=0.67).
Main Effects of the Food Environment on Food Acceptance and BMI z-score
Food environment did not have a main effect on children's reported liking of fruits (p=0.40), vegetables (p=0.99) or unhealthy foods, both when treated as a group (p=0.80), or when broken down into sweet/sweet fats (p=0.89) and savory fats (p=0.42). Food environment also had no effect on reported disliking of fruits (p=0.62), vegetables (p=0.55), or unhealthy foods, both when treated as a group (p=0.90), or when broken down into sweet/sweet fats (p=0.99) and savory fats (p=0.68). After adjusting for covariates, there was a main effect of the food environment on child BMI z-score (F(df)=4.6(1,95);p<0.05). Children who lived in healthy food environments had BMI z-scores of 0.8 ± 1.2, while children who lived in unhealthy food environments had BMI z-scores of 1.3 ± 1.1.
Interaction of PROP status and the Food Environment on Food Acceptance
The interaction between PROP status and the food environment did not significantly affect children's liking for fruits (p=0.30), but there was a significant interaction on liking of vegetables [F(df) = 9.2(1,110);p<0.005]. According to Scheffé post-hoc analysis, non-taster children living in a healthy environment had significantly higher reported liking of vegetables than taster children living in healthy environments (p≤0.005). On average, non-tasters living in healthy food environments reportedly liked two additional vegetables (out of 8 total vegetables) than tasters living in the same environment, with means ± SDs equal to 4.1 ± 2.1 and 2.3 ± 1.8, respectively. There were no differences in vegetable liking between PROP taster groups living in unhealthy food environments [Table 2].
Table 2.
Mean (S.D.) reported food likes for each food group as a function of PROP taster status and the food environment
| Food Group | Non-tasters in Healthy Food Environments (n=18) (mean ± SD) | Non-tasters in Unhealthy Food Environments (n=17) (mean ± SD) | Tasters in Healthy Food Environments (n=38) (mean ± SD) | Tasters in Unhealthy Food Environments (n=47) (mean ± SD) | P-value μ |
|---|---|---|---|---|---|
|
| |||||
| Fruits/Fruit Juices (out of 4 total foods) | 3.5 ± 0.7 | 3.4 ± 0.8 | 3.3 ± 0.8 | 3.6 ± 0.7 | 0.28 |
| Vegetables¥(out of 8 total foods) | 4.1 ±2.1a | 2.8 ± 1.5ab | 2.3 ± 1.8b | 3.5 ±2.0ab | 0.004 |
| Unhealthy Foods (out of 14 total foods) | 12.2 ± 1.6 | 11.4 ± 2.4 | 11.6 ± 1.4 | 12.4 ± 1.4 | 0.07 |
| Unhealthy Food -Sweet/Sweet-fats (out of 9 total foods) | 7.6 ± 1.4 | 7.2 ± 1.6 | 7.2 ± 1.1 | 7.9 ± 1.0 | 0.06 |
| Unhealthy Foods -Savory Fats (out of 5 total foods) | 4.6 ± 0.6 | 4.3 ±1.0 | 4.4 ± 0.7 | 4.5 ±0.7 | 0.21 |
P-values for the interaction of PROP status by food environment are adjusted for child ethnicity, family income, and population density.
PROP status and the food environment interacted to affect the number of vegetables children reported liking [F(df) =8.6 (1,110); p<0.005.] Non-taster children living in healthy food environments reportedly liked more vegetables than did taster children living in healthy food environments (p<0.005; Scheffé post-hoc of the interaction). Superscripts above numbers are used to signify mean values that are significantly different from one another (e.g. “a” is different from “b”).
There was a trend for PROP status to interact with the food environment for liking for unhealthy foods (F(df)=3.6(1,94);p=0.07). However, these differences did not withstand post-hoc analysis. Liking of unhealthy foods as a function of PROP status and the food environment is reported in Table 2. PROP status and the food environment did not interact to influence liking of sweet/sweet-fats (p=0.08) or savory fats (p=0.40).
