Abstract
Objective
Reduced nitric oxide (NO) concentrations are found in the airways of many patients with cystic fibrosis (CF) and are associated with increased airflow obstruction. We determined whether upregulated whole body de novo arginine synthesis and protein breakdown are present as a compensatory mechanism to meet the increased demand for arginine and nitric oxide production in pediatric patients with CF and nutritional failure.
Study design
In 16 children with CF, studied at the end of antibiotic treatment for a pulmonary exacerbation, and 17 healthy controls, whole body arginine, citrulline, and protein turnover were assessed by stable isotope methodology and de novo arginine synthesis, arginine clearance, NO synthesis, protein synthesis and breakdown, and net protein balance were calculated. The plasma isotopic enrichments and amino acid concentrations were measured by LC-MS/MS.
Results
Increased arginine clearance was found in patients with CF (p<0.001) whereas whole body NO production rate and plasma arginine levels were not different. Whole body arginine production (P<0.001), de novo arginine synthesis, and protein breakdown and synthesis (P<0.05) were increased in patients with CF, but net protein balance was comparable. Patients with CF with nutritional failure (n=7) had significantly higher NO production (P<0.05), de novo arginine synthesis, citrulline production (P<0.001), and plasma citrulline concentration (P<0.05) and lower plasma arginine concentration (P<0.05) than those without nutritional failure (n=9).
Conclusions
Nutritional failure in CF is associated with increased NO production. However, upregulation of de novo arginine synthesis and citrulline production was not sufficient to meet the increased arginine needs leading to arginine deficiency.
Keywords: CF, protein turnover, arginine kinetics, NO synthesis, pediatrics
Low exhaled nitric oxide (NO) concentrations (FeNO) are often found in patients with Cystic Fibrosis (CF) (1) despite the presence of airway inflammation. Several studies found positive correlations between pulmonary function and FeNO in CF (1, 2), suggesting that the reduced NO levels in CF airways contribute to functional changes of the airways. It is well possible that diminished local or systemic arginine (Arg) availability is an important factor negatively affecting pulmonary NO production in CF. Increased conversion of arginine by the enzyme arginase in sputum (3) may account for substrate limitation for NO production in stable CF. If an increased need for Arg exists in stable CF patients, Arg production should be upregulated to meet its needs. Plasma Arg levels are preserved in stable CF but reduced during an acute inflammatory exacerbation (1, 4). Arg is considered a conditionally essential amino acid especially during growth and stressful metabolic conditions (ie, sepsis) (5), when Arg production from endogenous (de novo) Arg production (from Citrulline) and protein breakdown may not be sufficient and will reduce NO synthesis and muscle protein synthesis (6). We have found that in several inflammatory states, alterations in Arg metabolism contribute to muscle wasting (6) and that the presence of malnutrition reduces the response of Arg-NO metabolism to inflammation (7). We hypothesize that an upregulated de novo arginine synthesis and a stimulated protein breakdown are present in stable CF patients to meet the increased demand for arginase and NO production and that this is related to enhanced muscle protein wasting, particularly in those patients with nutritional failure. In the present study, we investigated whether whole body NO synthesis and (de novo) Arg production is increased in CF patients. Furthermore, we studied whether changes in whole body Arg and Cit metabolism in CF patients with and without nutritional failure were associated with alterations in protein metabolism.
METHODS
Sixteen pediatric subjects with CF admitted to Arkansas Children’s Hospital for a pulmonary exacerbation and 17 healthy controls were studied. The patients with CF were studied in clinically stable condition during the last days of a 14 days IV antibiotic treatment course. Lung function (forced expiratory volume in 1 second (FEV1)) was back or close to baseline values (highest FEV1 in past year). Exclusion criteria included diabetes mellitus and unstable metabolic (i.e liver and renal) diseases. The healthy subjects were recruited in the local community. Written informed consent was obtained and the study was approved by the Institutional Review Board.
Body weight was measured by a digital beam scale, and height by a stadiometer and both were expressed as percentiles. Whole body fat (FM) and fat-free mass (FFM) of the CF patients were obtained by dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500/Version 12.7.3.1; Bedford, MA) and in healthy subjects by bioelectrical impedance spectroscopy (Xitron 4000B, Xitron Technologies) and standardized for height to obtain FFM and FM index (%). The anthropometric and body composition data were compared with published reference data (8). Dietary intake was estimated by food frequency questionnaires. In the CF group, nutritional failure was defined as FFM index<5th percentile and/or BMI<10th percentile (age ≤ 20 years) (8). FEV1 and forced vital capacity (FVC) were measured by spirometry (nSpire Health, Longmont, CO) (9).
