Abstract
Decreased elastin in mice (Eln+/−) yields a functioning vascular system with elevated blood pressure and increased arterial stiffness that is morphologically distinct from wild-type mice (WT). Yet, function is retained enough that there is no appreciable effect on life span and some mechanical properties are maintained constant. It is not understood how the mouse modifies the normal developmental process to produce a functioning vascular system despite a deficiency in elastin. To quantify changes in mechanical properties, we have applied a fiber-based constitutive model to mechanical data from the ascending aorta during postnatal development of WT and Eln+/− mice. Results indicate that the fiber-based constitutive model is capable of distinguishing elastin amounts and identifying trends during development. We observe an increase in predicted circumferential stress contribution from elastin with age, which correlates with increased elastin amounts from protein quantification data. The model also predicts changes in the unloaded collagen fiber orientation with age, which must be verified in future work. In Eln+/− mice, elastin amounts are decreased at each age, along with the predicted circumferential stress contribution of elastin. Collagen amounts in Eln+/− aorta are comparable to WT, but the predicted circumferential stress contribution of collagen is increased. This may be due to altered organization or structure of the collagen fibers. Relating quantifiable changes in arterial mechanics with changes in extracellular matrix (ECM) protein amounts will help in understanding developmental remodeling and in producing treatments for human diseases affecting ECM proteins.
Keywords: Constitutive modeling, Elastin, Developmental remodeling, Arteries, Arterial mechanics
1 Introduction
Elastin is an important extracellular matrix (ECM) protein responsible for providing elasticity to large arteries. It functions together with collagen to produce the classic nonlinear behavior observed in the arteries of vertebrates with closed circulatory systems (Shadwick 1999). The elasticity of the large arteries reduces the work done by the heart in expanding the arterial wall as the stroke volume is ejected during systole (Greenwald 2007). A major challenge in understanding how elastin and collagen contribute to the functional properties of the arterial wall is identifying how their unique material properties together influence the ability of the wall to respond to hemodynamic stress.
Mathematical constitutive modeling has been informative in defining the contribution of elastin and collagen to arterial mechanics. Early constitutive models were phenomenological in nature, treating the arterial wall as a homogeneous material [reviewed in (Vito and Dixon 2003)]. Later efforts focused on developing constitutive models that were based on the microstructure of the arterial wall [reviewed in (Holzapfel and Ogden 2010). Holzapfel et al. (2000) proposed a constitutive model using two families of collagen fibers symmetrically oriented with respect to the circumferential axis and embedded in an isotropic, elastin matrix. The model was later extended to include two additional collagen fiber families in the circumferential and axial direction (Baek et al. 2007). Modified versions of the four-fiber-family model have been used to fit mechanical data of arteries from wild-type (WT) (Hansen et al. 2009) and genetically modified mice with alterations in ECM proteins (Gleason et al. 2008; Eberth et al. 2009; Wan et al. 2010). While these models have been successfully applied to mature arteries, they have not yet been utilized to study arterial properties during development where hemodynamics as well as elastin and collagen amounts are changing.
In almost all animals with a closed circulatory system, elastin and collagen production in the artery wall begins with the onset of pulsatile blood flow and increases dramatically until blood pressure stabilizes postnatally. In WT mice, elastin and collagen expression in the aorta begins around embryonic day (E) 14, peaks around postnatal day (P) 14, and returns to low baseline levels by P30 (Kelleher et al. 2004). During this time, pressure and cardiac output increases significantly before reaching adult values around P30 (Huang et al. 2006; Ishii et al. 2001; Ishiwata et al. 2003; Wiesmann et al. 2000). Therefore, the protein amounts and consequent mechanical properties of the arterial wall change simultaneously with the applied hemodynamic loads.
This study uses a fiber-based constitutive model to quantify changes in the mechanical properties of the aorta in WT and elastin haploinsufficient (Eln+/−) mice from birth through adulthood. Including Eln+/− animals allows us to determine how changing the mechanical behavior of the arterial wall by genetically reducing elastin amount influences the functional contribution of both elastin and collagen throughout the developmental time course. Eln+/− mice live until adulthood, but have smaller arterial diameters and thinner walls (Wagenseil et al. 2005). These mice also have increased lamellar units and increased systemic blood pressure, similar to human patients with supravalvular aortic stenosis (SVAS) (Li et al. 1997). Despite these changes, the stress–strain behavior of adult Eln+/− arteries is similar to WT(Wagenseil et al. 2005). Collagen levels are not increased to compensate for reduced elastin in adult Eln+/− arteries (Faury et al. 2003). The unique structural and mechanical adaptation of Eln+/− arteries suggests that the developing arteries are able to remodel and reach a new cardiovascular set point or homeostatic state that is “normal” for these animals, preventing the pathological remodeling expected with adult onset disease (Wagenseil and Mecham 2009).
We previously gathered mechanical data for developing WT and Eln+/− aorta, and some of these data were presented in Le et al. 2011). Parameter estimates in this study were made by fitting the model equations to the complete set of experimental data with constraints on the relative elastin and collagen stress contributions in the circumferential direction. The constraints ensure that elastin contributes at least 30 % at low pressures and collagen contributes at least 75 % at high pressures, as shown in different adult arteries from various species (Dobrin and Canfield 1984; Ferruzzi et al. 2011; Fonck et al. 2007). The constraints also allow the definition of “low” and “high” pressure to be defined separately for each age and genotype. As elastin levels decrease from 100 to 50 to 35 % of normal in adult mice, the overall shape of the arterial pressure-diameter curve changes minimally, except that the onset of arterial stiffening occurs earlier in animals with less elastin, implying that collagen fibers may be engaged at different pressures in the different genotypes (Hirano et al. 2007).
