Abstract
Background
During vein graft adaptation to the arterial circulation, vascular endothelial growth factor (VEGF)-A expression transiently increases before becoming down-regulated; however the role of VEGF-A in venous remodeling is not clear. In addition, although VEGF-A stimulates angiogenesis and determines arterial identity in nascent arterial endothelial cells (EC), the role of VEGF-A in regulating identity in adult venous EC is also not clear.
Materials and Methods
EC, wild type (EphB4+/+) or heterozygous knockout (EphB4+/−), were stimulated with VEGF-A (0–100ng/ml) and examined with qPCR and Western blotting.
Results
VEGF-A (100ng/ml) inhibited expression of EphB4 and stimulated expression of dll4 but did not stimulate either notch or EphrinB2 expression in adult venous EC. Pretreatment with VEGFR2 neutralizing antibody abolished VEGF-stimulated down-regulation of EphB4 but not the up-regulation of Dll4. Pretreatment with PD98059 or wortmannin showed that VEGF-A down-regulation of EphB4 and up-regulation of dll4 are MEK-ERK-dependent but PI3k-Akt-independent. Compared to VEGF-induced EphB4 down-regulation and Dll4 up-regulation in control EC, reduced EphB4 signaling in EphB4+/− EC showed even further down-regulation of EphB4 and up-regulation of dll4.
Conclusions
Despite the genetic programming of arterial and venous EC fate, VEGF-A can repress venous identity in adult venous EC without induction of arterial identity. These changes in adult EC in vitro recapitulate the changes in identity described during vein graft adaptation to the arterial environment in vivo.
Keywords: VEGF-A, EphB4, EphrinB2, dll4, endothelial cells
Vein graft implantation into the arterial environment for surgical bypass is the gold standard to treat severe cardiovascular occlusive disease. After placement of a vein into the higher pressure, flow, and oxygen tension of the arterial circulation, the vein adapts to the arterial environment.1–2 Vein graft adaptation is characterized by wall thickening with deposition of smooth muscle cells and extracellular matrix; this thickening occurs in all layers of the vein graft and especially in the intima.3 We have previously shown that vein graft adaptation is also characterized by the loss of venous identity without gain of arterial identity, i.e. diminished EphB4 expression without induction of EphrinB2 expression.4–5 EphB4, a member of the trans-membrane receptor tyrosine kinase family is a determinant of venous fate during embryonic development, whereas its ligand EphrinB2 is a determinant of arterial fate; interestingly, both EphB4 and EphrinB2 persist on adult veins and arteries, respectively.6–7 Although the functions of EphB4 and EphrinB2 in adult cells are unknown, we have previously shown that EphB4 inhibits neointimal thickening of vein grafts, suggesting an active role for EphB4 in the limitation of venous wall thickness in adult veins.8
Vascular endothelial growth factor (VEGF) is a family of indispensable signal proteins particularly prominent in all aspects of vascular development, with their normal function to stimulate both angiogenesis as well as arteriogenesis.9–10 VEGF-A is the earliest discovered member of the VEGF family, and plays critical functions in both vasculogenesis and angiogenesis. VEGF-A is an upstream stimulus of EphrinB2-EphB4 signaling,11–13 and is a critical determinant of arterial endothelial specification during embryogenesis14 and arteriogenesis in adult organisms.15 However, the role of VEGF-A, as well as its potential ability to regulate EphrinB2 and EphB4 during vein graft adaptation to the arterial environment is not well understood. We have previously shown that vein graft adaptation is characterized by both sustained downregulation of EphB4 expression, as well as by transient upregulation of VEGF-A expression (24–72 hours) prior to subsequent downregulation.8 Furthermore, Luo et al have shown that VEGF-A reduces vein graft intimal hyperplasia in a rabbit model.16 Based on this data, we hypothesized that VEGF-A is an upstream inhibitor of EphB4 expression and venous identity during vein graft adaptation. Therefore we examined the response of adult endothelial cells (EC) to VEGF-A treatment in vitro.
