Abstract
The seasonal changes in thermal physiology and torpor expression of many heterothermic mammals are controlled by photoperiod. As function at low body temperatures during torpor requires changes of tissue lipid composition, we tested for the first time whether and how fatty acids are affected by photoperiod acclimation in hamsters, Phodopus sungorus, a strongly photoperiodic species. We also examined changes in fatty acid composition in relation to those in morphology and thermal biology. Hamsters in short photoperiod had smaller reproductive organs and most had a reduced body mass in comparison to those in long photoperiod. Pelage colour of hamsters under short photoperiod was almost white while that of long photoperiod hamsters was grey-brown and black. Short photoperiod acclimation resulted in regular (28% of days) torpor use, whereas all hamsters in long photoperiod remained normothermic. The composition of total fatty acids differed between acclimation groups for brown adipose tissue (5 of 8 fatty acids), heart muscle (4 of 7 fatty acids) and leg muscle (3 of 11 fatty acids). Importantly, 54% of all fatty acids detected were correlated (r2 = 0.60 to 0.87) with the minimum surface temperature of individuals, but the responses of tissues differed. While some of the compositional changes of fatty acids were consistent with a ‘homeoviscous’ response, this was not the case for all, including the sums of saturated and unsaturated fatty acids, which did not differ between acclimation groups. Our data identify a possible nexus between photoperiod acclimation, morphology, reproductive biology, thermal biology and fatty acid composition. They suggest that some of the changes in thermal physiology are linked to the composition of tissue and organ fatty acids.
Introduction
Although some textbooks still describe mammals as generically ‘homeothermic’ endotherms, e.g. [1], many species belonging to more than half of mammalian orders are, in fact, ‘heterothermic’ endotherms and use torpor for energy conservation [2]–[5]. Torpor is characterized by controlled reductions of metabolic rate, body temperature (Tb) and other physiological functions [6]. Usually, torpor is used more extensively in winter than in summer, and often the seasonal changes in torpor occurrence are controlled by photoperiod [7].
Seasonal expression of torpor is also affected by essential polyunsaturated dietary fatty acids, which have been shown to enhance the occurrence, duration and depth of torpor [8]–[15]. Data from these studies suggest that the observed diet-induced changes in the composition of tissue and cellular membrane fatty acids, which either occurred alongside, or caused the changes in thermal physiology, were to a large extent due to dietary uptake. In contrast, other recent studies have shown that seasonal changes in somatic fatty acid composition of heterothermic mammals also can occur independently of the dietary intake of fats, and may either be promoted by an endogenous circannual rhythm [16] or photoperiod acclimation [17]. Nevertheless, the available evidence on compositional changes of tissue fatty acids induced by photoperiod acclimation is essentially restricted to leg muscle of deer mice (Peromyscus maniculatus), which, like other members of the genus, are not strongly seasonal in their use of torpor [18]–[20].
Djungarian, or Siberian hamsters, Phodopus sungorus, show extreme seasonal phenotypes. During summer, they have dark brown fur, are reproductively active, with a high body mass and do not enter torpor. In autumn, they undergo a change in morphology and physiology such that by winter they have almost completely white fur, are reproductively quiescent, have a lower body mass and food intake and frequently use spontaneous (when food is available) daily torpor [7], [21]–[24]. The seasonal change in the occurrence of torpor in P. sungorus in autumn is controlled predominantly by the shortening photoperiod, but it can be modified somewhat by environmental temperature and food quantity and quality [7], [10], [25], [26].
Because both morphological and functional traits are so strongly influenced by photoperiodic change in P. sungorus, we tested the hypothesis that, even without dietary manipulations, changes in torpor use and depth induced by photoperiod acclimation are accompanied by changes of fatty acids composition of brown adipose tissue (BAT), heart, and leg muscle. BAT was investigated, because it plays an important role in non-shivering thermogenesis [27], [28], heart muscle was investigated because the heart is the most active organ during torpor [29], and leg muscle was examined to provide a comparison to previous work on deer mice [17].
Materials and Methods
Sixteen adult P. sungorus, born in summer of the previous year, were held at an ambient temperature (Ta) of 23±1°C, and a photoperiod that was adjusted weekly to the natural photoperiod of Marburg, Germany (50°48′ N, 8°45′ E). They were maintained on water and hamster chow (Altromin 7014) ad libitum. On 16 September they were divided into two groups (n = 8 each) of matched body mass and similar sex ratio. The Short Photoperiod (SP) group remained under natural photoperiod and at Ta 23°C. The Long Photoperiod (LP) group was exposed to a constant long summer solstice photoperiod of LD16∶8 at Ta 23°C. On 22 January when the natural photoperiod was short (LD 8.8∶15.2) for the SP group (the LP group remained under a LD16∶8 photoperiod), the Ta was reduced to 18°C for both SP and LP groups. Animals were kept under these conditions for eight days during which time torpor use was quantified as is outlined below. The study was carried out in strict accordance with German Animal Welfare Legislation under the umbrella of the Research Centre SFB#305, which had permission to conduct the kind of work reported here. A specific permit for this project was not required because animals were held with food ad libitum under mild thermal conditions and different photoperiods and then humanely sacrificed. There were no other experimental manipulations.