Our model predictions are compared to data for elastin and collagen protein amounts during development and in WT versus Eln+/− aorta. Our results show that changes in the constitutive model parameter for elastin correlate with changes in elastin amounts through development and in WT versus Eln+/− aorta. The model also predicts alterations in collagen fiber orientation and mechanical behavior that must be confirmed in future studies.
2 Methods
2.1 Experimental data
Mechanical data for mouse ascending aortas were previously gathered using a pressure myograph (Danish Myotechnology). The methods and results for a single inflation cycle at the in vivo length are presented in Le et al. 2011). Briefly, male C57BL/6J WT and Eln+/− mice (Li et al. 1998) at approximately postnatal day (P) 3, 7, 14, 21, 30, and 60 were used for all studies. The actual ranges after P3 were P7–8, P14–15, P21–24, P30–34, and P60–64 (N = 5–10 for each age and genotype). All protocols were approved by the Institutional Animal Care and Use Committee. Each aorta was excised and stored in physiologic saline solution (PSS) for 0–3 days before testing (Amin et al. 2011). For testing, each aorta was mounted in the myograph in PSS at 37 °C (Wagenseil et al. 2005) and subjected to three constant-length inflation cycles and three constant-pressure axial stretch cycles (Table 1). In the constant-length inflation cycles, the aorta was held at a constant length and the myograph system was programmed to cyclically inflate the aorta three times step-wise from 0 mmHg to a maximum of 90–175 mmHg, depending on age, at a rate of 1–2 mmHg/s. The rate is considerably slower than the physiologic loading rate of an adult mouse aorta (about 330 mmHg/s for a 40 mmHg pulse pressure at 600 bpm), but soft biologic tissues are generally insensitive to loading rates over about three orders of magnitude (Fung 1993). In the constant-pressure axial stretch cycles, the aorta was inflated to a constant pressure and cyclically extended three times from near the in vivo stretch ratio to above the in vivo stretch ratio by manually turning the micrometer attached to one of the artery mounting rods at a rate of approximately 20 µm/s. The in vivo stretch ratio (Supplemental Table 1) was determined by measuring the ratio of the in vivo and ex vivo lengths of each aorta from the base of the heart to the innominate artery using images taken before and after excision. The constant-pressure and maximum stretch values varied with the age of the mouse. Due to experimental difficulties, some aortas were subjected to more or less than six protocols, but parameter fitting was only performed for aortas subjected to a minimum of five and a maximum of seven loading protocols. Lumen pressure, outer diameter, axial force, and calculated axial stretch were recorded for each protocol at 1 Hz. Axial stretch with respect to the unloaded configuration was calculated based on the distance traveled by the artery mounting rod. After testing, the aorta was removed, cut into rings approximately 0.2 mm thickness and imaged to measure the unloaded dimensions (Supplemental Table 1). Assuming the aorta acts as an incompressible cylinder with no shear, the mean arterial wall stresses in the circumferential (σθθ) and axial (σzz) direction can be calculated from experimentally measured values using the relations:
| (1) |
| (2) |
where P is the internal pressure, f is the measured axial force, ri is the inner radius, and ro is the outer radius of the inflated aorta. The inner radius was not measured directly but was calculated by:
| (3) |
where Ro and Ri are the inner and outer radii of the unloaded aorta and λz is the axial stretch as defined in Eq. 7.
Table 1.
Mechanical test protocols for different aged specimens
| Age (days) |
Average systolic P from (Le et al. 2011) |
Constant-length inflation protocols | Constant-pressure axial stretch protocols |
PL and PH values (mmHg) for parameter fitting |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | Eln+/− | Max P (mmHg) |
Wait time (s) |
Step P (mmHg) |
Typical axial stretch |
P (mmHg) | Typical axial stretch |
WT | Eln+/− | |
| 3 | 31 | 33 | 90 | 8 | 9 | 1.1, 1.2, 1.3 | 20, 40, 60 | 1.1–1.3 | PL = 9 | PL = 9 |
| PH = 45 | PH = 45 | |||||||||
| 7 | 46 | 48 | 120 | 8 | 12 | 1.1, 1.2, 1.3 | 25, 50, 75 | 1.1–1.3 | PL = 12 | PL = 12 |
| PH = 60 | PH = 48 | |||||||||
| 14 | 57 | 65 | 140 | 9 | 14 | 1.1, 1.2, 1.3 | 30, 60, 90 | 1.1–1.3 | PL = 14 | PL = 14 |
| PH = 98 | PH = 70 | |||||||||
| 21 | 84 | 90 | 160 | 10 | 20 | 1.1, 1.25, 1.4 | 40, 80, 120 | 1.1–1.4 | PL = 20 | PL = 20 |
| PH = 120 | PH = 100 | |||||||||
| 30 | 99 | 108 | 175 | 12 | 25 | 1.1, 1.3, 1.5 | 50, 100, 150 | 1.1–1.5 | PL = 25 | PL = 25 |
| PH = 125 | PH = 100 | |||||||||
| 60 | 112 | 127 | 175 | 12 | 25 | 1.1, 1.3, 1.5 | 50, 100, 150 | 1.1–1.5 | PL = 25 | PL = 25 |
| PH = 125 | PH = 100 | |||||||||
For constant-length inflation cycles, the myograph system (Danish Myotechnology) was programmed to cyclically inflate the aorta three times from 0 mmHg to the maximum (max) pressure (P) in discrete pressure steps. Deflation occurred in a single step over 60 s for all aortas, allowing the aorta to fully return to the starting dimensions, while minimizing total cycle time. The maximum pressure avoids damage to the aorta from overinflation and ranges from 1.5 to 3 times the average systolic pressure for each age (Le et al. 2011). The wait time provides enough time for the system to stabilize at each pressure and allows operator intervention if necessary to correct diameter tracking problems. The pressure steps provide an overall rate of 1–2 mmHg/s from a starting pressure of 0 mmHg. The constant-length values were chosen to provide a range of axial stretch ratios at and above the in vivo value and were varied for each aorta. Typical values are presented above. The average in vivo axial stretch ratio is approximately 1.1 for all ages and genotypes (Supplemental Table 1). For constant- pressure axial stretch cycles, the aorta was cyclically lengthened manually three times from the same minimum and maximum axial stretches used in the inflation cycles by turning a micrometer attached to one of the artery mounting rods at a constant rate of approximately 20 µm/s. The constant-pressure values were chosen to provide a range of values above and below the physiologic value. PL and PH values were used to constrain the elastin and collagen circumferential stress contributions in the constitutive model for the “low” and “high” pressure regions. Note that the PL values are similar between genotypes, but PH is lower in Eln+/− aorta compared with WT at all ages above P3
For an axisymmetric cylinder subjected to nonlinear, large elastic deformation in the absence of shear, the inflation and extension of the aorta can be described by the deformation gradient (F), right Cauchy–Green strain (C), and the Green strain tensors (E):
| (4) |
| (5) |
| (6) |
where λi are the stretch ratios in each direction (r = radial, ϑ = circumferential, and z = axial)) defined by:
| (7) |
and r and R are the radii of the deformed and undeformed configurations, and l and L are the axial lengths in the deformed and undeformed configurations.