Materials and Methods
Antibodies and Reagents
Human recombinant VEGF-A165 was purchased from Peprotech (Rocky Hill, NJ). Phospho-VEGF Receptor2 (Tyr1054/1059) antibody was purchased from Cell Application, Inc (San Diego, CA). Neutralizing VEGFR2 antibody was purchased from R&D Systems (Minneapolis, MN). The following reagents and antibodies were purchased from Cell Signaling Technology (Boston, MA): PD98059, wortmannin, phospho-VEGF Receptor 2 antibody, phospho-extracellular signal-regulated kinase (ERK)-1/2 antibody, GAPDH antibody. All the antibodies and reagents above were used according to the manufacturer’s instructions.
Cell culture
Mouse lung EC were isolated from 3-wk-old C57BL/6 mice (Harlan) as previously described.17 EphB4 heterozygous-knockout (EphB4+/−) EC were similarly isolated from EphB4+/− mice (The Jackson Laboratory).5 Primary EC were immortalized by infection with retrovirus encoding the middle T antigen, and propagated in EBM2/EGM-2 MV SingleQuot Kit Supplement & Growth Factors (Lonza) supplemented with 15% fetal bovine serum (Hyclone), Penicillin-streptomycin, and L-glutamine in 5% CO2 at 37°C. Culture media was changed every 2 days. For each experiment EC were split into 6-well plates (35mm diameter per well) at a density of 2×105/well and used when the cell density appeared to be approximately 90% confluence. EC were starved with EBM2 containing 0.2%–0.5% fetal bovine serum for 24h before VEGF-A treatment.
RNA extraction and RT quantitative PCR
Total RNA was isolated from cells and RNA was cleaned using the RNeasy Mini kit (QIAGEN). For quantification of total RNA, each sample was measured using the Nanodrop 2000c (Thermo Scientific), and RNA quality was confirmed by the 260/280nm ratio. RT was performed using the SuperScript III First-Strand Synthesis Supermix (Invitrogen) according to the manufacturer’s instructions. Real-time quantitative PCR was performed using SYBR Green Supermix (Bio-Rad Laboratories) and amplified for 39 cycles using the iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories). Correct target amplification and exclusion of nonspecific amplification was confirmed by 1.5% agarose gel electrophoresis, and primer efficiencies were determined by melting curve analysis. All samples were normalized to GAPDH and/or beta-actin. Primers are listed in Table 1.
Table 1.
Primers used for Gene Expression Analysis by Real-Time PCR
| Gene | Forward Primers, 5′-3′ | Reverse Primers, 5′-3′ |
|---|---|---|
| EphB4 | CAGGTGGTCAGCGCTCTGGAC | ATCTGCCACGGTGGTGAGTCC |
| EphrinB2 | CTGTGCCAGACCAGACCAAGA | CAGCAGAACTTGCATCTTGTC |
| Dll4 | AAGGTGCCACTTCGGTTACAC | AATGACACATTCGTTCCTCTCTT |
| VEGFR2 | AGTCTACGCCAACCCTCC | CATTCTTTACAAGCATACGG |
| Notch1 | ATGACTGTGCCAGTGCCGCC | AGGGTTGGCACCCAGAGCAC |
| Notch4 | TGCGAACATGGCGGCTCCTG | GCGCCGGTGAATCCAGGAAGG |
| Osteopontin | GTCCCTCGATGTCATCCCTG | TGATCAGAGGGCATGCTCAG |
| ERK1 | TTTGGCCTGGCCCGGATTGC | TCCTCGGCCACTGGCTCATCTG |
| ERK2 | GTGATGAGCCCATTGCTGAAGCG | ACGGGCTGAAGACAGGACCAGG |
| Neuropilin1 | GCGCTTTCCGCAGCGACAAAT | GAACTTCCCCCACAGGCGGC |
| Neuropilin2 | GAGTGGAAGCACGGGCGCAT | CATGGGTTTCTGGGATGTCCACTTT |
| GAPDH | AACGACCCCTTCATTGAC | TCCACGACATACTCAGCAC |
| Belta-actin | TTCTTTGCAGCTCCTTCGTTGCCG | TGGATGGCTACGTACATGGCTGGG |
Western blotting
Cells were harvested with extraction buffer including protease inhibitors (Thermo Scientific #78420) and sonicated for 5 seconds and centrifugation (13500rpm, 20 min). Equal amounts of protein from each treatment group were loaded for SDS-PAGE, and transferred onto nitrocellulose membranes followed by Western blot analysis. The membranes were probed with antibodies as described in Antibodies and reagents, without any clustering treatments. Membrane signals were detected using the ECL detection reagent (HyGlo Quick Spray E2400 Denville Scientific) and the film processor SRX-101A (Konica Minolta).