Reported dislikes as a function of the interaction between PROP status and the food environment are presented in Table 3. The interaction had no effect on reported disliking of fruits/fruit juices (p=0.60), or unhealthy foods (p=0.20), but did influence disliking of vegetables [F(df)=5.6(1,110);p<0.05]. According to Scheffé post-hoc analysis, non-tasters living in healthy food environments disliked 2.5 ± 1.9 vegetables, and this was fewer than the number of dislikes reported by tasters living in healthy food environments, 4.0 ± 2.4 (p<0.05).
Table 3.
Mean (S.D.) reported food dislikes for each food group as a function of PROP taster status and the food environment
| Food Group | Non-tasters in Healthy Food Environments (n=18) (mean ± SD) | Non-tasters in Unhealthy Food Environments (n=17) (mean ± SD) | Tasters in Healthy Food Environments (n=38) (mean ± SD) | Tasters in Unhealthy Food Environments (n=47) (mean ± SD) | P-value μ |
|---|---|---|---|---|---|
|
| |||||
| Fruits/Fruit Juices (out of 4 total foods) | 0.5 ± 1.1 | 0.5 ± 0.7 | 0.6 ± 0.8 | 0.5 ± 0.7 | 0.12 |
| Vegetables¥(out of 8 total foods) | 2.5 ±1.9a | 3.9 ± 2.1ab | 4.0 ± 2.4b | 3.2 ±2.2ab | 0.03 |
| Unhealthy Foods (out of 14 total foods) | 1.2 ± 1.3 | 1.7 ± 1.9 | 1.9± 1.5 | 1.3 ± 1.4 | 0.12 |
| Unhealthy Food -Sweet/Sweet-fats (out of 9 total foods) | 0.9 ± 1.1 | 1.2 ± 1.3 | 1.2 ± 1.1 | 0.8 ± 0.8 | 0.16 |
| Unhealthy Foods -Savory Fats (out of 5 total foods) | 0.4 ± 0.6 | 0.7 ±1.0 | 0.6 ± 0.7 | 0.5 ± 0.7 | 0.25 |
P-values for the interaction of PROP status by food environment are adjusted for child ethnicity, family income, and population density.
PROP status and the food environment interacted to affect the number of vegetables children reported disliking [F(df) = 5.1 (1,110); p<0.05.] Non-taster children living in healthy food environments reportedly disliked fewer vegetables than taster children living in healthy food environments (p<0.05; Scheffé post-hoc of the interaction). Superscripts above numbers are used to signify mean values that are significantly different from one another (e.g. “a” is different from “b”).
Interaction of PROP status and the food environment on BMI z-score
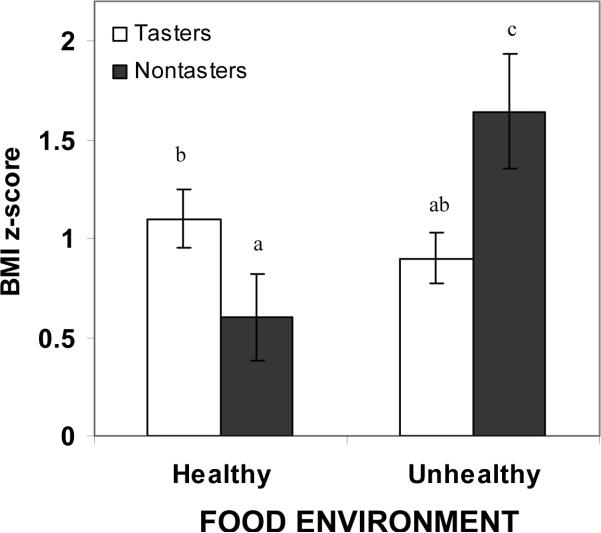
After adjusting for covariates, PROP status also interacted with the food environment to influence child BMI z-score (Figure 3, F(df) = 8.2(1,94) ; p≤0.005). According to Scheffé post-hoc analysis, non-taster children living in unhealthy food environments had significantly higher BMI z-scores than tasters living in healthy (p<0.01), and unhealthy food environments (p<0.005), and non-tasters living in healthy environments (p<0.005). Mean ± SD BMI z-scores of non-tasters living in healthy and unhealthy environments were 0.6 ± 0.9 and 1.6 ± 1.1, while BMI z-scores of tasters living in healthy and unhealthy environments were 1.1 ± 0.8 and 0.9 ± 1.0, respectively. These results remained significant when food acceptance variables that were associated with BMI z-score (fruits/fruit juices liked and disliked; sweet/sweet fats liked) were added to the models.