2.2 Model equations
This study uses a fiber-based constitutive model, similar to the four-fiber-family model that has been used to compare the passive mechanical data from carotid arteries of WT mice to genetically modified mice with alterations in ECM proteins (Gleason et al. 2008; Eberth et al. 2009; Wan et al. 2010). We have reduced the number of collagen fiber families from four (circumferential, axial, and symmetrically angled with respect to the circumferential axis) to two (symmetrically angled fibers only) to reduce the number of fitted parameters. The motivation for the reduction in parameters is to focus on a set of parameters that will be more directly relatable to physiologic changes in the aortic wall and to decrease the chance of obtaining non-unique parameter values. The original fiber-family model considered only two collagen fiber families, although with separate material properties for the medial and adventitial layers (Holzapfel et al. 2000).We have also included a passive smooth muscle cell (SMC) fiber family oriented in the circumferential direction that has been used to model SMC mechanics in human cerebral (Baek et al. 2007) and mouse carotid arteries (Hansen et al. 2009). SMCs have been shown to have a preferential circumferential orientation in mammalian aortas (Clark and Glagov 1985). We assume that the SMCs are completely passive during the mechanical tests, as similar tests on adult mouse arteries after killing the SMCs with KCN showed no significant effects on the mechanical behavior (Faury et al. 1999). We assume that the two collagen fiber families and the SMC fibers are embedded in an amorphous, isotropic matrix dominated by elastin.
For an incompressible cylinder, the relevant principle stresses can be calculated as:
| (8) |
where i = r, θ, or z and p is the Lagrange multiplier. Inflation and extension in the absence of shear require σrθ = σrz = σθz = 0.
The total strain energy function is represented by the sum of the individual structural constituents elastin (e), collagen (c), and SMCs (m):
| (9) |
Elastin is modeled as an isotropic, neo-Hookean solid:
| (10) |
where c1 is a material parameter and the first invariant is .
Collagen is modeled as two fiber families with exponential behavior:
| (11) |
where c2 and c3 are material parameters and I4 is the fourth invariant for the kth fiber family as defined by
| (12) |
where α represents the angle of the fibers in the unloaded configuration with respect to the circumferential direction. It is assumed that the two collagen fiber families are oriented at angles ±α. SMCs are modeled as a single, circumferentially oriented fiber family with exponential behavior:
| (13) |
where c4 and c5 are material properties and I4 is the fourth invariant, as defined in Eq. 12, for the single SMC fiber family oriented at α = 0°.
2.3 Material parameters
The material parameters (c1−c5) and unloaded collagen fiber angle (α) were determined by constrained nonlinear regression to minimize the error between the experimental and predicted circumferential and axial stress values for every data point, i, using the fmincon function in Matlab R2010b. The error is defined as:
| (15) |
The material parameters were constrained to the positive domain, and α was only allowed to vary between 0° and 90° with 0° aligning with the circumferential axis. Parameter fitting was performed on data for each individual aorta. Multiple initial parameter estimates were randomly generated to ensure a global minimum was found. Additionally, constraints on the relative stress contributions of elastin and collagen were implemented to ensure physiologically relevant contributions from these constituents. It was assumed that for the circumferential direction, elastin contributes 30% of the total stress at low pressures and collagen contributes 75 % of the total stress at high pressures. Specifically, the constraints:
| (16) |
were imposed where is the circumferential stress from elastin, σθθ is the total circumferential stress, is the circumferential stress from collagen, PL is the maximum pressure for the low-pressure region, and PH is the minimum pressure for the high-pressure region. The values of 30 and 75 % for the elastin and collagen contributions to the total circumferential stress were chosen from a previous study using a four-fiber-family model for mouse carotid arteries (Hansen et al. 2009). While the exact percentages and pressure limits may vary for different species and artery types, it is well-accepted that elastin and collagen contribute at different pressure ranges (Dobrin 1997). PL and PH were assumed to bracket the linear region of the pressure–diameter curve and were defined as the second derivative of an empirical equation (Fonck et al. 2007) fitted to the pressure–diameter data for each aorta at the in vivo length:
| (17) |
| (18) |
where dout is the external diameter, P is the measured pressure, and a, b, c, and d are fitted constants. The second derivative was calculated for each discrete pressure step for every aorta and averaged for each age and genotype. The pressure steps that corresponded with the maximum and minimum of the average second derivative values were used to obtain PL and PH, respectively, for each group. The average values (Table 1) were subsequently used to enforce the relative stress contributions when fitting material parameters for each aorta. To enforce the constraints in Eq. 16, a penalty function was included in the final minimization function:
| (19) |
where penalty(i) is a penalty function applied to each data point, i, to enforce the minimum circumferential stress contributions of elastin and collagen in the appropriate pressure range. The penalty function is:
| (20) |
Without the penalty limits on elastin, parameter estimates may result in the material parameter, c1, being zero because the overall material behavior is not isotropic and neo-Hookean. While c1 = 0 may provide a good mathematical fit to the data, this result is biologically irrelevant because it is known that elastin contributes significantly to arterial mechanics in vertebrate animals (Dobrin 1997).Without the penalty limits on collagen, the nonlinear contribution to the mechanical stress in the circumferential direction can be contributed either by the SMCs or by the collagen fibers. Because the collagen fibers must also contribute in the axial direction, the best fit may be axially aligned collagen fibers with one set of parameters and circumferentially aligned SMCs with a different set of parameters. It is known that collagen fibers are not only aligned axially (Holzapfel 2006) and that SMCs are not the sole component contributing to the nonlinear circumferential behavior, so constraints are necessary to enforce physiologically relevant parameters.