Statistics
Western-blot results were quantitated with Image J software (NIH). Statistical significance was determined using the t-test or analysis of variance (ANOVA) with appropriate post-hoc testing between groups. All tests were 2-tailed and a p-value ≤ 0.05 was considered statistically significant (Prism 5.0, Graphpad Software).
Results
VEGF-A down-regulates EphB4 expression without inducing EphrinB2 expression
We have previously shown that adult mouse lung EC are characterized by EphB4 expression.5 To determine whether other Eph receptors are expressed in EC, we examined the expression of all 9 Eph-A and all 5 Eph-B receptors using RT-qPCR; EphB4 was the only Eph-B receptor mRNA detected, with only minimal detection of EphA2 mRNA (Figure 1A). Conventional PCR confirmed expression of both EphB4 and EphrinB2 expression (Figures 1B, 1C), consistent with a small vessel venous phenotype.18
Figure 1.
Expression of EphB4 and EphrinB2 in EC. (A) Bar graph shows detection of mRNA transcripts for Eph receptors using real-time quantitative PCR (qPCR). (B) Bar graph shows relative numbers of EphB4 and EphrinB2 transcripts. (C) Photomicrograph of 1.5% agarose gel confirms correct target amplification. Results were representative of samples from n=3 independent experiments.
To determine the effect of VEGF-A on EphB4 expression we stimulated adult EC with soluble VEGF-A (0–100ng/ml) for 6 hours. VEGF-A inhibited Eph-B4 expression in a dose-dependent fashion (Figure 2A). EphB4 mRNA expression was also down-regulated by VEGF-A in a time-dependent manner with its maximal effect at 12 hours (Figure 2B). Similarly, EphB4 protein expression was also decreased after 72 hours (Figure 2E). Although VEGF-A stimulates EphrinB2 expression in embryonic or stem cells,19–20 VEGF-A stimulation of adult EC resulted in no change in either EphrinB2 mRNA expression or protein expression (Figures 2C, 2F). Since osteopontin is highly upregulated during vein graft adaptation,5,21 we examined the effect of VEGF-A on osteopontin expression. VEGF-A stimulated osteopontin expression (Figure 3A). These results, e.g. reduced EphB4, no change in EphrinB2, and increased osteopontin expression, are similar to those seen during vein graft adaptation and suggest that VEGF-A actively represses venous identity in EC.8,5
Figure 2.
Effects of VEGF-A on expression of arterial and venous markers in adult EC. (A) Bar graph shows EphB4 mRNA transcript numbers, after treatment of VEGF-A (0–100 ng/ml) for 6 hours. n=3. (B–D) Bar graphs shows mRNA transcript numbers of (B) EphB4, (C) EphrinB2 and (D) dll4. n=5. (E–G) Representative Western blots and bar graphs showing densitometry of (E) EphB4, (F) EphrinB2, and (G) dll4 after VEGF-A treatment. Cells were continuously given VEGF-A (100ng/ml) for 72 hours and VEGF-A solution was replaced every 24 hours; the control group had no VEGF-A stimulation. n=5–9 independent experiments. * p<0.05,** p<0.01,*** p<0.001. A.U, arbitrary units.
Figure 3.