Figure 3.
Mean ± SE BMI z-score for tasters (white bars) and non-tasters (black bars) living in healthy and unhealthy food environments. Mean ± SD for tasters vs. non-tasters living in healthy environments were 1.1 ± 0.8 and 0.6 ±0.9, respectively. Mean ± SD for tasters vs. non-tasters living in unhealthy food environments were 0.9 ± 1.0 and 1.6 ± 1.1, respectively. Letters above graphs are used to signify significant differences in BMI z-score as a function of PROP status, food environment, and their interaction F(df)=8.2 (1,94); interaction effect p<0.005), with different letters used to denote differences between means (e.g. “a” is different from “b”). Sheffé post-hoc tests revealed that non-tasters living in unhealthy food environments (n=17) had higher BMI z-scores than tasters living in both healthy (n=38) (n=38;p<0.01) and unhealthy food environments (n=47) (n=47;p<0.005), and non-tasters living in healthy (n=18) (n=17;p<0.005) food environments.
Exploratory Analyses to Adjust for Sex Differences in PROP Taster Groups
Due to the higher prevalence of female non-tasters observed in this study, we ran exploratory analyses to adjust for sex differences in all of the above models. In each case, including sex as a covariate did not change the results. In addition, we ran three-way ANOVA to test the interaction between PROP status, the food environment, and sex on each of the outcomes. For all of the food acceptance variables, the interaction between all three variables was not significant (p values ranging from 0.70 – 0.90). The interaction between PROP status, food environment, and sex also did not influence child BMI z-score (p=0.31).
DISCUSSION
To our knowledge, this is the first study to test the interaction between the food environment, categorized in a systematic fashion, and a well-known genetic marker of taste response, PROP status. Two novel findings are reported. First, PROP non-taster children who live in healthy food environments reported higher acceptance of vegetables when compared to taster children who live in healthy food environments. No differences were seen between tasters and non-tasters who live in unhealthy food environments. It is also interesting to note that reported dislikes followed a similar pattern, with non-tasters living in healthy food environments reporting fewer vegetable dislikes than tasters living in healthy food environments. The second novel finding was that non-taster children living in unhealthy food environments had higher BMI z-scores than all other groups of children. Mean BMI z-score of non-taster children living in unhealthy food environments was over 1.6, and this corresponds to the 95th percentile of BMI-for-age, classifying them as obese (25). This difference was significant after adjusting for income, ethnicity, and measures of fruit acceptance that were associated with children's weight status. Because Chi-square tests identified a higher frequency of female compared to male non-tasters, exploratory analyses were done to determine if this sex difference skewed the results with respect to BMI outcomes, but this was not the case. If anything, male non-tasters appeared to drive this relationship, a finding supported by past studies in this age group (14, 15), however, the interactions between PROP status, the food environment, and sex were not significant. Consequently, the primary take home message is that that the food environment interacts with genetic taste predispositions to impact both food acceptance and weight status independently in children.