Throughout the experimental period hamsters were fed on Altromin hamster chow 7014, which contained 22.5% protein, 4.7% fat, 4.5% fiber, 39% carbohydrates, 11% water, minerals and vitamins. The fatty acid composition of fat is provided in Table 1 (from Altromin Tier-Labor-Service, Lage). Animals were weighed to the nearest 0.1 g with an electronic balance. The pelage index was determined by a staging system using fur coloration following [21]. The extreme values in this staging system are “1” for brown summer animals and “6” for white winter animals. Torpor was quantified between 0930 and 1030 hours by measuring body surface temperatures (Ts) using an infrared radiation thermometer (Heiman KT 17; accuracy ±0.2°C at a distance of 1 to 40 cm; measured area 16–18 mm diameter). For each Ts measurement, conducted on four of the eight days the hamster were exposed to Ta 18°C, the back and head surface of each animals was scanned from a 2–5 cm distance and the maximum Ts was recorded [23]. Animals with a Ts <25.0°C at Ta 18°C were considered torpid because these Tss correspond with Tbs of <31°C [10].
Table 1. Percent fatty acid composition of hamster chow.
| Fatty acid | Dietary fat % |
| 14∶0 | 0.3 |
| 16∶0 | 12.7 |
| 16∶1 | 0.3 |
| 18∶0 | 3.6 |
| 18∶1 | 21.8 |
| 18∶2n6 | 49.6 |
| 18∶3n3 | 6.8 |
| 20∶0 | 0.4 |
| 20∶1 | 0.9 |
| 20∶4n6 | 1.2 |
| 20∶5n6 | 0.5 |
| Rest | 1.9 |
The left column shows the numbers of carbons in the chain before the colon, followed by the number of double bonds after the colon and, for n3 and n6 polyunsaturated fatty acids, the position of the first double bond in the chain with respect to the terminal methyl group.
On 31 January, after animals had been at Ta 18°C for 8 days, they were humanely sacrificed with carbon dioxide and dissected for removal of inter-scapular BAT, hearts, and upper leg muscle (biceps femoralis) of n = 4 males from each group. White adipose tissue (WAT) was not examined because it showed limited response to photoperiod acclimation in P. maniculatus [17]. Tissues were removed and immediately frozen at −20°C for fatty acid analyses. Ovary and uterus, paired testes, epididymis and hearts were also removed and weighed to the nearest 0.01 g.
Fatty acid analyses were conducted within one week of tissue preparation. Total fatty acids were extracted from homogenized tissues and analyzed by gas chromatography as described [10]. Each sample was run twice and the mean values for fatty acids present at >0.1% are reported in the Tables. Unidentified fatty acids amounted to <2% of those identified.
Data of groups did not differ significantly between sexes for occurrence of torpor and pelage scoring (see Table 1). Therefore data were pooled and occurrence of torpor and pelage index between experimental groups were tested for differences using a Kruskal-Wallis test. General linear models were used to examine Ts, heart mass and body mass. T-tests were used to compare means of masses of reproductive organs between treatment groups. Fatty acid percentage values were arcsine-transformed before testing means for differences by t-tests on normally distributed data (Anderson-Darling test). Bonferroni adjustments were not made because they substantially reduce the power of rejecting an incorrect null hypothesis in each test. To examine possible correlations between thermal biology (i.e. Ts) and fatty acid composition, linear regressions were fitted using the least squares method. Numeric values are expressed as means ±1 SD; ‘n’ is the number of individuals, ‘N’ the number of measurements.
Results
Morphology and Torpor Use
Body mass changed with season and photoperiod acclimation (group p<0.01; Group × Initial vs Final p<0.01; Table 1). Body mass change was also significantly different between sexes, with males generally heavier than females at the end of the experiments, although body masses did not differ at the beginning of the experiments (Sex, p = 0.02; Sex × Initial vs Final p<0.05; Table 1). Heart mass (0.19±0.01 g) did not differ between sexes or treatment groups.
Mean mass of paired testes differed substantially (p = 0.001) between the SP (0.24±0.22 g) and LP (0.95±0.10 g) groups (Table 1). The mean epididymis mass in the SP group was only 0.06±0.02 g, but was about 4-times heavier (0.25±0.06 g) in the LP group. Similar to the reproductive organs in males, the combined uterus and ovary mass in females was only 0.06±0.02 g in the SP group and was heavier under LP at 0.21±0.03 g.
The pelage index was 1 (brown) for all individuals of both groups of hamsters in September. In January it remained at 1 in the LP group, but had increased significantly to a mean of 5.1 (largely white) in the SP group (Table 1).