Effects of VEGF-A on mRNA transcript expression. (A–F) Bar graphs show mRNA transcript numbers of (A) osteopontin, (B) notch-1, (C) notch-4, (D) VEGFR2, (E) neuropilin-1, and (F) neuropilin-2. n=3–5 independent experiments. * p<0.05,** p<0.01,*** p<0.001. A.U, arbitrary units.
VEGF-A induces Dll4 but not Notch expression
We next examined the effects of VEGF-A on the delta-notch pathway in adult EC, since VEGF-A stimulation of Ephrin-Eph signaling is mediated by this intermediate signaling pathway, at least during embryonic development.22–24 We have previously shown that, in a rat model, vein graft adaptation is characterized by unchanged expression of the delta-notch pathway.8 Expression of dll4 mRNA was increased in a time-dependent manner by VEGF-A (Figure 2D); similarly, dll4 protein was also increased (Figure 2G). However, neither notch1 nor notch4 mRNA expression was induced by VEGF-A (Figures 3B, 3C).
VEGF-induced down-regulation of EphB4 is VEGFR2-dependent
VEGF-A signaling is transduced by two high-affinity VEGF tyrosine kinase receptors, VEGFR1 (flt1) and VEGFR2 (flk-l/KDR).25 Although the function of VEGFR1 is still not clear,26 VEGFR2 is the principal receptor of VEGF-A in EC.27 Therefore we examined the effect of VEGF-A on VEGFR2 expression; no change of VEGFR2 expression was detected (Figure 3D). However, neuropillin-1 and neuropillin-2, co-receptors for VEGFR2, were both transiently up-regulated but afterwards down-regulated by VEGF-A (Figures 3E, 3F).
EC were stimulated with VEGF-A in the presence or absence of a VEGFR2 neutralizing antibody that inhibits VEGFR2 phosphorylation. This neutralizing antibody abolished VEGF-stimulated down-regulation of EphB4 expression (Figure 4A) but did not inhibit the VEGF-stimulated up-regulation of dll4 expression (Figure 4B). These results suggest that VEGF-stimulated down-regulation of EphB4 is VEGFR2-dependent but also suggests that VEGF-stimulation of dll4 expression may involve a VEGFR2-independent pathway in adult EC.
Figure 4.
Involvement of VEGFR2 and ERK but not Akt signaling. (A,B) Bar graph shows (A) EphB4 and (B) dll4 transcripts after pretreatment (1 hr) with VEGFR2 neutralizing antibody (1.0ug/ml) and then VEGF-A (100ng/ml) treatment (6 hr). (C,D) Bar graph shows (A) EphB4 and (B) dll4 transcripts after pretreatment (1 hr) with PD98059 (100uM) and then VEGF-A (100ng/ml) treatment (6 hr). (E,F) Bar graph shows (A) EphB4 and (B) dll4 transcripts after pretreatment (1 hr) with wortmannin (1uM) and then VEGF-A (100ng/ml) treatment (6 hr). n=3–5 independent experiments. ** p<0.01, *** p<0.001. A.U, arbitrary units.
VEGF-induced down-regulation of EphB4 and up-regulation of Dll4 are both ERK dependent but PI3k-AKT independent
Since extracellular-regulated kinase (ERK)-1/2 and phosphatidylinositol-3 kinase (PI3K)-Akt are intracellular signaling pathways downstream of VEGF signaling, and they both play key roles in the determination of artery/vein specification28 as well as in the regulation of growth of arterial vessels,15 we examined whether ERK1/2 or PI3k-Akt signaling are active during VEGF-stimulated down-regulation of EphB4 and up-regulation of dll4 expression. EC were stimulated with VEGF-A in the presence or absence of PD98059, a MEK1/ERK inhibitor, or in the presence or absence of wortmannin, an inhibitor of PI3K phosphorylation. These two inhibitors had no effect on basal EphB4 and dll4 gene expression (Figures 4C–F). Pre-treatment with PD98059 abolished the down-regulation of EphB4 expression and up-regulation of dll4 expression (Figures 4C, 4D). However, wortmannin had no effect on VEGF-stimulated down-regulation of EphB4 expression nor on the up-regulation of dll4 expression (Figures 4E, 4F). These results show that VEGF-induced down-regulation of EphB4 and up-regulation of Dll4 in adult venous EC are both ERK-dependent but PI3k-Akt-independent.