2.4 Protein quantification
Desmosine, a cross-link specific to mature elastin, and hydroxyproline, an amino acid abundant in collagen, were quantified to determine changes in elastin and collagen amounts with age and genotype. Female littermates to the male mice used for mechanical testing were used for the protein quantification. Ascending aorta segments from the aortic valve to the innominate artery were harvested from WT and Eln+/− mice at each age from P3 to P60 (N = 5–6 for each group) and frozen at −20 °C until use. Vapor-phase acid hydrolysis for each aorta was performed with 6N constant-boiling HCl (Thermo Scientific) at 105 °C for 36 h. Acid was removed using a vacuum centrifuge and the samples suspended in Milli-Q ultra pure water and filtered using 0.45 µm Ultrafree-MC microcentrifuge tubes (Amicon). Hydrolyzed samples were prepared for analysis by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate using the AccQ-Fluor reagent kit (Waters).
Desmosine quantification was carried out using a competitive ELISA according to the methods developed by Rahman 2009). Purified desmosine, isodesmosine, rabbit primary antibody to desmosine/isodesmosine, and desmosine/isodesmosine conjugated to ovalbumin were provided by Dr. Barry Starcher (University of Texas Health Science Center, Tyler, Texas). Secondary peroxidase labeled goat anti-rabbit IgG antibody and microtiter plates were purchased as part of a commercially available ELISA kit (KPL).
Quantification of hydroxyproline was carried out by reverse phase HPLC. A Beckman HPLC, System Gold, equipped with a Programmable Solvent Module 126, Diode Array Detector Module 168, and Autosampler 508 was used in conjunction with a Waters AccQ.Tag 3.9 × 150 mm C18 Reverse phase Silica base analytical HPLC Column run at 39 °C. Sample detection was at 260 and 275 nm. A modification of the Waters amino acid analysis method was developed for the simultaneous separation and quantification of hydroxyproline with other amino acids in the protein hydrolyzate. Eluent A consisted of 400 ml of Waters 10× eluent buffer A, containing 19 % NaAc+ 1–3 % Triethylamine diluted in 4 l of mQ water (pH 5.13). Eluent B was 100 % Acetonitrile. The column was run at a flow rate of 1 ml/min starting with 100%eluent A followed by eluent B in a series of linear gradients as follows: from 1 to 2.5 % beginning at 0.5 min, from 2.5 to 5 % beginning at 16 min, from 5 to 9 % at beginning 21 min, from 9 to 17 % beginning at 22 min, and from 17 to 20%beginning at 32.5min. At 38min, eluent A was replaced by water. At 40 min, eluent B was increased to 60 % over 1 min, and at 43 min, eluent B was lowered to 17 % over 1 min. After 1 additional minute, water was replaced with eluent A, and at 45 min, eluent B was reduced to 0 % to re-equilibrate the column until data acquisition at 60 min.
2.5 Statistics
A general linear model (GLM) was used to determine the effects of age, genotype, and interactions between age and genotype on the fitted material parameters and protein quantification data. Comparisons between ages were performed using ANOVA followed by the Tukey’s HSD post-hoc test. Additional comparisons between genotypes at each age were performed using two-tailed t tests assuming unequal variance. Averages are presented as mean ± SD. All analyses were performed with SPSS software (IBM). P < .05 is considered
3 Results
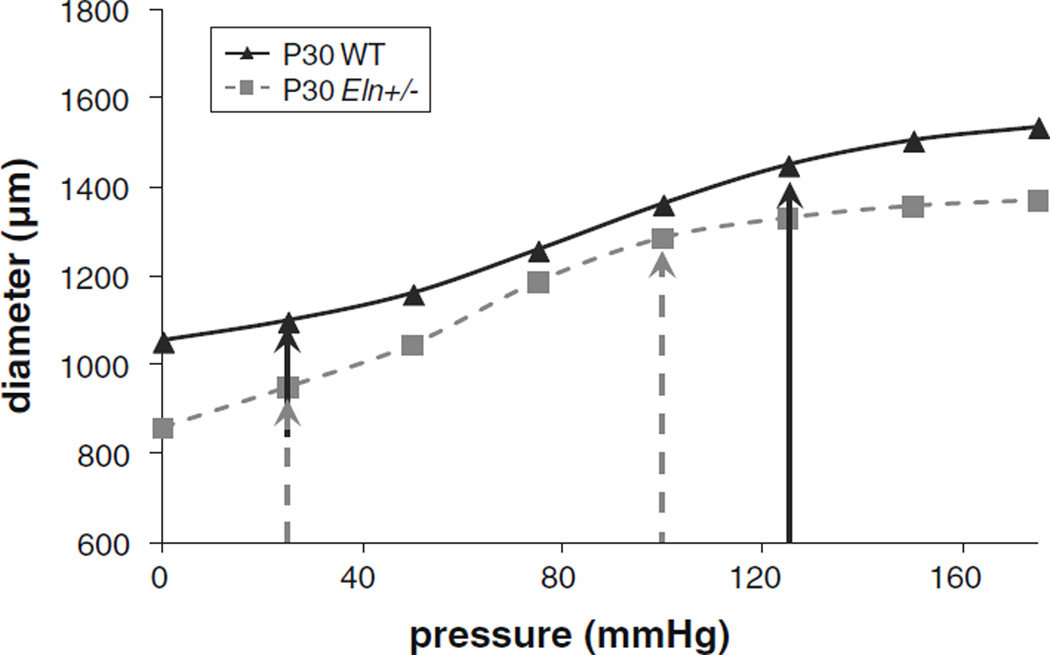
A pressure–diameter plot of representative P30 WT and Eln+/− aortas at the in vivo length shows the difference in mechanical behavior between the two genotypes (Fig. 1). Eln+/− aortas have significantly smaller diameters at most pressures. Arrows indicate the inflection points, PL and PH, used to define the pressure limits for the constraints on elastin and collagen contributions in the model. PL is similar between genotypes for all ages, while PH is 12–25 mmHg lower in Eln+/− aorta than WT for all ages above P3 (Table 1). PL and PH values were determined from the second derivative of an empirical equation (Eqs. 17–19) fitted to the pressure–diameter data of each aorta at the in vivo length. The penalty constraints enforce a minimum 30 % contribution of elastin toward the total circumferential stress in the low- to mid-pressure range (between PL and PH) and a minimum 75%contribution of collagen at high pressures (greater than PH).