Previous studies that have not included measures of the built environment have demonstrated that non-taster children tend to like (13, 33) and consume (32) more bitter-tasting vegetables in laboratory studies compared to taster children. The reason attributed to this is that non-tasters have fewer fungiform papillae and taste buds on these papillae, thus they are less sensitive to the bitter flavors in vegetables that many children and adults find offensive. However, other studies in children (14) and adults (34) have found no differences in liking of vegetables and/or other bitter foods as a function of PROP status. Additionally, previous studies looking at PROP status and BMI in children have suggested an association between the two, but the results have been inconsistent (13, 14, 35). The differences reported in the present study, however, suggest that the food environment moderates the role of PROP status on acceptance of vegetables. For example, healthier food environments might provide greater access to fresh vegetables, and as a result, children who live in these environments may have greater familiarity and experiences with these foods. Previous research has demonstrated that repeated exposure to foods can increase children's acceptance for these foods (36). However, we speculate that non-tasters' food acceptance patterns may be more responsive to environmental influences and they may require fewer exposures to vegetables before developing a liking for them. In contrast, tasters' may have a stronger aversion to vegetables because the bitter flavor notes in these foods are perceived as strong, and consequently, even when healthy foods are readily accessible, their aversions to these flavors are more difficult to overcome. This speculation warrants investigation in future studies.
By the same mechanism noted above, non-taster children who live in unhealthy food environments may be more likely to acquire unhealthy eating habits that leave them vulnerable to obesity. Our findings revealed that non-taster children living in unhealthy food environments had higher BMI z-scores than all other groups of children. In fact, mean BMI in this group classified children in the obese range. Previous work has shown that non-taster adults are less discriminative of fat content in foods (11) and have greater liking for some high-fat foods than do tasters (12). Furthermore, non-taster children report higher consumption of discretionary sources of fat, like butter, spreads, and oils (13), and among males, higher rates of obesity (14, 37). The present results suggest that children, regardless of taster status, living in food environments where fast food and other unhealthful choices are plentiful may be more susceptible to obesity compared to children living in healthier food environments, as evidenced by a main effect of the food environment on BMI z-score. However, non-taster children may have an even greater susceptibility to these food environments due to a predisposed tendency to like higher fat foods. As such, we observed a robust interaction between taster status and food environment on BMI z-score and this effect was stronger than the main effect due to food environment alone. When the food acceptance measures that were associated with BMI z-score (fruit/fruit juice liking and disliking; sweet/sweet fat liking) were included in these analyses, the interaction between PROP status and the food environment on BMI z-score remained significant. This suggests that the food acceptance measures taken in the present study did not mediate the relationship between BMI z-score, PROP status, and the food environment. It is possible that the dichotomous scale used to measure food acceptance was not sensitive enough, or that other variables, such as actual food consumption, may have been better predictors of children's weight status.
Given that non-tasters living in unhealthy food environments had higher rates of obesity than all other groups, one might have expected to find greater acceptance for unhealthy foods in this group as well. This was not observed. In fact, trends suggested the opposite: that tasters living in unhealthy food environments reported greater acceptance for unhealthy foods. There are a couple proposed reasons for these findings. First, the foods contained in this category are generally well-liked and familiar to most children and variance across subjects was lower than for fruits and vegetables. Because these foods are highly liked by most children, small effects due to the food environment or taster status may have been masked by their high palatability. Second, the observance of a trend for taster children living in unhealthy food environments to prefer more unhealthy foods may be explained by the fact that many foods in this category were sweet. Previous studies have shown that taster children report higher consumption of sweet-fat foods (14) and higher liking of sweets (24), even though they do not appear to be at greater risk for obesity than non-tasters. Because many of the foods included in the unhealthy foods category had sweet taste qualities, it is possible that taster children with greater access to these foods had greater acceptance for them compared to non-tasters. In exploratory analyses that split sweet/sweet-fat foods from savory foods, the relationship between PROP status and the food environment was stronger between the former than the latter, thus supporting that sweet foods were driving this relationship. These results should be interpreted with caution, however, because of the high acceptance of unhealthy foods found across all children.