EphB4 is a negative regulator of VEGF signaling
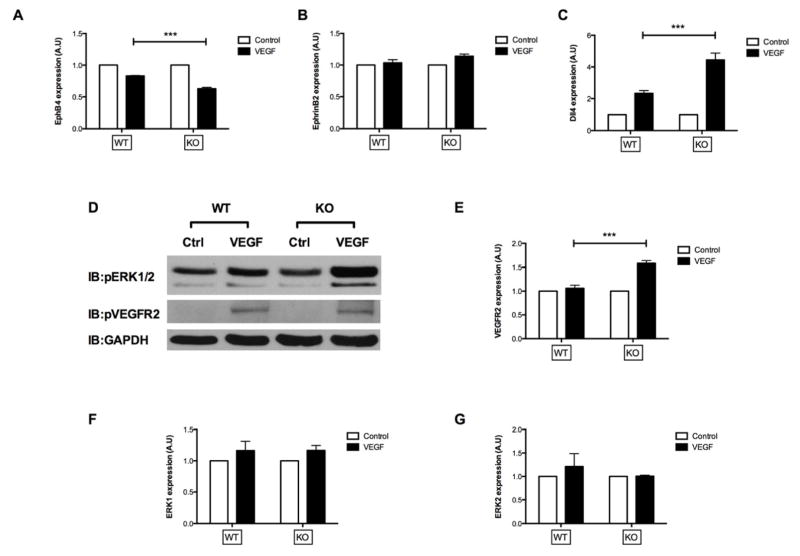
Since we hypothesize that EphB4 is a negative inhibitor of smooth muscle cell accumulation during vein graft adaptation,5 we also hypothesize that EphB4 should be a negative inhibitor of VEGF-A signaling in EC. To test the role of EphB4 as an inhibitor of VEGF signaling, we used heterozygous EphB4 knockout (KO) cells;29 these cells have previously been shown to have approximately 50% diminished EphB4 signaling.29,5 Compared to the VEGF-stimulated down-regulation of EphB4 expression in WT EC, VEGF-stimulation in KO cells resulted in even further down-regulation of EphB4 expression (Figure 5A). Similarly, compared to the VEGF-stimulated up-regulation of dll4 in WT EC, VEGF-stimulation in KO cells resulted in even further up-regulation of dll4 (Figure 5C). Interestingly, EphrinB2 expression was not changed by VEGF-A in either WT or KO cells (Figure 5B).
Figure 5.
Comparison of the effect of VEGF-A on WT (EphB4+/+) and KO (EphB4+/−) adult EC. (A–C, E–G) Bar graphs show mRNA transcript numbers of (A) EphB4, (B) EphrinB2, (C) dll4, (E) VEGFR2, (F) ERK1, and (G) ERK2 in response to VEGF-A stimulation (6 hr) in WT and KO cells. n=3–7 independent experiments. (D) Representative Western blots showing phosphorylation of ERK1/2 and VEGFR2 in WT and KO cells after VEGF-A stimulation (10 min). n=3. WT, wide type; KO, knockout. ** p<0.01, *** p<0.001. A.U, arbitrary units.
Since VEGFR2 and ERK1/2 signaling mediate VEGF-stimulated EphB4 and dll4 expression levels (Figure 4), we examined the effect of EphB4 on VEGF-stimulated ERK and VEGFR2 phosphorylation. There was no difference in baseline levels of phosphorylated ERK1/2 and VEGFR2 between WT and KO cells (Figure 5D). Although VEGF-A stimulated both ERK1/2 and VEGFR2 phosphorylation in both WT and KO cells, only ERK1/2 phosphorylation, but not VEGFR2 phosphorylation, was enhanced in EphB4 KO EC compared to WT EC (Figure 5D). Interestingly, effects on mRNA expression were different from those of protein phosphorylation, with enhanced VEGFR2 mRNA expression (Figure 5E) but not ERK1/2 expression (Figures 5F, 5G) in KO EC. Taken together, these results suggest that EphB4 is a negative inhibitor of some VEGF-A functions in adult venous EC.