Fig. 1.
Representative pressure versus diameter curves for a single inflation cycle at the in vivo length of P30 aortas from WT and Eln+/− mice (Le et al. 2011). Arrows indicate PL and PH as determined from the second derivative of an empirical equation fit to the data for each aorta (Eqs. 17–19). PL values are similar between genotypes, but PH is lower in Eln+/− aorta compared with WT. PL and PH were averaged for each age and genotype and used for constraining elastin and collagen contributions in the circumferential direction for the constitutive model. Axial contributions and SMC contributions were not constrained in the model
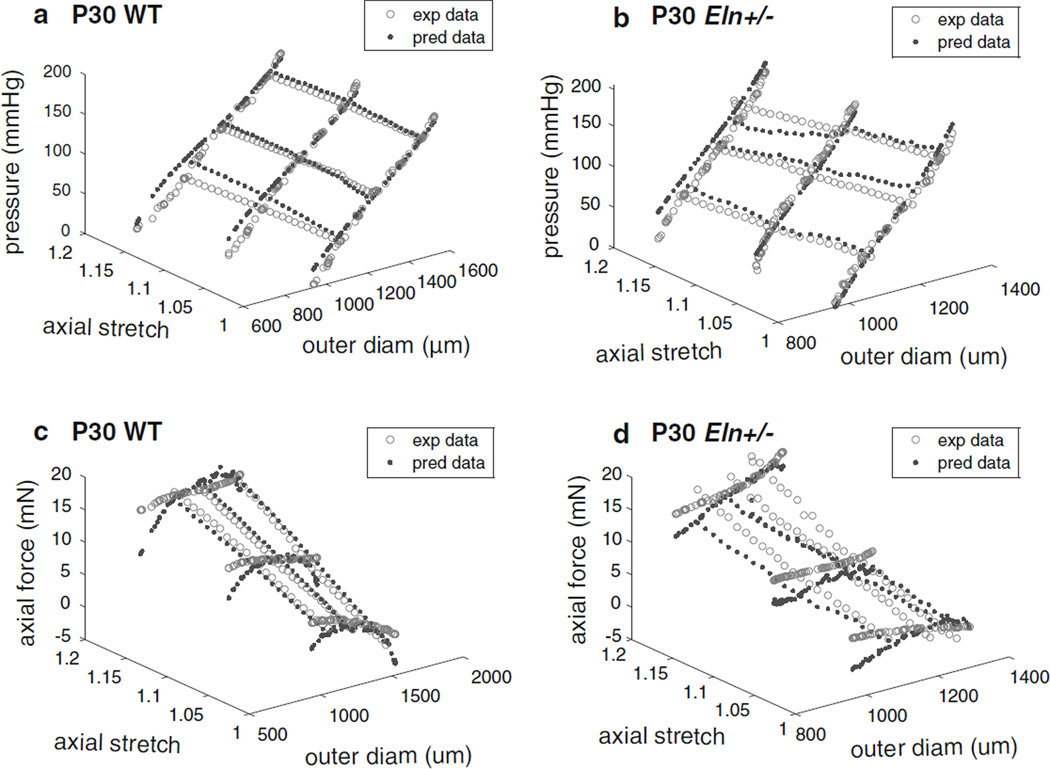
The experimental data and modeling results for all six mechanical testing protocols for representative P30 WT and Eln+/− aortas are shown in Fig. 2. Similar plots for all ages are shown in Supplemental Figs 1–4. Overall, the predicted pressure values follow the experimental pressures and seem to fit better at the older ages. The predicted force values often underestimate the experimental forces at the lowest and highest diameter values and also seem to fit better at the older ages. The underestimation of forces at high circumferential stretch values was also observed in the original two fiber-family model (Holzapfel et al. 2000) and may be a consequence of assuming isotropic elastin behavior or not including an individual family of collagen fibers oriented in the axial direction.
Fig. 2.