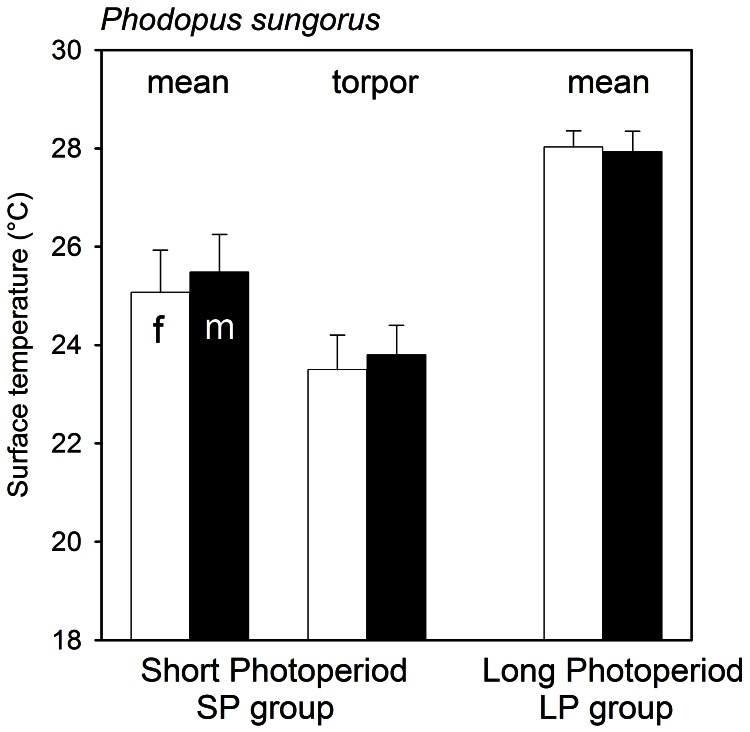
Torpor use at Ta 18°C differed significantly (H1 = 8.52, p<0.01) between LP (0% occurrence N = 32) and SP (28.1±20.9% occurrence, N = 32) groups (Table 2). Torpor use did not differ between sexes. Similarly, the mean of all Ts measured in the SP group (males 25.5±0.8°C; females 25.1±0.9°C) was significantly lower (F1,15 = 72.3, p<0.001) than that for the LP groups (males 27.9±0.4°C; females 28.0±0.3°C), but did not differ between sexes (F1,15 = 0.24, p = 0.63). The mean Ts in torpid individuals exclusively, which only was observed in the SP group (Fig. 1), was almost identical in females and males (females Ts 23.5±0.7°C; males Ts 23.8±0.6°C).
Table 2. Morphological variables, pelage index and torpor occurrence of Phodopus sungorus acclimated to short (SP group) and long (LP group) photoperiods, number of individuals reflecting all sample sizes in parentheses.
| Body mass Initial | Body mass Final | Testes | Epididymis | Uterus+Ovary | Pelage index | Torpor occurrence (%) | |
| (g) | (g) | (g) | (g) | (g) | |||
| SP females | 32.1±0.7 (3) | 23.4±3.6* | 0.06±0.02 | 5.0±0.0 | 33.3±28.9 | ||
| SP males | 31.1±2.3 (5) | 33.5±12.3 | 0.24±0.22 | 0.06±0.02 | 5.2±0.5 | 25.0±17.7 | |
| LP females | 30.1±1.6 (4) | 36.0±6.6 | 0.21±0.03 | 1.0±0 | 0±0 | ||
| LP males | 32.8±3.9 (4) | 46.3±5.4* | 0.95±0.10 | 0.25±0.06 | 1.0±0 | 0±0 | |
| Statistics | group F 1,31 = 7.83 p<0.01 | T7 = 6.49 | T6 = 5.92 | T5 = 8.16 | H1 = 14.22 | H1 = 8.52 | |
| Sex F1,31 = 6.19 p = 0.02 | p = 0.001 | p = 0.01 | p = 0.001 | p<0.0001 | p = 0.004 | ||
| Initial vs Final F1,31 = 2.21 p = 0.15 | |||||||
| Group × Sex F1,31 = 0.18 p = 0.66 | |||||||
| Group × Initial vs Final F1,31 = 8.34 p<0.01 | |||||||
| Sex × Initial vs Final F1,31 = 4.39 p<0.05 | |||||||
| Group × Sex × Initial vs Final F1,31 = 0.15 p = 0.70 | |||||||
| *Indicates final body mass significantly different from initial body mass at P<0.05 | |||||||
Data are means ±1 standard deviation.
Figure 1. Mean surface temperatures (±1SD) female (f) and male (m) Phodopus sungorus acclimated to short and long photoperiods and maintained at Ta 18°C.
Values for torpid individuals are shown only for the short photoperiod group because none of the long photoperiod animals displayed torpor.
Fatty Acid Composition
Eight fatty acids were present at >0.1% in BAT (Table 3). Five of these differed significantly between the SP and LP group. All fatty acids with 16 carbons or less were present in lower concentrations in the SP than LP group. In contrast, stearic acid (18∶0) and eicosenoic acid (20∶1) were present at higher concentrations in the SP than LP group. Sums of saturated (SFA), unsaturated (UFA), polyunsaturated (PUFA), and of n3 and n6 fatty acids did not differ between treatments.
Table 3. Percent fatty acid composition (>0.1%) of brown adipose tissue (BAT).