Discussion
We show that VEGF-A decreases expression of EphB4 in adult venous EC, consistent with diminished venous identity, without concomitant induction of EphrinB2 expression (Figure 2); these changes recapitulate those changes during vein graft adaptation in human, rat, and mouse vein grafts.8,5 Interestingly, osteopontin expression is induced during vein graft adaptation21,5 and osteopontin expression is stimulated by VEGF-A in EC (Figure 3A). We also show that VEGF-A stimulates dll4 (Figure 2) but nothing downstream of it (Figure 3), consistent with a block in the delta-notch pathway in adult EC. In addition, we show that VEGF-A down-regulation of EphB4 is VEGFR2- and ERK1/2-dependent, but is Akt-independent (Figure 4). Lastly we show that EphB4 negatively regulates this pathway (Figure 5), although the exact point of regulation is not clear (Figure 6). These results confirm the importance of VEGF-A not just in promotion of arterial identity, but in repression of venous identity.
Figure 6.

Diagram of proposed signaling pathways that modulate phenotypic changes in venous EC in response to VEGF-A. Figure 2 shows the main pathway of VEGF downregulation of EphB4 and upregulation of dll4. Figure 3 shows the delta-notch block (red X). Figure 4 shows the possible non-canonical activation of dll4, questioning the activity of the canonical pathway in these cells (red ?). Figure 5 shows the negative feedback of EphB4 on the VEGF downregulation of EphB4 and upregulation of VEGFR2 and dll4, although the proposed site of feedback (red dashed line) is not clearly established.
VEGF-A down-regulation of EphB4 has been previously reported in ES cells, HUVEC, and transformed EC;19–20 however, we show that VEGF-A can also down-regulate EphB4 in adult EC. Interestingly, we also show that VEGF-A stimulates dll4 expression, but neither notch nor EphrinB2 expression, in adult EC. These results are consistent with some plasticity of adult venous EC, with ability to lose venous identity, but inability to gain arterial identity. Although VEGF-A stimulates arterial differentiation and prevents venous fate in embryogenesis,19,11,20,30 VEGF-A fails to stimulate arterial identity in adult EC. The VEGF-A-dll4-notch-EphrinB2 pathway appears to be blocked at the dll4-notch step. These results show that, at least in adult EC, loss of venous identity does not automatically confer an arterial identity. Our study is limited in that we only tested a single isoform of VEGF-A; it is possible that other VEGF family members may participate in Eph regulation or may stimulate notch and EphrinB2 expression. However, VEGF-A is the major isoform implicated in arterial differentiation31 and a delta-notch block has been previously reported in adult cells.32 Since both dll4 and notch-4 quiescence have been reported during vein graft adaptation in aged vein grafts,33 we believe that the differences between these findings may reflect differences between the in vitro and the in vivo models; nevertheless, both models suggest that delta-notch signaling may be active during vein graft adaptation.
Our finding that VEGF-A induced down-regulation of EphB4 is VEGFR2-dependent (Figure 4A) is not surprising, since VEGFR2 is the primary signaling receptor of VEGF-A in EC.34 However, our finding that VEGF-A induced up-regulation of dll4 expression is not inhibited by VEGFR2 inhibition suggests that a non-canonical VEGF-A signaling pathway that stimulates dll4 may exist. The transient upregulation and then downregulation patterns of neuropilin-1 and -2 induced by VEGF-A stimulation (Figure 3) may suggest that these receptors may have functions in this pathway, although it is not clear whether they are involved as either arterial or venous markers of phenotype or not.35 The VEGF165 isoform binds to neuropilins36 and these all function coordinately during angiogenesis and tumor development.37–39 However our data does not suggest a clear function for neuropilins in adult EC.