Experimental data (exp data) and model predictions (pred data) for the three constant-length inflation and three constant-pressure axial stretch mechanical test protocols for the same representative P30 aortas as Fig. 1 for WT (a, c) and Eln+/− (b, d) mice. Panels a and b show pressure versus outer diameter (diam) and axial stretch ratio, while panels c and d show axial force versus outer diameter and axial stretch ratio. Representative graphs for all ages are shown in Supplemental Figs 1–4
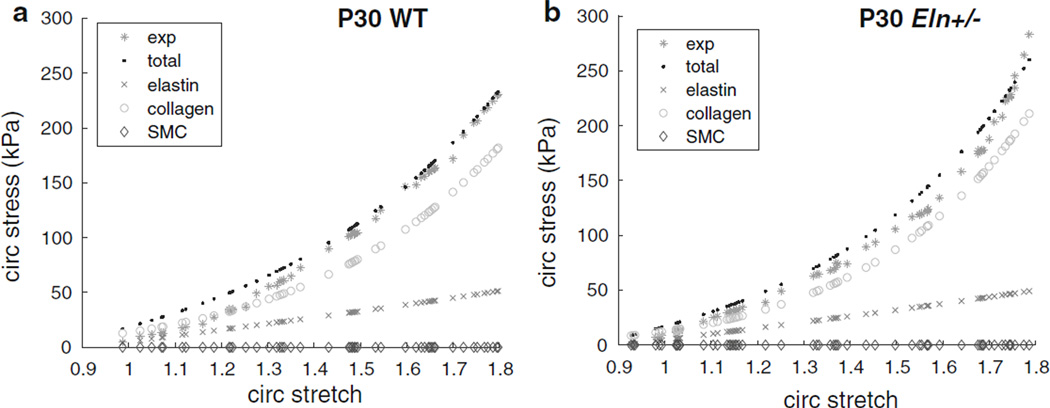
A plot of the predicted circumferential stresses for the same aortas as Fig. 2 against circumferential stretch for the inflation protocol at the in vivo length shows the contributions from individual artery constituents (elastin, collagen, and SMCs) compared with the total predicted and experimental stresses (Fig. 3). At the in vivo length, the aorta is stretched axially, decreasing the diameter compared with the unloaded state, so the starting circumferential stretch ratio can be <1. Similar plots for all ages are shown in Supplemental Figs 5 and 6. At circumferential stretches <1.4, the model overestimates the experimental stresses by 5–40 %, depending on the individual aorta. This is most likely due to the enforced minimum 30 % contribution of the linear, isotropic elastin to the total circumferential stress at low pressures and hence low stretch. At higher pressures and stretches, when the anisotropic, nonlinear collagen begins to contribute an enforced minimum of 75%to the total circumferential stress, the model usually matches the experimental stresses within 10 %. Predicted circumferential stress values for the SMCs are near zero, showing that as modeled, they do not contribute to the passive mechanical behavior of the mouse aorta. We chose to focus on the circumferential stress behavior at the in vivo length, because that is the most physiologically relevant loading configuration. However, predicted axial stress contributions for each constituent can also be compared. Graphs of the axial stress versus axial stretch ratio for the axial stretch protocols at different constant pressures show similar behavior to the circumferential direction (not shown). SMCs do not contribute to the axial stress, because they are assumed to be oriented in the circumferential direction. Elastin contributes linearly and is isotropic, so the axial behavior is identical to the circumferential behavior. Collagen contributes nonlinearly, and although the minimum contribution was not constrained, the model predicts that collagen contributes 60–80 % of the total axial stress at the higher axial stretch ratios.
Fig. 3.
Circumferential (circ) stress versus circumferential stretch ratio for the inflation cycle at the in vivo length for the same representative P30 aortas as Fig. 1 for WT (a) and Eln+/− (b) mice. Experimental stress (exp) and total predicted stress (total), as well as the predicted stress contributions of each constituent (elastin, collagen, and SMCs), are shown. The elastin contribution is slightly lower, while the collagen contribution is higher in Eln+/− aorta compared with WT. The predicted SMC contribution is minimal in both genotypes. Representative graphs for all ages are shown in Supplemental Figs 5–6