Patients with CF were studied in their hospital room whereas the healthy controls were examined on the metabolic ward after overnight fasting. For infusion of the stable isotopes (Table I; available at www.jpeds.com), an indwelling venous access port or antecubital vein was used. A blood sample was taken and the primed constant continuous tracer infusion started with a calibrated pump. A second catheter for blood sampling was placed in a superficial hand vein and a hotbox was used to obtain arterialized-venous blood (10). Infusion lasted for 2–3 hours with 3 blood samples in the last 30 min. Blood was treated as reported previously (5). Analysis for enrichment and concentrations was done by LC-ESI-MS (QTrap 5500MS; AB Sciex, Foster City, CA) with ExpressHT Ultra LC (Eksigent AB Sciex, Foster City, CA) after derivatization with 9-fluorenylmethoxycarbonyl (Fmoc) (11). Fmoc was fragmented to obtain specific and high sensitivity fragments. Whole body arginine, citrulline, and protein turnover were assessed by stable isotope methodology and de novo arginine synthesis, arginine clearance, protein synthesis and breakdown, and net protein balance were calculated. Plasma arginine (Arg) and citrulline (Cit) fluxes (Q) were calculated from the isotope enrichment values of L-[guanidine-15N2]Arg and L-[ureido-13C-2H2]Cit. NO synthesis was calculated by calculating the arginine to citrulline flux = QCit * TTRCit M+1/TTRArg M+2 where QCit is the plasma citrulline flux, estimated from the infusions of the L-[ureido-13C-2H2]Cit tracer, and TTRCit and TTRArg are the respective tracer-tracee ratios of L-[guanidino-15N]Cit and L-[guanidino-15N]Arg. All metabolic data were determined under steady-state conditions and subsequently calculated (5).
Table I.
online only. Infusion rates of stable isotopes
| Isotope | Priming (μmol/kg BW) | Infusion rate (μmol/kg BW/h) | |
|---|---|---|---|
| L-[guanidine-15N2]arginine | 15N2- Arg | 3.75 | 3.75 |
| L-[ureido-15N-2H2]citrulline | 15N-2H2-Cit | 0.88 | 0.30 |
| L-[ring-2H5]-phenylalanine | 2H5-Phe | 3.60 | 3.60 |
| L-[ring-2H2]-tyrosine | 2H2-Tyr | 1.14 | 1.14 |
| L-[ring-2H4]-tyrosine | 2H4-Tyr | 0.31 | - |
Primed, constant, and continuous infusion of the stable isotopes was done to measure whole body rate of appearance of Arg, Cit, Phe, and Tyr as well as the conversion of Phe to Tyr, Arg to Cit (nitric oxide production) and Cit to Arg (de novo Arg synthesis) (6, 13). The stable isotopes were purchased from Cambridge Isotopic Laboratories (Woburn, MA).
Statistical analyses
Results are expressed as mean ± standard error. The mean value of the measures of arginine and protein kinetics at the triple sample points was used. Data failing the normality or equal variance test were log-transformed where appropriate. One-way ANOVA was used to determine differences between the CF groups with and without nutritional failure and the control group, and Newman-Keuls was used for posthoc analysis. Unpaired Student t test was used to determine differences in clinical changes between the CF group with and without nutritional failure. The level of significance was set at p<0.05. The statistical package within Graphpad Prism (Version 5.04) and SPSS (version 20) was used for data analysis.
RESULTS
Age and height did not differ significantly between the CF groups with and without nutritional failure (Table II) but were lower in both groups as compared with the control group (P<0.01). BMI percentile was significantly lower in the CF group with nutritional failure as compared with those without nutritional failure (P<0.01). FFMI and FMI (as % of normal value) were not significantly different between the CF without nutritional failure and the healthy control groups but were lowest in the CF group with nutritional failure (P<0.01). FEV1 was not different between the CF groups with and without nutritional failure although FVC tended to be lower in the group with nutritional failure (P=0.06).
Table II.