| Fatty acid | Short Photoperiod (n = 4) | Direction of Change | Long Photoperiod (n = 4) | T-test P-value |
| 14∶0 | 0.8±0.1 | < | 1.2±0.2 | <0.05 |
| 16∶0 | 16.7±1.0 | < | 21.1±1.3 | <0.01 |
| 16∶1 | 2.4±0.9 | < | 5.3±1.2 | <0.05 |
| 18∶0 | 14.8±1.5 | > | 9.5±1.4 | <0.01 |
| 18∶1 | 41.4±1.9 | 40.7±1.3 | ns | |
| 18∶2n6 | 21.7±0.6 | 21.4±1.2 | ns | |
| 18∶3n3 | 0.8±0.1 | 0.9±0.1 | ns | |
| 20∶1 | 0.6±0.1 | > | 0.1±0.2 | <0.01 |
| Rest | 0.9±0.6 | 0.1±0.2 | ||
| SFA | 32.3±1.2 | 31.7±1.3 | ns | |
| UFA | 66.9±1.5 | 68.3±1.3 | ns | |
| PUFA | 22.5±0.7 | 22.2±1.2 | ns | |
| n3 | 0.8±0.1 | 0.9±0.1 | ns | |
| n6 | 21.7±0.6 | 21.3±1.2 | ns |
In heart muscle seven fatty acids were identified at >0.1% (Table 4). Of these, four differed significantly between treatments, with palmitic acid (16∶0) and docosahexaenoic acid (22∶6) present at lower concentrations in the SP than LP group, whereas the PUFAs linoleic acid (18∶2) and arachidonic acid (20∶4) were present at higher concentrations in the SP than LP group. Moreover, the sum of n3 fatty acids, represented only by 22∶6, was lower in the SP than LP group and the opposite was the case for the sum of n6 fatty acids. Sums of saturated and unsaturated fatty acids did not differ.
Table 4. Percent fatty acid composition (>0.1%) of heart muscle.
| Fatty acid | Short Photoperiod (n = 4) | Direction of Change | Long Photoperiod (n = 4) | T-test P-value |
| 16∶0 | 17.9±0.6 | < | 20.4±0.9 | <0.01 |
| 16∶1 | 0.5±0.5 | 0.7±0.5 | ns | |
| 18∶0 | 21.0±2.2 | 19.8±1.5 | ns | |
| 18∶1 | 18.6±2.9 | 17.6±0.8 | ns | |
| 18∶2n6 | 22.3±0.9 | > | 20.4±1.0 | <0.05 |
| 20∶4n6 | 5.5±1.3 | > | 3.2±0.2 | <0.05 |
| 22∶6n3 | 12.7±1.5 | < | 16.9±1.3 | <0.01 |
| Rest | 1.7±1.4 | 1.1±0.9 | ||
| SFA | 38.9±2.2 | 40.1±0.7 | ns | |
| UFA | 59.4±0.9 | 58.9±1.1 | ns | |
| PUFA | 40.4±2.7 | 40.5±0.6 | ns | |
| n3 | 12.7±1.5 | < | 16.9±1.3 | <0.01 |
| n6 | 27.7±1.2 | > | 23.6±1.1 | <0.01 |
Leg muscle contained eleven fatty acids at >0.1% (Table 5). Only three of these differed significantly between the SP and LP group and for these three cases (stearic acid 18∶0, arachidonic acid 20∶4, docosahexaenoic acid 22∶6) concentrations were greater in the SP than LP group. Sums of SFA, UFA, PUFA, and of n3 and n6 fatty acids did not differ between treatments.
Table 5. Percent fatty acid composition (>0.1%) of leg muscle.
| Fatty acid | Short Photoperiod (n = 4) | Direction of Change | Long Photoperiod (n = 4) | T-test P-value |
| 14∶0 | 0.6±0.4 | 1.1±0.1 | ns | |
| 16∶0 | 21.6±3.2 | 21.6±0.6 | ns | |
| 16∶1 | 5.2±2.3 | 8.1±1.5 | ns | |
| 18∶0 | 7.3±1.5 | > | 4.4±0.8 | <0.05 |
| 18∶1 | 33.9±3.3 | 35.9±1.3 | ns | |
| 18∶2n6 | 23.3±7.5 | 23.1±1.5 | ns | |
| 18∶3n3 | 0.8±0.2 | 1.0±0.2 | ns | |
| 20∶1 | 0.4±0.4 | 0.1±0.3 | ns | |
| 20∶4n6 | 2.0±0.3 | > | 1.0±0.3 | <0.01 |
| 22∶1 | 0.7±0.9 | 0.3±0.5 | ns | |
| 22∶6n3 | 4.1±0.4 | > | 2.8±0.8 | <0.05 |
| Rest | 0.2±0.2 | 0.8±0.2 | ||
| SFA | 29.5±4.1 | 27.1±1.2 | ns | |
| UFA | 70.3±4.3 | 72.2±1.0 | ns | |
| PUFA | 30.1±7.6 | 27.9±2.3 | ns | |
| n3 | 4.9±0.5 | 4.1±0.6 | ns | |
| n6 | 25.3±7.4 | 24.1±1.7 | ns |
Discussion
Our study provides the first evidence that, in the absence of any alteration of dietary fats, the composition of fatty acids of BAT, heart muscle and leg muscle of P. sungorus changes in response to photoperiod acclimation and is correlated with the depth of torpor expressed. It shows that the seasonal change of thermal physiology and morphology in this species is largely controlled by photoperiod, in agreement with other studies, and suggests that some of the functional changes are linked to the fatty acid composition of tissues and organs.
Morphology and Torpor Use
Changes in morphological variables such as body mass and pelage index in response to photoperiod acclimation in P. sungorus were pronounced. Hamsters in long summer photoperiod had a high body mass and dark fur, and acclimation to short photoperiod generally resulted in a decrease in body mass and a change in fur colour to largely white, similar to previous studies [10], [21]–[23], [30]. Interestingly, body masses of male and female hamsters in our study responded differently to short photoperiod acclimation. Whereas SP females decreased body mass as expected by about 30%, males did not show a significant change in body mass (to some extent because the data were skewed by one heavy male), although overall body mass was lower than in the LP group. The pelage index changed as predicted with SP acclimation to nearly white in both sexes.