Our data suggests that VEGF induction of EphB4 down-regulation and Dll4 up-regulation are MEK/ERK dependent but PI3K/AKT independent (Figure 4). Previous studies have uncovered the opposing roles of PI3K and ERK1/2 in artery/vein specification and arteriogenesis. For example, ERK1/2 signaling is critical both in embryonic vascular development40 and arterial morphogenesis in adult tissues.41–43 Deficient ERK1/2 activity results in defective arterial morphogenesis, and restoration of which by suppressing PI3K is sufficient for recovery.15 Conversely, inhibiting PI3K promotes arterial specification whereas inhibition of ERK inhibits arterial specification, and expression of active Akt promotes venous specification during embryonic development.28 Our results are consistent with these reports, as inhibition of ERK1/2 signaling restores venous identity and inhibits gain of arterial identity; however, inhibition of PI3K/Akt signaling does not appear to be functional in adult EC, which may be an artifact of cell immortalization with middle T antigen.44 We used immortalized EC since primary mouse venous EC are difficult to keep alive and healthy in cell culture, and immortalized mouse venous EC are useful to understand mechanisms of mouse vein graft adaptation.5 However, it is possible that examination of primary venous EC may reveal additional pathways that are relevant, such as the PI3K/Akt pathway.
We also show that EphB4 participates in a negative feedback loop to VEGF-A, with reduced EphB4 signaling associated with augmented VEGFR2 expression, down-regulation of EphB4 and up-regulation of dll4 (Figure 5). We used EphB4 heterozygous knockout EC, isolated from mice heterozygous for EphB4, since homozygous deletion is embryonic lethal.29 The mutated EphB4 is prevented from membrane insertion,29 and we have previously confirmed approximately 50% reduction of extracellular EphB4 detection.5 These cells also show increased VEGF-induced ERK1/2 phosphorylation but not VEGF-induced VEGFR2 phosphorylation, consistent with EphB4 negative regulation of this pathway.18 However, it is not clear whether this negative regulation is functional in vivo during vein graft adaptation.
Our data suggests that VEGF-A inhibits venous identity in adult EC, recapitulating the change seen during vein graft adaptation in vivo. Vein graft adaptation to the arterial environment may depend on the plasticity of adult EC, and their ability to integrate VEGF signaling pathways, in order to properly modify the vessel phenotype. In addition we show that EphB4 is a negative repressor of VEGF-induced reduced EphB4 expression and increased dll4 expression. This finding may provide a basis for EphB4 retention of venous identity in adult EC.
Acknowledgments
This work was supported by the National Institute of Health (R01-HL095498 to A.D.); the American Vascular Association William J. von Liebig Award; as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson VJ, Cohen RG, Mitchell RS, Kosek JC, Miller DC. Biochemical (functional) adaptation of “arterialized” vein grafts. Ann Surg. 1986;203:339–45. doi: 10.1097/00000658-198604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush HL, Jr, Jakubowski JA, Curl GR, Deykin D, Nabseth DC. The natural history of endothelial structure and function in arterialized vein grafts. J Vasc Surg. 1986;3(2):204–15. doi: 10.1067/mva.1986.avs0030204. [DOI] [PubMed] [Google Scholar]
- 3.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74(8):1501–12. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fancher TT, Muto A, Fitzgerald TN, Magri D, Gortler D, Nishibe T, Dardik A. Control of Blood Vessel Identity: From Embryo to Adult. Ann Vasc Dis. 2008;1(1):28–34. doi: 10.3400/avd.AVDrev07011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto A, Yi T, Harrison KD, Dávalos A, Fancher TT, Ziegler KR, Feigel A, Kondo Y, Nishibe T, Sessa WC, Dardik A. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med. 2011;208(3):561–75. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 7.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 Selectively Marks Arterial Vessels and Neovascularization Sites in the Adult, with Expression in Both Endothelial and Smooth-Muscle Cells. Dev Biol. 2001;230(2):151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 8.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous Identity Is Lost but Arterial Identity Is Not Gained During Vein Graft Adaptation. Arterioscler Thromb Vasc Biol. 2007;27:1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi S, Asahara T, Masuda H, Isner JM, Losordo DW. Functional ephrin-B2 expression for promotive interaction between arterial and venous vessels in postnatal neovascularization. Circulation. 2005;111(17):2210–8. doi: 10.1161/01.CIR.0000163566.07427.73. [DOI] [PubMed] [Google Scholar]