General characteristics of the healthy control group and the CF groups with a normal nutritional status and with nutritional failure
| Healthy controls (n=17) | CF no nutritional failure (n=9) | CF nutritional failure (n=7) | |
|---|---|---|---|
| Sex (M/F) | 8/9 | 5/4 | 5/2 |
| Age (yrs) | 22.2 ± 5.6 | 15.5 ± 5.9*** | 15.2 ± 6.2*** |
| Genotypes (#) | DF508/DF508 (11), G551D/DF508 (2), DF508/G1244E (1), DF508/1717-1G TO, A (1), DF508/3272-26A>G (1). | ||
| Tanner stage (n: 1, 2–4, 5) | 1/5/3 | 0/6/1 | |
| Weight (kg) | 69.4 ± 17.4 | 54.6 ± 20.6 | 42.2 ± 17.2 |
| BMI (percentile) | 54.8 ± 20.7 | 7.9 ± 3.2## | |
| (kg/m2) | 22.0 ± 5.5 | ||
| Height (m) | 1.77 ± 0.44 | 1.61 ± 0.61 | 1.62 ± 0.66 |
| (percentile) | 37.0 ± 14.0 | 36.3 ± 14.8 | |
| FFMI (% norm) | 99.3 ± 24.8 | 99.0 ± 40.4 | 81.8 ± 33.4 |
| FMI (% norm) | 106.8 ± 26.7 | 104.7 ± 39.6 | 79.0 ± 32.2 |
| FEV1 (% pred) | 88.9 ± 33.6 | 74.6 ± 30.4 | |
| FVC (% pred) | 104.0 ± 39.3 | 88.7 ± 36.2 | |
| Weight change 3 mo. before admission (kg) | 0.7 ± 0.2 | −1.7 ± 0.7# | |
| Weight change admission until study day (kg) | 1.9 ± 0.7 | 1.9 ± 0.8 | |
| Exacerbations past year (nr) | 0.8 ± 0.3 | 3.6 ± 1.5# | |
| Hospitalizations past year (nr) | 0.4 ± 0.1 | 2.0 ± 0.8# | |
Values are means ± SEM. CF: cystic fibrosis; BMI: body mass index; FFMI: fat-free mass index; FMI: fat mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity. Significance of difference as compared with healthy controls:
P < 0.01,
P < 0.001, and to CF with no nutritional failure:
P < 0.05,
P < 0.01,
P < 0.001.
Clinical status and dietary intake in CF subgroups
Absolute change of body weight (Table II) in the preceding 3 months before hospital admission was higher in the CF group with nutritional failure (P<0.05) than in those with a normal nutritional status. Six CF patients with nutritional failure (86%) and 2 (25%) of with a normal nutritional status were characterized by weight loss prior to hospital admission. Weight change during hospital stay was not different between the groups. The number of exacerbations and hospital admissions in the preceding year before hospital admission were both significantly higher in the CF group with nutritional failure than in those with a normal nutritional status (P<0.05). Dietary intake (energy in kcal, protein in g and %, and carbohydrates and fat in %) between enrollment and study days and before hospital admission was not different between the CF groups with and without nutritional failure (Table III; available at www.jpeds.com).
Table III.
Dietary intake of the CF groups with and without nutritional failure