Reproductive organs of male P. sungorus also changed enormously with photoperiod acclimation. Testes and also epididimys mass in the SP group decreased by ∼75% in comparison to the LP group, similar to that previously documented [31]. Few data are available detailing the adjustments in reproductive organs of females due to photoperiod acclimation in P. sungorus [32], but it appears that, like for males, female reproductive organs change in mass and activity. In our study, the combined uterus and ovary mass decreased by ∼70% in the SP in comparison to the LP group. It is known that in the season, in which torpor is expressed in heterothermic mammals, reproductive activity is often halted and reproductive organs regress, but this is not the case for all species [33]–[38].
Associated with the morphological changes due to acclimation to short photoperiod observed here, torpor occurrence in P. sungorus increased and torpor was deeper, but animals entered daily torpor exclusively like other daily heterotherms [10], [22], [23]. Interestingly however, male hamsters in the SP group showed the same response in Ts reduction as females (Fig. 1) despite the lack of a uniform reduction in body mass in the former, to some extent due to the skewed data from one fat male who did not enter torpor. Nevertheless, the data suggests that a reduction in body mass in autumn is not always a prerequisite for torpor expression in the species in winter, cf. [24]. Overall, occurrence of spontaneous torpor in the LP group of about 30% of days measured, as observed here, is as expected under the thermal conditions investigated [10], [22], [23]. However, this may represent an underestimate because only a single reading/day was made in the morning, which might have missed some torpor bouts that, on average, last from 3.5 to 7.4 h and commence in the early morning [23]. In contrast, it is unlikely that torpor bouts were missed in the LP group, because P. sungorus do not express spontaneous torpor when long day acclimated [10], [22].
Fatty Acid Composition
The compositional changes of tissue fatty acids induced by photoperiod acclimation were substantial, but differed among tissues, as did the composition of tissues. Especially for brown adipose tissue (BAT), more than half (63%) of all detected fatty acids showed a significant change in concentration between the SP and LP group. This finding is especially interesting because in a previous similar study on white adipose tissue (WAT) in deer mice (Peromyscus maniculatus), only two fatty acids of those detected were found to differ between SP and LP acclimation groups [17]. This suggests that with regard to compositional changes in fatty acids to photoperiod acclimation, the response of BAT is more pronounced than that of WAT, which appears appropriate considering the important function of BAT in thermogenesis [27], [28] that go well beyond storage of energy, as is the case for WAT.
The second most pronounced compositional changes of fatty acids were observed for heart muscle, with 57% of fatty acids showing a significant change. There are few data for comparison, although seasonal changes in M. marmota heart phospholipids, especially for arachidonic acid (20∶4) and docosahexaenoic acid (22∶6) [16], are similar. Heart mitochondrial phospholipids also respond strongly to dietary fatty acids in hibernators [39] and P. maniculatus [17] and this compositional change is accompanied by pronounced changes in torpor patterns [40], as was observed here for P. sungorus under different photoperiods.
The least compositional changes were observed for leg muscle with only 27% of fatty acids showing significant differences between photoperiod groups. This observation is somewhat surprising, considering that P. maniculatus leg muscle was highly responsive to photoperiod acclimation, with 50% of its fatty acids showing significant compositional changes [17]. Perhaps the difference in the response to photoperiod acclimation reflects different extent of movement displayed by these species during torpor [41] because muscle fatty acid composition and locomotor performance are linked [42].
As in the previous study on photoperiod acclimation in P. maniculatus [17],, compositional changes were not observed for sums of SFA, UFA and MUFA (mono-unsaturated fatty acids) in any of the tissues investigated. However, changes for sums of n3 and n6 fatty acids were observed for heart muscle of P. sungorus, in agreement with data from P. maniculatus leg muscle. Other tissues of P. sungorus did not change sums of n3 or n6 fatty acids or their ratios.