The present findings demonstrate how a genetic marker of taste response can interact with the food environment to impact food acceptance and obesity risk. It should be acknowledged, however, that sensitivity to PROP is not only influenced by genetic variation at the TAS2R38 receptor (37), but also by anatomical variations in fungiform papillae density (38) and possibly by incidence of otitis media, a common childhood illness (39). We have previously demonstrated that TAS2R38 and the PROP phenotype interact with child sex to influence body weight in children (15). The fact that incidence of otitis media, taste bud density, and TAS2R38 genotype were not measured in the present study is a limitation.
In addition, there are several limitations to this type of ecological, built-environment research. The use of a secondary source, namely ReferenceUSA, for characterization of neighborhood food environments presents opportunity for measurement error between the data and what actually exists “on the ground.” For future research, it would be appropriate to control for this by undergoing a neighborhood audit in which the establishments found via internet databases are compared with actual ground level observation. Additionally, in recent years, New York City has made important strides in improving access to healthy foods by increasing fruit and vegetable availability in corner stores, and bringing in mobile fruit and vegetable vendors and farmers' markets to low-income neighborhoods. These changes could not be accounted for using the retail codes available in this study and may undermine the validity of our food environment index. Another limitation is that we do not know how families in the present study actually interact with their food environments. Classification of subjects by food environment assumes that a one-half mile buffer around the home comprehensively reflects the area in which food shopping is done. While studies have shown that a half-mile buffer captures an area of significance regarding food availability (27), future research in this field would benefit from the inclusion of a separate questionnaire for parents regarding access to foods in order to characterize the “food environment” in a more comprehensive manner.
In conclusion, research into the relationship between food acceptance, BMI and the built environment could benefit from identifying study populations by genetic markers of eating behaviors, such as PROP status. Inclusion of this variable could serve as an index of general taste perception and a marker for oral sensory acuity that could provide insight into biological differences in one's susceptibility to the food environment. Future studies that incorporate both genetic and environmental components will further elucidate the complexities of childhood eating behaviors and the aspects of the environment that can be manipulated to prevent childhood obesity.
Acknowledgements
Funding for this study came from NIH grant K01DK068008 and an NIH/NIDDK Pilot and Feasibility Award (KLK). Additional support came from the Obesity Research Center Grant (NIH grant 5P30DK026687-27). Additionally, the authors received GIS consultation from James Quinn, Geographer and Senior Geographic Information Systems (GIS) Analyst, with the Built Environment and Health Research Group at Columbia University.
Footnotes
Disclosure There are no conflicts of interest on behalf of any of the authors.
Reference List
- 1.Hill JO. Genetic and environmental contributions to obesity. The Medical clinics of North America. 2000;84(2):333–46. doi: 10.1016/s0025-7125(05)70224-8. [DOI] [PubMed] [Google Scholar]
- 2.Reedy J. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assn. 2010;110(10):1477–84. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch LL. Dimensions of preschool children's food preferences. J Nutr Educ. 1979;11:91–5. [Google Scholar]
- 4.Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obesity Res. 2004;12(11):1711–22. doi: 10.1038/oby.2004.212. [DOI] [PubMed] [Google Scholar]
- 5.Kratt P, Reynolds K, Shewchuk R. The role of availability as a moderator of family fruit and vegetable consumption. Health Educ & Behav. 2000;27(4):471–82. doi: 10.1177/109019810002700409. [DOI] [PubMed] [Google Scholar]