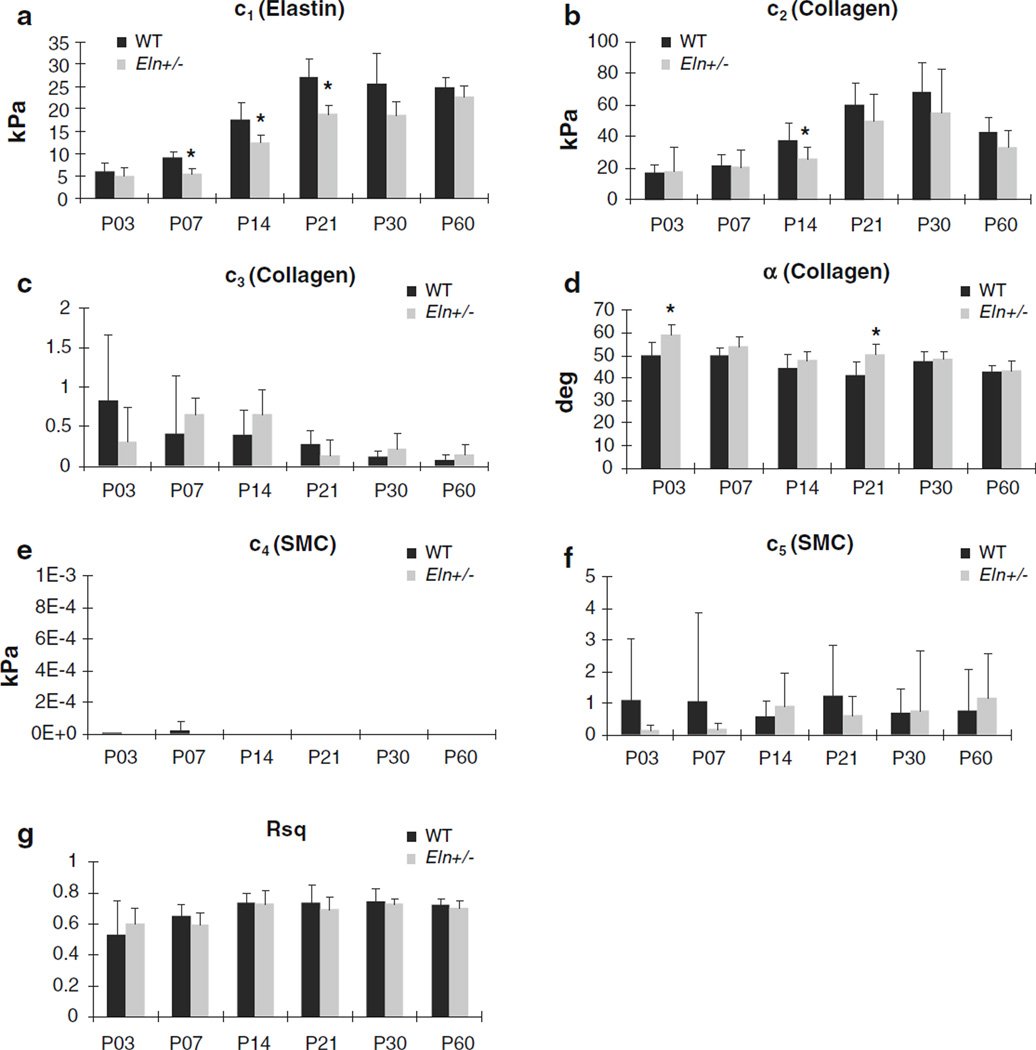
The fitted parameters for each individual aorta are shown in Supplemental Table 1. The average fitted material parameters and R2 values are shown in Fig. 4. The average material parameters for elastin, c1, and two of the parameters for collagen, c2 and α, are significantly affected by age (P < .001). For elastin, the parameter c1 increases between successive ages from P3 to P21 in WT (P < .02) and from P7 to P21 and P30 to P60 in Eln+/−aorta (P < .02). The parameter c1 approximately quadruples in both genotypes from P3 to P21. For collagen, the parameter c2 increases between successive ages from P7 to P21 in WT(P < .004) and from P14 to P21 in Eln+/− aorta (P < .002). The parameter c2 approximately triples in both genotypes from P3 to P21. From P30 to P60, c2 decreases 40 % in WT aorta (P < .02). The unloaded collagen fiber angle, α, decreases in angle, toward a more circumferential orientation, with age, and the effect is more pronounced in Eln+/− than in WT aorta. For example, α in P3 Eln+/−aorta is significantly different between every age after P7 (P < .0007), while α in P3 WT aorta is only significantly different between P14 and P60 (P < .04). Overall, α decreases from 50° at P3 to 43° at P60 in WT aorta and from 60° at P3 to 43° at P60 in Eln+/− aorta. The average R2 value is 0.69 ± 0.11 for all ages, showing a reasonable fit of the model to the experimental data. As suggested by Supplemental Figs 1–4, the average R2 value is 20 % lower for P3 and P7 (average = 0.58 ± 0.17) than the older ages (average = 0.72 ± .07) (P < .001), indicating that modifications to the model assumptions may be necessary to provide a better fit at the younger ages.
Fig. 4.
Average predicted parameter (a–f) and R2 (g) values for the constitutive model. Parameters were determined for individual WT and Eln+/− aortas via constrained nonlinear least squares regression and then averaged for each age and genotype. Parameters for each individual aorta are provided in Supplemental Table 1. For clarity, significant differences between ages are not shown, but are discussed in the text. The parameters c1, c2, and α are significantly affected by age (P < .001). * P < .05 between WT and Eln+/−. N = 5−10 for each age and genotype. Mean ± SD
In comparing material parameters across genotypes, the elastin parameter, c1, for Eln+/− aorta is 30–40 % less than WT beginning at P7 and continuing through P21 (P < .002). One of the collagen parameters, c2, is 30%lower in Eln+/− aorta compared with WT at P14 (P < .002). The unloaded collagen fiber angle, α, is 15–20 % higher in Eln+/− aorta compared with WT at P3 and P21 (P < .002).We found significant interactions between age and genotype for the elastin parameter, c1, and the unloaded collagen fiber angle, α (P < .03). Similar trends in parameter values, including differences between genotypes and ages, were observed when the model was fit to mechanical data from the left common carotid artery of WT and Eln+/− mice from P3 to P60.