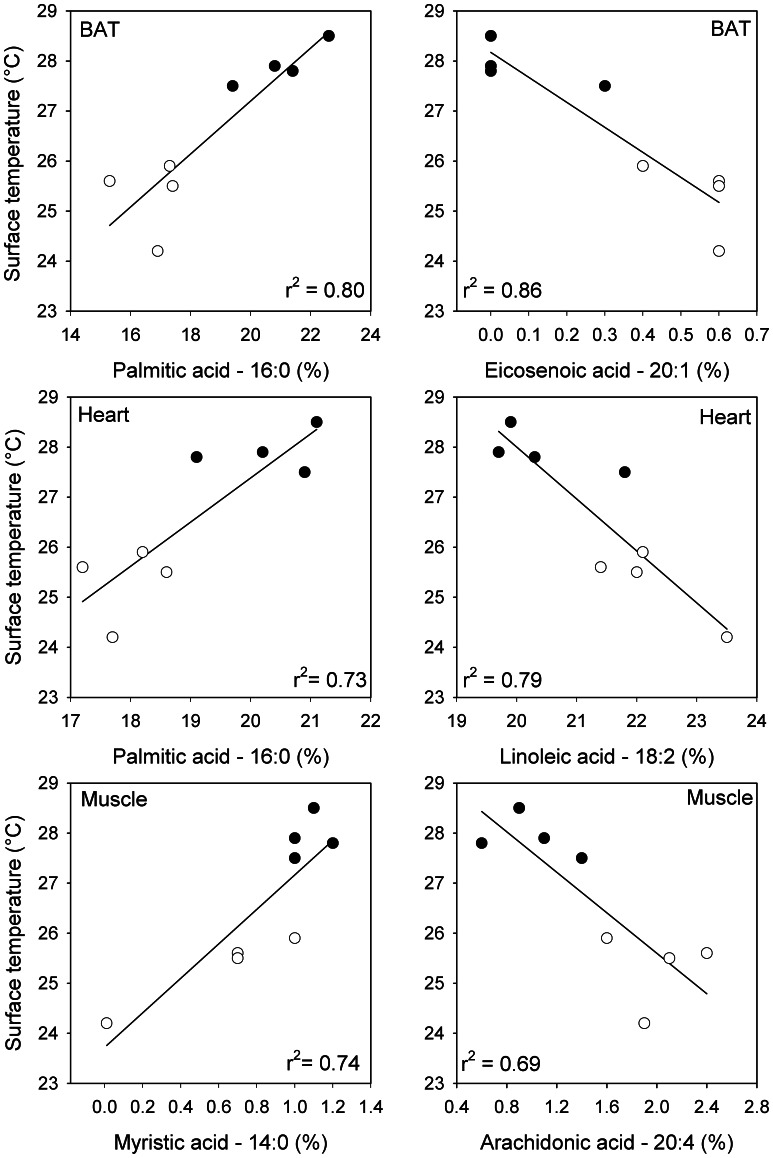
Changes in the amount of individual fatty acids in response to photoperiod acclimation were clearly evident, and often were highly correlated (r2 = 0.60 to 0.87) with the minimum Ts of individuals (Fig. 2; Table 6). In the absence of different dietary fatty acids these differences could be due to preferential uptake or storage, or preferential oxidation of saturated and sparing of unsaturated fatty acids during torpor [12], [43], [44], although the latter seems less likely here considering the shallow bouts of torpor expressed over a short period of time.
Figure 2. Linear regressions of mean surface temperature (Ts) of individual P. sungorus as a function of fatty acid percent.
Equations are provided in Table 6 and the two regressions with the greatest r2–values for each tissue are shown. Black dots represent long photoperiod (LP) hamsters, circles represent short photoperiod (SP) hamsters.
Table 6. Linear regression analyses of mean surface temperature (Ts) as a function of fatty acid concentration.
| Tissue/organ | Fatty acid | a | b | p | r2 |
| BAT | 14∶0 | 21.5 | 5.27 | 0.005 | 0.762 |
| 16∶0 | 16.6 | 0.529 | 0.003 | 0.798 | |
| 16∶1 | 24.0 | 0.683* | 0.009 | 0.709 | |
| 18∶0 | 31.2 | −0.381* | 0.017 | 0.64 | |
| 20∶1 | 28.2 | −5.0 | 0.001 | 0.855 | |
| Heart | 16∶0 | 9.95 | 0.871 | 0.007 | 0.733 |
| 18∶2 | 48.8 | −1.04 | 0.003 | 0.793 | |
| 20∶4 | 30.1 | −0.797 | 0.025 | 0.595 | |
| 22∶6 | 19.6 | 0.475* | 0.013 | 0.671 | |
| n3 | 19.6 | 0.475* | 0.013 | 0.671 | |
| n6 | 41.5 | −5.79 | 0.001 | 0.874 | |
| Leg muscle | 14∶0 | 23.8 | 3.41 | 0.006 | 0.741(+) |
| 16∶1 | 23.1 | 0.532* | 0.013 | 0.669(+) | |
| 18∶0 | 30.2 | 0.612 | 0.021 | 0.618 | |
| 20∶4 | 29.6 | −2.02 | 0.011 | 0.685 | |
| 22∶6 | 31.2 | −1.33 | 0.018 | 0.635 |
14 fatty acids showed significant correlations with Ts, 2 additional (+) to those that were different in t-tests. The equation is in the form of y = a+bx. Slopes that do not conform with ‘homeoviscous’ responses are indicated by asterisks (*). Examples are shown in Figure 2.
Of the five fatty acids of BAT, three changed as predicted by the ‘homeoviscous’ responses of lipids to increase in unsaturation with decreasing temperatures (14∶0 and 16∶0 were lower and 20∶1 was higher in the SP than in the LP group). In contrast, stearic acid (18∶0) and palmitoleic (16∶1) showed the opposite response, both in the comparison of means and in the regression analyses (Fig. 2; Table 6). For heart muscle, three fatty acids (16∶0, 18∶2, 20∶4) changed as predicted by a homeovicous response (Table 6), whereas docosahexaenoic acid (22∶6) was positively correlated with Ts and was lower in the SP than LP group, as was also observed for leg muscle of P. maniculatus [17]. Two of the three fatty acids showing changes to photoperiod acclimation of leg muscle went as predicted with an increase in PUFAs (20∶4 and 22∶6) in the SP group, but again stearic acid (18∶0) moved in the opposite direction in the comparison of means and palmitic acids (16∶1) also showed a positive slope as for BAT.