| CF no nutritional failure (n=9) | CF nutritional failure (n=7) | |
|---|---|---|
| Habitual dietary intake prior to hospital admission | ||
| Energy (kcal) | 3603 ± 1362 | 3548 ± 1448 |
| Protein (%) | 13.1 ± 5.0 | 11.7 ± 4.8 |
| (g) | 124.3 ± 47.0 | 102.8 ± 42.0 |
| CHO (%) | 58.0 ± 21.9 | 56.3 ± 23.0 |
| Fat (%) | 28.8 ± 10.9 | 32.7 ± 13.4 |
| Dietary intake between enrollment and study days | ||
| Energy (kcal) | 3185 ± 1300 | 2804 ± 1145 |
| Protein (%) | 12.3 ± 5.0 | 11.9 ± 4.8 |
| (g) | 100.7 ± 41.1 | 86.9 ± 35.5 |
| CHO (%) | 56.4 ± 23.0 | 56.7 ± 23.3 |
| Fat (%) | 31.3 ± 12.8 | 32.0 ± 13.1 |
Values are means ± SEM. CF: cystic fibrosis; CHO: carbohydrates. Significance of difference as compared to CF group with no nutritional failure
Arginine and Protein metabolism
Healthy controls vs. CF
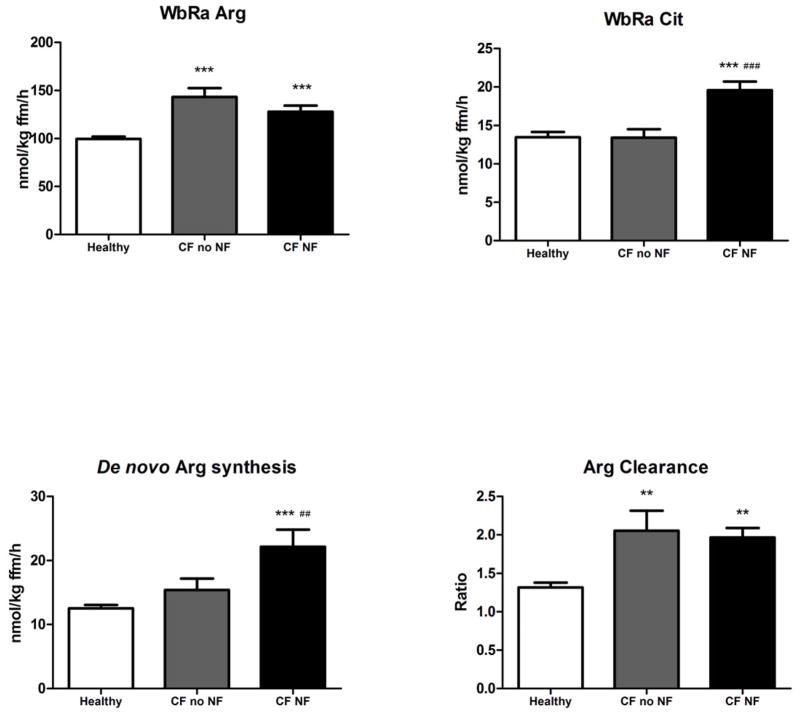
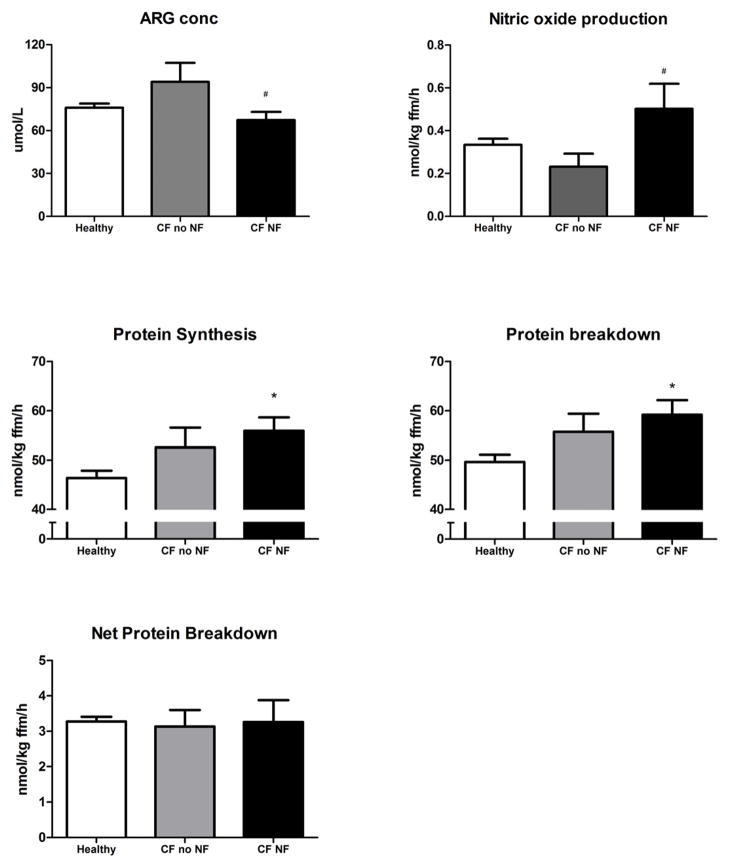
Plasma Arg concentration was not different between the whole CF and the healthy control groups (Table IV). Significantly higher values were found in the CF group as compared with the healthy subjects for whole body rate of appearance (WbRa) of Arg, Arg clearance (P<0.001), and the conversion of Cit to Arg (de novo arginine synthesis) (P<0.01). WbRa Cit tended to be higher in the CF group (P=0.08). WbPB and WbPS rates were higher in the CF group than in the healthy subjects (P<0.01) indicating elevated protein turnover. Net protein breakdown rates and the conversion of Arg to Cit (nitric oxide synthesis rate) were not significant different between the groups.
Table IV.
Arginine and protein kinetics in the healthy control group and the whole CF group
| Healthy controls (n=17) | CF (n=16) | |
|---|---|---|
| Arg concentration (μM) | 76 ± 3 | 78 ± 8 |
| WbRa Arg (nmol/kg FFM/h) | 99.58 ± 2.25 | 142.40 ± 8.70*** |
| WbRa Cit (nmol/kg FFM/h) | 13.44 ± 0.72 | 15.78 ± 1.12 (P=0.08) |
| QCit to Arg (nmol/kg FFM/h) | 12.55 ± 0.52 | 18.54 ± 1.75** |
| Arg clearance | 1.32 ± 0.06 | 2.01 ± 0.16*** |
| QArg to Cit (nmol/kg FFM/h) | 0.33 ± 0.02 | 0.36 ± 0.07 |
| Protein breakdown (nmol/kg FFM/h) | 49.63 ± 1.50 | 57.33 ± 2.36** |
| Protein synthesis (nmol/kg FFM/h) | 46.35 ± 1.49 | 54.13 ± 2.43** |
| Net protein breakdown (nmol/kg FFM/h) | 3.28 ± 0.13 | 3.19 ± 0.37 |
Values are means ± SEM. CF: cystic fibrosis; WbRa: whole body rate of appearance; Arg: arginine; Cit: citrulline; Q Cit to Arg: de novo arginine synthesis rate; QArg to Cit: nitric oxide synthesis rate; FFM: fat-free mass. Significance of difference between the healthy control and CF group:
P < 0.01,
P<0.001.