- 12.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 13.Kume T. Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol. 2010;25(5):637–646. doi: 10.14670/hh-25.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act up-stream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 15.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120(4):1217–28. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Z, Asahara T, Tsurumi Y, Isner JM, Symes JF. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. J Vasc Surg. 1998 Jan;27(1):167–73. doi: 10.1016/s0741-5214(98)70304-0. [DOI] [PubMed] [Google Scholar]
- 17.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–27. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL, Yancopoulos GD, Gale NW, Koh GY. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin-1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 2002;16(9):1126–8. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- 19.Hainaud P, Contrerès JO, Villemain A, Liu LX, Plouët J, Tobelem G, Dupuy E. The Role of the Vascular Endothelial Growth Factor–Delta-like 4 Ligand/Notch4-Ephrin B2 Cascade in Tumor Vessel Remodeling and Endothelial Cell Functions. Cancer Res. 2006;66(17):8501–10. doi: 10.1158/0008-5472.CAN-05-4226. [DOI] [PubMed] [Google Scholar]
- 20.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear Stress Increases Expression of the Arterial Endothelial Marker EphrinB2 in Murine ES Cells via the VEGF-Notch Signaling Pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 21.Abeles D, Kwei S, Stavrakis G, Zhang Y, Wang ET, García-Cardeña G. Gene expression changes evoked in a venous segment exposed to arterial flow. J Vasc Surg. 2006;44(4):863–70. doi: 10.1016/j.jvs.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–9. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 23.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128(19):3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 24.Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98(10):5643–8. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggests FIk-l as a major regulator of angiogenesis and vasculogenesis. Cell. 1993;72(6):835–46. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95(16):9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18(5):713–24. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16(13):1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 30.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107(3):931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104(5):576–88. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 32.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 33.Kondo Y, Muto A, Kudo FA, Model L, Eghbalieh S, Chowdhary P, Dardik A. Age-related Notch-4 quiescence is associated with altered wall remodeling during vein graft adaptation. J Surg Res. 2011;171(1):e149–60. doi: 10.1016/j.jss.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19(10):2003–12. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109(1):115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- 36.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275(38):29922. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 37.Mac Gabhann F, Popel AS. Targeting Neuropilin-1 to inhibit VEGF signaling in cancer: Comparison of therapeutic approaches. PLoS Comput Biol. 2006;2(12):e180. doi: 10.1371/journal.pcbi.0020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narazaki M, Segarra M, Tosato G. Neuropilin-2: A New Molecular Target for Antiangiogenic and Antitumor Strategies. J Natl Cancer Inst. 2008;100(2):81–3. doi: 10.1093/jnci/djm305. [DOI] [PubMed] [Google Scholar]
- 39.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312(5):584–93. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE. 2006;355:pe39. doi: 10.1126/stke.3552006pe39. [DOI] [PubMed] [Google Scholar]
- 41.Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(2):227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 42.Sumpio BE, Yun S, Cordova AC, Haga M, Zhang J, Koh Y, Madri JA. MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J Biol Chem. 2005;280(12):11185–11191. doi: 10.1074/jbc.M414631200. [DOI] [PubMed] [Google Scholar]
- 43.Eitenmüller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99(6):656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 44.Dahl J, Jurczak A, Cheng LA, Baker DC, Benjamin TL. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J Virol. 1998;72(4):3221–6. doi: 10.1128/jvi.72.4.3221-3226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]