Astonishingly, the fatty acids 14∶0 and 16∶1, which did not show differences between groups when means were compared, were significantly correlated with Ts (Table 6) although only the slope for 14∶0 conforms with the concept of homeoviscosity. Thus, although pronounced compositional changes were observed for a substantial number of fatty acids, the changes induced by photoperiod acclimation are only partially explained by a homeoviscous response. It is therefore likely that interactions of specific fatty acids with membrane proteins [14], [45], [46] are responsible for appropriate adjustments that allow transient function both at low and high Tbs during a torpor/arousal cycle. Although the observed correlation between fatty acid composition and torpor depth suggests a functional nexus, more work is needed to understand why different tissues differ in composition and the mechanisms by which tissue fatty acid composition and whole animal thermal physiology are linked.
Acknowledgments
We would like to thank Daniela Gerber for help with animal maintenance and technical assistance, Gerhard Heldmaier for providing animals and facilities, and Petra Degen, Willi Lühs and R. Marquard for help with the fatty acid analyses.
Funding Statement
This study received financial support from Australian Research Council, University of New England. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hickman CP, Roberts LS, Keen SL, Eisenhour DJ, Larson A, et al.. (2011) Integrated Principles of Zoology. New York. McGraw-Hill.
- 2. Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181. [DOI] [PubMed] [Google Scholar]
- 3. Geiser F, Turbill C (2009) Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96: 1235–1240. [DOI] [PubMed] [Google Scholar]
- 4.Lovegrove BG (2012) A single origin of heterothermy in mammals. In: Ruf T, Bieber C, Arnold W, Millesi E eds. Living in a seasonal world. Springer Verlag, Berlin, Heidelberg. 3–12.
- 5. Stawski C, Geiser F (2012) Will temperature effects or phenotypic plasticity determine the thermal response of a heterothermic tropical bat to climate change? PLoS ONE 7 (7): e40278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyer BB, Barnes BM (1999) Molecular and metabolic aspects of mammalian hibernation. Bioscience 49: 713–724. [Google Scholar]
- 7.Steinlechner S, Heldmaier G, Weber C, Ruf T (1986) Role of photoperiod: pineal gland interaction torpor control. In: Heller HC, Musacchia XJ and Wang LCH eds. Living in the Cold. New York. Elsevier. 301–307.
- 8. Geiser F, Kenagy GJ (1987) Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. Am J Physiol 252: R897–R901. [DOI] [PubMed] [Google Scholar]
- 9. Frank CL (1994) Polyunsaturate content and diet selection by ground squirrels (Spermophilus lateralis). Ecology 75: 458–463. [Google Scholar]
- 10. Geiser F, Heldmaier G (1995) The impact of dietary fats, photoperiod, temperature and season on morphological variables, torpor patterns, and brown adipose tissue fatty acid composition of hamsters, Phodopus sungorus . J Comp Physiol B 165: 406–415. [DOI] [PubMed] [Google Scholar]
- 11. Schalk G, Brigham RM (1995) Prey selection by insectivorous bats: are essential fatty acids important? Can J Zool 73: 1855–1859. [Google Scholar]
- 12. Florant GL (1998) Lipid metabolism in hibernators: the importance of essential fatty acids. Amer Zool 38: 331–340. [Google Scholar]
- 13. Dark J (2005) Annual lipid cycles in hibernators: integration of physiology and behaviour. Annu Rev Nutr 25: 469–497. [DOI] [PubMed] [Google Scholar]
- 14. Ruf T, Arnold W (2008) Effects of polyunsaturated fatty acids on hibernation and torpor: a review and a hypothesis. Am J Physiol 294: R1044–R1052. [DOI] [PubMed] [Google Scholar]
- 15. Hulbert AJ, Abbott SK (2012) Nutritional ecology of essential fatty acids: an evolutionary perspective. Aust J Zool 59: 369–379. [Google Scholar]
- 16. Arnold W, Ruf T, Frey-Roos F, Bruns U (2011) Diet-independent remodelling of cellular membranes precedes seasonally changing body temperature in a hibernator. PLoS ONE 6(4): e18641 Doi:10.1371/journal.pone.0018641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geiser F, McAllan BM, Kenagy GJ, Hiebert SM (2007) Photoperiod affects daily torpor and tissue fatty acid composition in deer mice. Naturwissenschaften 94: 319–325. [DOI] [PubMed] [Google Scholar]
- 18. Hill RW (1975) Daily torpor in Peromyscus leucopus on an adequate diet. Comp Biochem Physiol A 51: 413–423. [DOI] [PubMed] [Google Scholar]
- 19. Lynch GR, White SE, Grundel R, Berger MS (1978) Effects of photoperid, melatonin administration and thyroid block on spontaneous daily torpor and temperature regulation in the white-footed mouse, Peromyscus leucopus . J Comp Physiol B 125: 157–163. [Google Scholar]
- 20. Tannenbaum MG, Pivorun EB (1989) Summer torpor in montane Peromyscus maniculatus . Am Midl Nat 121: 194–197. [Google Scholar]
- 21. Figala J, Hoffmann K, Goldau G (1973) Zur Jahresperiodik beim djungarischen Zwerghamster Phodopus sungorus Pallas. Oecologia 12: 89–118. [DOI] [PubMed] [Google Scholar]
- 22. Heldmaier G, Steinlechner S (1981) Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus . Oecologia 48: 265–270. [DOI] [PubMed] [Google Scholar]
- 23. Ruf T, Klingenspor M, Preis H, Heldmaier G (1991) Daily torpor in the Djungarian hamster (Phodopus sungorus): interactions with food intake, activity, and social behaviour. J Comp Physiol B 160: 609–615. [Google Scholar]
- 24.Diedrich V, Steinlechner S (2012) Spontaneous daily torpor versus fasting-induced torpor in the Djungarian hamster (Phodopus sungorus): two sides of a medal or distinct phenomena? In: Ruf T, Bieber C, Arnold W, Millesi E eds. Living in a seasonal world. Berlin, Heidelberg. Springer Verlag. 231–242.