Effect of nutritional failure in CF
WbRa of Arg as well as Arg clearance (Figure 1, A and D) were higher in the CF group than in the control group independent of the presence of nutritional failure (P<0.001 vs. P<0.01, resp.). Both WbRa of Cit and de novo Arg production were highest in the CF group with nutritional failure (P<0.01; Figure 1, B and C). NO production (Figure 2, B) was higher in the CF group with nutritional failure as compare with those without nutritional failure (P<0.05). WbPS and WbPB were higher in the CF group with nutritional failure as compared with the healthy control subjects (P<0.05), but no differences were found in net protein breakdown between the 3 groups (Figure 2, C–E).
Figure 1.
A, Whole body arginine turnover, B, citrulline turnover, C, conversion of citrulline to arginine (de novo arginine production), and D, arginine clearance in healthy subjects (n=17, white bar), patients with CF without (n=9, gray bar) and with nutritional failure (n=7, black bar). Mean values ± SE are shown. Significance of difference as compared with the healthy group (**: P<0.01, ***: P<0.001) and as compared with the CF group with no nutritional failure (##: P<0.01, ###: P<0.001).
Figure 2.
A, Plasma arginine concentration, B, conversion of arginine to citrulline (NO synthesis, C, whole body protein synthesis, D, whole body protein breakdown, and E, net protein breakdown (=PB-PS) in healthy subjects (n=17, white bar), patients with CF without (n=9, gray bar) and with nutritional failure (n=7, black bar). Mean values ± SE are shown. Significance of difference as compared with the healthy group (*: P<0.05) and as compared with the CF group with no nutritional failure (#: P<0.05).
Plasma amino acid values
Lower values for Arg concentration (Figure 2, A) and higher values for Cit (P<0.05; Table V available at www.jpeds.com) were found in the group with nutritional failure as compared with those without nutritional failure. Higher Cit values were found in the CF group with nutritional failure than in the healthy group (P<0.05). Highest values were present for Orn in the CF group with normal nutritional status (P<0.05). Arg/Orn ratio (P<0.05) was lower in both CF compared with the healthy control group, and there was a tendency towards lower values for Arg/Orn+Lys ratio (P=0.07). Sum AA was elevated in both CF groups (P<0.01).
Table V.
online only. Differences in plasma amino acids concentrations between the healthy control group and the CF groups with a normal nutritional status and with nutritional failure
| Healthy controls (n=17) | CF no nutritional failure (n=9) | CF nutritional failure (n=7) | |
|---|---|---|---|
| Cit (μmol/L) | 34 ± 9 | 29 ± 11 | 42 ± 17*,# |
| Gln (μmol/L) | 586 ± 146 | 653 ± 247 | 643 ± 263 |
| Orn (μmol/L) | 51 ± 13 | 65 ± 24** | 57 ± 23 |
| Lys (μmol/L) | 156 ± 40 | 162 ± 61 | 174 ± 71 |
| Arg/Orn | 1.54 ± 0.39 | 1.26 ± 0.47* | 1.19 ± 0.49* |
| Arg/Orn+Lys | 0.38 ± 0.09 | 0.36 ± 0.13 | 0.30 ± 0.12 |
| Leu (μmol/L) | 110 ± 27 | 90 ± 34 | 98 ± 39 |
| BCAA (μmol/L) | 367 ± 92 | 324 ± 123 | 356 ± 145 |
| Sum EAA (μmol/L) | 961 ± 240 | 961 ± 363 | 973 ± 397 |
| Sum AA (μmol/L) | 2342 ± 585 | 2709 ± 1024** | 2657 ± 1085** |
Values are means ± SEM. CF: cystic fibrosis. Cit: citrulline; Gln: glutamine; Orn: ornitine; Lys: lysine; Arg: arginine; Leu: leucine. BCAA is the sum of valine, leucine and isoleucine. Sum EAA: Sum of essential amino acids represents the sum of histidine, isoleucine, leucine, valine, lysine, phenylalanine, Tyrosine, Threonine, Tryptophan, and Asparagine. Sum AA: sum of the concentration of the measurable α-amino acids (glutamine, glycine, threonine, histidine, citrulline, alanine, arginine, tyrosine, valine, methionine, isoleucine, phenylalanine, tryptophan, leucine, ornithine and lysine). Significance of difference as compared to healthy controls:
P < 0.05,
P < 0.01. Significance of difference as compared to CF no nutritional failure:
P < 0.05.