- 6.Bartoshuk LM. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 7.Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Ann Rev Nutr. 2008;28:1–22. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 8.Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28(2):111–42. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller IJ, Reedy FE. Variations in human taste bud density and taste intensity perception. Physiol Behav. 1990;47:1213–9. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- 10.Essick GK, Chopra A, Guest S, McGlone F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol Behav. 2003;80:289–302. doi: 10.1016/j.physbeh.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol Behav. 1997;61:949–954. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 12.Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Ann NY Acad Sci. 1998;30(855):802–804. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 13.Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 14.Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obesity Res. 2004;12:904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- 15.Keller KL, Reid AR, MacDougall M, et al. Sex differences in the effects of bitter thiourea sensitivity on body weight in 4–6 year-old children. Obesity. 2010;18(6):1194–200. doi: 10.1038/oby.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tepper BJ, Ullrich NV. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol Behav. 2002;75:305–312. doi: 10.1016/s0031-9384(01)00664-3. [DOI] [PubMed] [Google Scholar]
- 17.Tepper BJ, Koelliker Y, Zhao L, et al. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity. 2008;16(10):2289–95. doi: 10.1038/oby.2008.357. [DOI] [PubMed] [Google Scholar]
- 18.Drewnowski A, Henderson SA, Cockroft JE. Genetic sensitivity to 6-n-propylthiouracil has no influence on dietary patterns, body mass indexes, or plasma lipid profiles of women. J Am Diet Assn. 2007;107:1340–48. doi: 10.1016/j.jada.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Laraia BA. Proximity of supermarkets is positively associated with diet quality index for pregnancy. Prevent Med. 2004;39(5):869–75. doi: 10.1016/j.ypmed.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Morland K. Supermarkets, other food stores, and obesity: the atherosclerosis risk in communities study. Am J Prevent Med. 2006;30(4):333–9. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Sturm R, Datar A. Body mass index in elementary school children, metropolitan area food prices and food outlet density. Public Health. 2005;119(12):1059–68. doi: 10.1016/j.puhe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen AW, Resurreccion AVA. Age appropriate hedonic scales to measure food preferences of young children. J Sens Stud. 1996;11:141–63. [Google Scholar]
- 24.Mennella JA, Pepino Y, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115(2):216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 26.Pikora TJ. Developing a reliable audit instrument to measure the physical environment for physical activity. Am J Prevent Med. 2002;23(3):187–94. doi: 10.1016/s0749-3797(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal AW, Schlossberg M, Irvin K. How far, by which route and why? Environ Behav. 2008;13(1):81–98. [Google Scholar]
- 28.InfoGroup ReferenceUSA. 1786. 2010 Reference Division, n.d. Web. [Google Scholar]
- 29.Kaufman L. Understanding the sociocultural roots of childhood obesity: food practices among Latino families of Bushwick, Brooklyn. Social Sci & Med. 2007;64(11):2177–88. doi: 10.1016/j.socscimed.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Duffey KJ. Differential associations of fast food and restaurant food consumption with 3-y change in body mass index: the Coronary Artery Risk Development in Young Adults Study. Am J Clin Nutr. 2007;85(1):201–8. doi: 10.1093/ajcn/85.1.201. [DOI] [PubMed] [Google Scholar]
- 31.New York City Department of Planning. Going to market: New York City's Neighborhood Grocery Store and Supermarket Shortage. 2008 [Google Scholar]
- 32.Bell KI, Tepper BJ. Short-term vegetable intake by young children classified by 6-n-propylthiouracil bitter-taste phenotype. Am J Clin Nutr. 2006;84:245–251. doi: 10.1093/ajcn/84.1.245. [DOI] [PubMed] [Google Scholar]
- 33.Turnbull B, Matisoo-Smith E. Taste sensitivity to 6-n-propylthiouracil predicts acceptance of bitter-tasting spinach in 3-6-y-old children. Am J Clin Nutr. 2002;76:1101–1105. doi: 10.1093/ajcn/76.5.1101. [DOI] [PubMed] [Google Scholar]
- 34.Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. J Am Diet Assn. 2000;100(6):647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 35.Lumeng JC, Cardinal TM, Sitto JR, Kannan S. Ability to taste 6-n-propylthiouracil and BMI in low-income preschool-aged children. Obesity. 2008;16(7):1522–1528. doi: 10.1038/oby.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(3):1–49. [PubMed] [Google Scholar]
- 37.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1224. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 38.Hayes JE, Bartoshuk LM, Kidd J, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008;33:255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- 39.Bartoshuk LM, Duffy VB, Reed DR, Williams A. Supertasting,earaches and head injury: genetic and pathology alter out taste. Neurosci Biobehav Rev. 1996;20:79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]