- 25. Stamper JL, Dark J, Zucker I (1999) Photoperiod modulates torpor and food intake in siberian hamsters challenged with metabolic inhibitors. Physiol. Behav 66: 113–118. [DOI] [PubMed] [Google Scholar]
- 26. Hiebert SM, Hauser K, Ebrahim AJ (2003) Djungarian hamsters exhibit temperature-dependent dietary fat choice in long day. Physiol Biochem Zool 76: 850–857. [DOI] [PubMed] [Google Scholar]
- 27. Klingenspor M (2003) Cold-induced recruitment of brown adipose tissue thermogenesis. Experimental Physiology 88 1: 141–148. [DOI] [PubMed] [Google Scholar]
- 28. Jastroch M, Withers KW, Taudien S, Frappell PB, Helwig M, et al. (2008) Marsupial uncoupling protein 1 sheds light on the evolution of mammalian nonshivering thermogenesis. Physiol Genomics 32: 161–169. [DOI] [PubMed] [Google Scholar]
- 29. Swoap SJ, Gutilla MJ (2009) Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol 297: R769–R774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klingenspor M, Klaus S, Wiesinger H, Heldmaier G (1989) Short photoperiod and cold activate brown fat lipase in the Djungarian hamster. Am J Physiol 257: R1123–R1127. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann K (1978) Effects of short photoperiod on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus). J Reprod Fert 54: 29–35. [DOI] [PubMed] [Google Scholar]
- 32. Schlatt S, Niklowitz P, Hoffmann K, Nieschlag E (1993) Influence of short photoperiod on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus . Biol Reprod 49: 243–250. [DOI] [PubMed] [Google Scholar]
- 33. Hoffman RA (1964) Speculations on the regulation of hibernation. Mammalian Hibernation II. Ann Acad Sci Fenn A IV. Biologica 71: 199–216. [Google Scholar]
- 34.Barnes BM (1996) Relationship between hibernation and reproduction in male ground squirrels. In: Geiser F, Hulbert AJ and Nicol SC eds. Adaptations to the Cold: Tenth International Hibernation Symposium. Armidale. University of New England Press. 71–80.
- 35. Mzilikazi N, Lovegrove BG (2002) Reproductive activity influences thermoregulation and torpor in pouched mice, Saccostomus campestris . J Comp Physiol B 172: 7–16. [DOI] [PubMed] [Google Scholar]
- 36. Morrow G, Nicol SC (2009) Cool sex? Hibernation and reproduction overlap in the echidna. PLoS ONE 4 (6): e6070 doi:10.1371/journal.pone.0006070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiser F, Brigham RM (2012) The other functions of torpor. In: Ruf T, Bieber C, Arnold W, Millesi E eds. Living in a seasonal world. Berlin, Heidelberg. Springer Verlag. Pp 109–121.
- 38. McAllan BM, Feay N, Bradley AJ, Geiser F (2012) The influence of reproductive hormones on the torpor patterns of the marsupial Sminthopsis macroura: Bet-hedging in an unpredictable environment. Gen Comp Endocrinol 179: 265–276. [DOI] [PubMed] [Google Scholar]
- 39. Geiser F (1990) Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain, and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim Biophys Acta 1046: 159–166. [DOI] [PubMed] [Google Scholar]
- 40. Geiser F (1991) The effect of unsaturated and saturated dietary lipids on the pattern of daily torpor and the fatty acid composition of tissues and membranes of the deer mouse Peromyscus maniculatus . J Comp Physiol B 161: 590–597. [DOI] [PubMed] [Google Scholar]
- 41. Rojas AD, Körtner G, Geiser F (2012) Cool running: locomotor performance at low body temperature in mammals. Biol Lett 8: 868–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valencak TG, Arnold W, Tataruch F, Ruf T (2003) High content of polyunsaturated fatty acids in muscle phospholipids of a fast runner, the European brown hare (Lepus europaeus). J Comp Physiol B 173: 695–702. [DOI] [PubMed] [Google Scholar]
- 43. Giroud S, Perret M, Gilbert C, Zahariev A, Goudable J, et al. (2009) Dietary palmitate and linoleate oxidations, oxidative stress, and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation. Am J Physiol 297: R950–R959. [DOI] [PubMed] [Google Scholar]
- 44. Price ER, Armstrong C, Gugliemo CG, Staples JF (2013) Selective mobilization of saturated fatty acids in isolated adipocites of hibernating 13-lined ground squirrels Ictidomys tridecemlineatus . Physiol Biochem Zool 86: 205–212. [DOI] [PubMed] [Google Scholar]
- 45. Aloia RC, Raison JK (1989) Membrane function in mammalian hibernation. Biochim Biophys Acta 988: 123–146. [DOI] [PubMed] [Google Scholar]
- 46. Phillips R, Ursell T, Wiggins P, Sens P (2009) Emerging roles for lipids and shaping membrane-protein function. Nature 459: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]