DISCUSSION
We found that children with CF were characterized by enhanced total and de novo arginine production and protein breakdown, and nutritional failure was associated with increased NO production. Despite further up-regulation of de novo arginine production in CF patients with nutritional failure, plasma arginine level was reduced suggesting an arginine deficient state. The CF group was studied at the end of 2 weeks of antibiotic treatment for a pulmonary exacerbation. Systemic inflammation is still expected as elevated circulating inflammatory mediators have previously been observed at the end of antibiotic treatment in CF (12). Moreover, the studied CF group was characterized by pulmonary inflammatory exacerbations in the year prior to the study. Inflammation is known to stimulate the activity of the NO and arginase enzymes (13). Arginase degrades Arg to urea and ornithine and can limit the availability of L-arginine for NO production in macrophages, suggesting a competition between arginases and NO synthases for their common substrate (14, 15). Previous studies showed higher sputum arginase activity in CF during a pulmonary exacerbation and after 2 weeks of intravenous antibiotic therapy (16). Although plasma arginase levels were elevated and Arg concentrations reduced during a pulmonary exacerbation, both were normalized after 2 weeks of antibiotic therapy (17). In line, we also found preserved plasma Arg concentrations in the CF group with a preserved nutritional status after antibiotic therapy. Furthermore, a significant reduction was found in the plasma concentration ratio of Arg to Orn, a tendency towards decreased values for plasma Arg/(Pro+Lys), as well as an increased Arg clearance in both CF groups suggesting an increased arginase activity in CF independent of the presence of nutritional failure.
Many studies have observed unchanged (18) or reduced exhaled NO (4, 19) in CF patients, suggestive for local airway NO synthesis deficiency. Low exhaled NO is associated with severity of pulmonary obstruction (20) in CF. Many factors are present in CF contributing toward a severely disturbed NO metabolism (4). NO synthase II (inducible NO synthase) expression is absent in bronchial epithelium of patients with CF (21), and increased levels for nitrotyrosine (4) and asymmetric dimethyl arginine (ADMA) were found in sputum of patients with CF (22). ADMA inhibits cellular arginine uptake and NO synthase activity and levels decrease during antibiotic therapy in association with improved lung function (22). Whole body NO production was comparable in the total CF and the healthy control groups, but interestingly higher values for whole body NO production were found in patients with CF with nutritional failure, suggesting that whole body NO production could still be upregulated in these patients. We measured the actual NO production in our patients at the end of treatment of an acute exacerbation, this after ADMA could have inhibited NO synthesis. As we found an increase in the NO synthesis in the depleted patients, we think that in the case of increased ADMA levels, this could have attenuated the observed increase in NO synthesis. Furthermore, the apparent discrepancy between the previously observed reduced FeNO (4, 19) and the observed unchanged or even increased systemic NO production in CF suggests that reduced exhaled NO levels in CF do not reflect systemic NO production. Other mechanisms (i.e. retention of NO in airways secretions, increased conversion of NO to metabolites and proteins, increased consumption of NO by denitrifying bacteria (23, 24) might therefore largely contribute to the observed reduction in FeNO in CF. Another potential explanation is that tissues outside the lungs are primarily responsible for the enhanced whole body NO production in patients with CF with nutritional failure. Systemic inflammatory mediators are known to be elevated in patients with CF and even more in those with low FFM (12). Our patients with CF with nutritional failure had more exacerbations in the preceding year compared with those with a normal nutritional status, and therefore the observed elevated whole body NO production in those patients might be related to increased systemic inflammation. More research is needed to quantify the relative contribution of pulmonary NO production to the enhanced whole body NO production in patients with CF with nutritional failure.
Plasma Arg levels were maintained in the patients with CF with preserved nutritional status indicating that Arg availability on a systemic level was maintained by stimulated whole body Arg production. Both arginine de novo production and enhanced whole body protein breakdown, the main sources of Arg production besides dietary Arg, were increased in the CF group with nutritional failure. The enhanced protein breakdown in these patients was balanced by an increased protein synthesis resulting in a comparable net protein loss in the CF and the healthy control groups. It has been suggested that in inflammatory conditions like CF, muscle proteins are broken down as an adaptation to provide amino acids for acute phase protein synthesis in the liver. The preserved plasma Arg levels in the CF group with preserved nutritional status suggest also an adaptive increase in total Arg production to meet the enhanced Arg requirements. Interestingly, nutritional failure in CF was associated with a further increase of de novo ARG production likely to generate more Arg for NO synthesis. In line, the production and concentration of Cit as precursor for de novo ARG production were elevated in this CF subgroup. Still, total Arg production was not different between the patients with CF with and without nutritional failure despite the higher Arg need for NO synthesis in the group with nutritional failure, which can be explained by the coupling between the enzymes of de novo ARG and NOS. Plasma Arg was significantly lower in the patients with CF with nutritional failure suggesting that the enhanced Arg need in this group is not balanced by enhanced production. Increased arginine requirements in CF could result from increased breakdown in the lung because of arginase released from immune cells, lung epithelium or bacteria from their stimulated NO production. To differentiate whether lung bacterial metabolism is an important factor in the observed reduced arginine levels was not possible in the present study. We previously observed similar findings in wound infection and reduced plasma arginine levels were found with unchanged wound fluid arginine levels (25). More research is needed regarding whether inadequate de novo Arg production is responsible for the reduced compromised plasma Arg levels (17) during periods of pulmonary inflammatory exacerbations in CF.
Nutritional failure commonly occurs in CF and is related to overall disease severity as reflected by impaired lung function, muscle weakness, and bone mineral loss (8, 26). Moreover, we found that nutritional failure in CF is associated with an increased frequency of exacerbations and hospitalizations. Protein breakdown is a major endogenous source of Arg whereas de novo Arg synthesis (from Cit) normally supplies about 10% to 15% of the total Arg flux (27). In the present study, higher values for whole body protein breakdown and synthesis were observed in subjects with CF indicating an elevated whole body protein turnover independent of the presence of nutritional failure. Net protein breakdown was comparable in both CF subgroups indicating no difference in net catabolism in the postabsorptive state. In previous studies, both reduced and elevated whole body protein breakdown and synthesis rates have been observed in patients with CF (28, 29). These conflicting data could be related to differences in the nutritional status as well as the clinical status of the patients and the tracer methodology that was used.
Limitation of the present study are that exhaled NO was not measured and the fact that no data were available on bioactive NO metabolites in sputum. In the conversion of arginine to NO, stochiometrical amounts of NO and citrulline are produced. Our stable isotope approach measures the synthesis of this citrulline and any further conversion of NO is not measured. FENO only reports a small fraction of NO production and therefore our data are not necessarily in conflict with previous data regarding reduced FENO in CF. Another potential limitation of the study is that the healthy controls were not well age-matched to the patients wih tCF (mean age was 22 vs. 15 y, respectively) and puberty in the CF group has an impact on anabolic/catabolic balance. However, it is unclear what age of control subjects would be the proper control group for the studied patients with CF as puberty sets in later in CF. In the present study, we conclude that stable patients with CF with nutritional failure are characterized by upregulated whole body NO synthesis. Despite the presence of accelerated de novo Arg synthesis, plasma Arg was reduced suggesting an Arg deficient state in these patients. Future studies are warranted to examine whether an Arg deficient state will also develop during pulmonary inflammatory exacerbations in the CF group with normal nutritional status and become even more severe in those with nutritional failure. If this is the case, a reduction in whole body and pulmonary NO synthesis is expected in CF, negatively affecting lung function and defense against bacterial infections.
Acknowledgments
Supported by the Arkansas Children’s Hospital Research Institute and the Arkansas Biosciences Institute (major research component of the Tobacco Settlement Proceeds Act of 2000), a partnership of scientists from Arkansas Children’s Hospital, Arkansas State University, the University of Arkansas for Medical Sciences, the University of Arkansas Division of Agriculture, the University of Arkansas, Fayetteville, and the National Institutes of Health (S10RR027047). Y. L. is an employee of Nutricia Advanced Medical Nutrition, Danone Research - Centre for Specialized Nutrition, Wageningen, The Netherlands.
Abbreviations
- Arg
arginine
- BMI
body mass index
- BCAA
branched-chain amino acids
- CF
Cystic Fibrosis
- Cit
citrulline
- DXA
dual-energy X-ray absorptiometry
- EAA
essential amino acids
- FEV1
forced expiratory volume in one second
- FFM
fat-free mass
- FM
fat mass
- FVC
forced vital capacity
- Gln
glutamine
- Leu
leucine
- Lys
lysine
- NetPS
net protein synthesis
- NO
nitric oxide
- Orn
ornithine
- PHE
phenylalanine
- Q Arg to Cit
nitric oxide production
- Q Cit to Arg
de novo Arg production
- Ra
rate of appearance
- SE
standard error
- SPE
splanchnic extraction of phenylalanine
- TAA
balanced mixture of essential and non-essential amino acids
- TYR
tyrosine
- TTR
tracer tracee ratio
- WbPB
whole body protein breakdown
- WbPS
whole body protein synthesis
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatric pulmonology. 1997;24:173–7. doi: 10.1002/(sici)1099-0496(199709)24:3<173::aid-ppul2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Grasemann H, Ioannidis I, Tomkiewicz RP, de Groot H, Rubin BK, Ratjen F. Nitric oxide metabolites in cystic fibrosis lung disease. Archives of disease in childhood. 1998;78:49–53. doi: 10.1136/adc.78.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. British Journal of Pharmacology. 2009;158:652–64. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrissey B, Schilling K, Weil J, Silkoff P, Rodman D. Nitric oxide and protein nitration in the cystic fibrosis airway. Arch Biochem Biophys. 2002;406:33– 9. doi: 10.1016/s0003-9861(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 5.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–52. doi: 10.3945/ajcn.2007.25765. [DOI] [PubMed] [Google Scholar]
- 6.Bruins MJ, Lamers WH, Meijer AJ, Soeters PB, Deutz NE. In vivo measurement of nitric oxide production in porcine gut, liver and muscle during hyperdynamic endotoxaemia. Br J Pharmacol. 2002;137:1225–36. doi: 10.1038/sj.bjp.0704993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poeze M, Bruins MJ, Luiking YC, Deutz NE. Reduced caloric intake during endotoxemia reduces arginine availability and metabolism. Am J Clin Nutr. 2010;91:992–1001. doi: 10.3945/ajcn.2009.27812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr. 2012;17 doi: 10.1016/j.clnu.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatric pulmonology. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 10.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–40. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 11.van Eijk HM, Suylen DP, Dejong CH, Luiking YC, Deutz NE. Measurement of amino acid isotope enrichment by liquid chromatography mass spectroscopy after derivatization with 9- fluorenylmethylchloroformate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:48–56. doi: 10.1016/j.jchromb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Ionescu AA, Nixon LS, Shale DJ. Cellular proteolysis and systemic inflammation during exacerbation in cystic fibrosis. J Cyst Fibros. 2004;3:253–8. doi: 10.1016/j.jcf.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. 2010;13:97–104. doi: 10.1097/MCO.0b013e328332f99d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher JL, Moali C, Tenu JP. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cellular and molecular life sciences: CMLS. 1999;55:1015–28. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, et al. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. British journal of pharmacology. 2002;136:391–8. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1523–8. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 17.Grasemann H, Schwiertz R, Grasemann C, Vester U, Racke K, Ratjen F. Decreased systemic bioavailability of L-arginine in patients with cystic fibrosis. Respir Res. 2006;7:87. doi: 10.1186/1465-9921-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho L, Innes J, Greening A. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J. 1998;12:1290– 4. doi: 10.1183/09031936.98.12061290. [DOI] [PubMed] [Google Scholar]
- 19.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1523– 8. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 20.Grasemann H, Kurtz F, Ratjen F. Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in cystic fibrosis patients. Am J Respir Crit Care Med. 2006;174:208–12. doi: 10.1164/rccm.200509-1439OC. [DOI] [PubMed] [Google Scholar]
- 21.Erskine JM, Lingard CD, Sontag MK, Accurso FJ. Enteral nutrition for patients with cystic fibrosis: Comparison of a semi-elemental and nonelemental formula. The Journal of pediatrics. 1998;132:265–9. doi: 10.1016/s0022-3476(98)70443-3. [DOI] [PubMed] [Google Scholar]
- 22.Grasemann H, Al-Saleh S, Scott JA, Shehnaz D, Mehl A, Amin R, et al. Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:1363–8. doi: 10.1164/rccm.201012-1995OC. [DOI] [PubMed] [Google Scholar]
- 23.Gaston B, Ratjen F, Vaughan JW, Malhotra NR, Canady RG, Snyder AH, et al. Nitrogen redox balance in the cystic fibrosis airway: effects of antipseudomonal therapy. Am J Respir Crit Care Med. 2002;165:387–90. doi: 10.1164/ajrccm.165.3.2106006. [DOI] [PubMed] [Google Scholar]
- 24.Grasemann H, Ratjen F. Cystic fibrosis lung disease: the role of nitric oxide. Pediatr Pulmonol. 1999 Dec;28:442–8. doi: 10.1002/(sici)1099-0496(199912)28:6<442::aid-ppul10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Debats IB, Booi D, Deutz NE, Buurman WA, Boeckx WD, van der Hulst RR. Infected chronic wounds show different local and systemic arginine conversion compared with acute wounds. J Surg Res. 2006;134:205–14. doi: 10.1016/j.jss.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Bolton CE, Ionescu AA, Evans WD, Pettit RJ, Shale DJ. Altered tissue distribution in adults with cystic fibrosis. Thorax. 2003;58:885–9. doi: 10.1136/thorax.58.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci U S A. 1996;93:11460–5. doi: 10.1073/pnas.93.21.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran A, Milla C, Ducret R, Nair KS. Protein metabolism in clinically stable adult cystic fibrosis patients with abnormal glucose tolerance. Diabetes. 2001;50:1336–43. doi: 10.2337/diabetes.50.6.1336. [DOI] [PubMed] [Google Scholar]
- 29.Hardin DS, LeBlanc A, Lukenbaugh S, Para L, Seilheimer DK. Proteolysis associated with insulin resistance in cystic fibrosis. Pediatrics. 1998;101:433–7. doi: 10.1542/peds.101.3.433. [DOI] [PubMed] [Google Scholar]