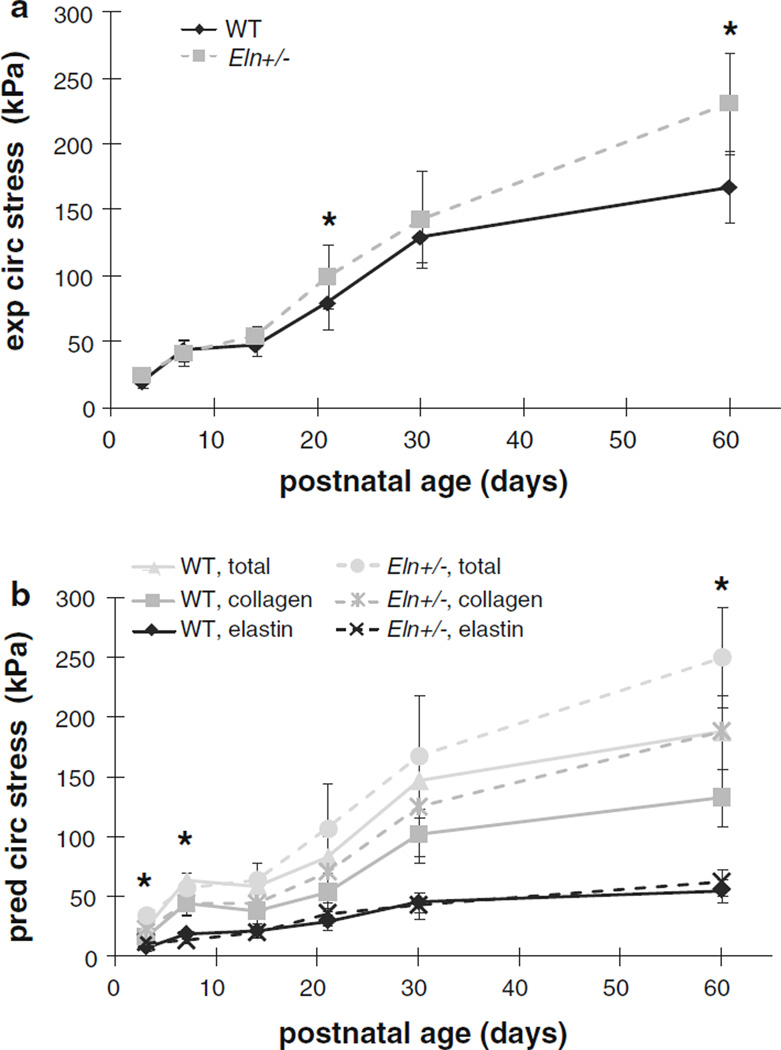
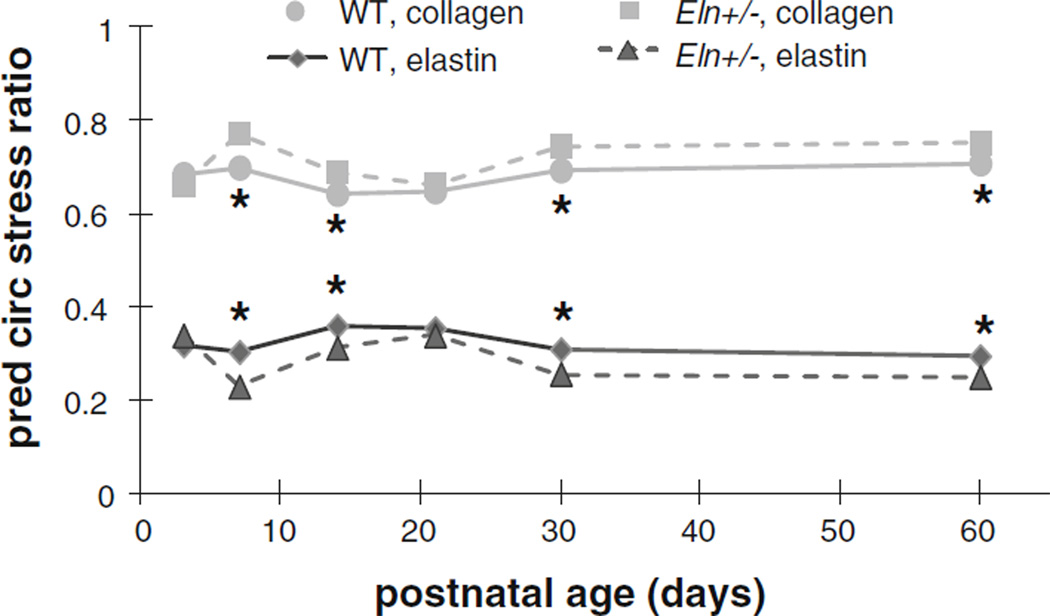
The circumferential stress calculated from the mechanical data at the average systolic arterial blood pressure for each age and genotype (Le et al. 2011) is similar in WT and Eln+/− aorta for most ages, until P60 when circumferential stress in the Eln+/− aorta is 30 % higher than WT (P < .001) (Fig. 5a). This behavior is also observed in the total predicted circumferential stress from the model (Fig. 5b). The 25 % increase in total predicted circumferential stress in P60 Eln+/− aorta (P < .001) is due to a 30 % increase in the predicted collagen stress contribution compared with WT (P < .001). Interestingly, the predicted circumferential stress contribution of elastin is higher, lower or the same in Eln+/− and WT aorta depending on the age. At P3, the predicted contribution of elastin is increased 35% in Eln+/− aorta compared with WT (P = .04), and at P7, the predicted contribution of elastin is increased 30 % in WT compared with Eln+/− aorta (P = .003). There are no significant differences in the predicted circumferential stress contribution of elastin at any of the older ages. The calculated and predicted axial stresses for the same loading conditions show similar behavior (not shown). A plot of the circumferential stress contributions from elastin and collagen as a ratio of the total circumferential stress reveals a 15–25 % lower contribution from elastin and 10 % higher contribution of collagen in Eln+/− aorta compared to WT (Fig. 6). The difference is significant at P7 and P14 (P < .01) and becomes insignificant at P21, only to diverge again and become significant at P30 and P60 (P < .001). Similar behavior is seen for the axial stress contributions of elastin and collagen as a ratio of the total axial stress (not shown).
Fig. 5.
Average experimental (exp) (a) (Le et al. 2011) and predicted (pred) (b) circumferential (circ) stress in WT and Eln+/− aorta at the in vivo length and average systolic pressure for each age and genotype. Systolic pressures are found in Table 1. Note that the predicted stress increases with age, but is similar in WT and Eln+/−aorta at most ages. The large increase in predicted circumferential stress for Eln+/− aorta compared with WT at P60 comes from an increase in the predicted collagen stress contribution. For panel a, * = P < .05 between WT and Eln+/−. For panel b, * = P < .05 between WT and Eln+/− at P3 and P7 for the elastin stress and at P60 for the collagen and total stress. N = 5 − 10 for each age and genotype. Mean ± SD
Fig. 6.
Mean predicted (pred) circumferential (circ) stress contributions from elastin and collagen as a ratio of the total circumferential stress for WT and Eln+/− aorta at the in vivo length and average systolic pressure for each age and genotype. Systolic pressures are found in Table 1. Predicted stress contributions from the SMCs are negligible. Note that Eln+/− aorta is predicted to have lower elastin and higher collagen circumferential stress ratio contributions. Error bars are omitted for clarity. * = P < .05 between WT and Eln+/−. N = 5 − 10 for each age and genotype
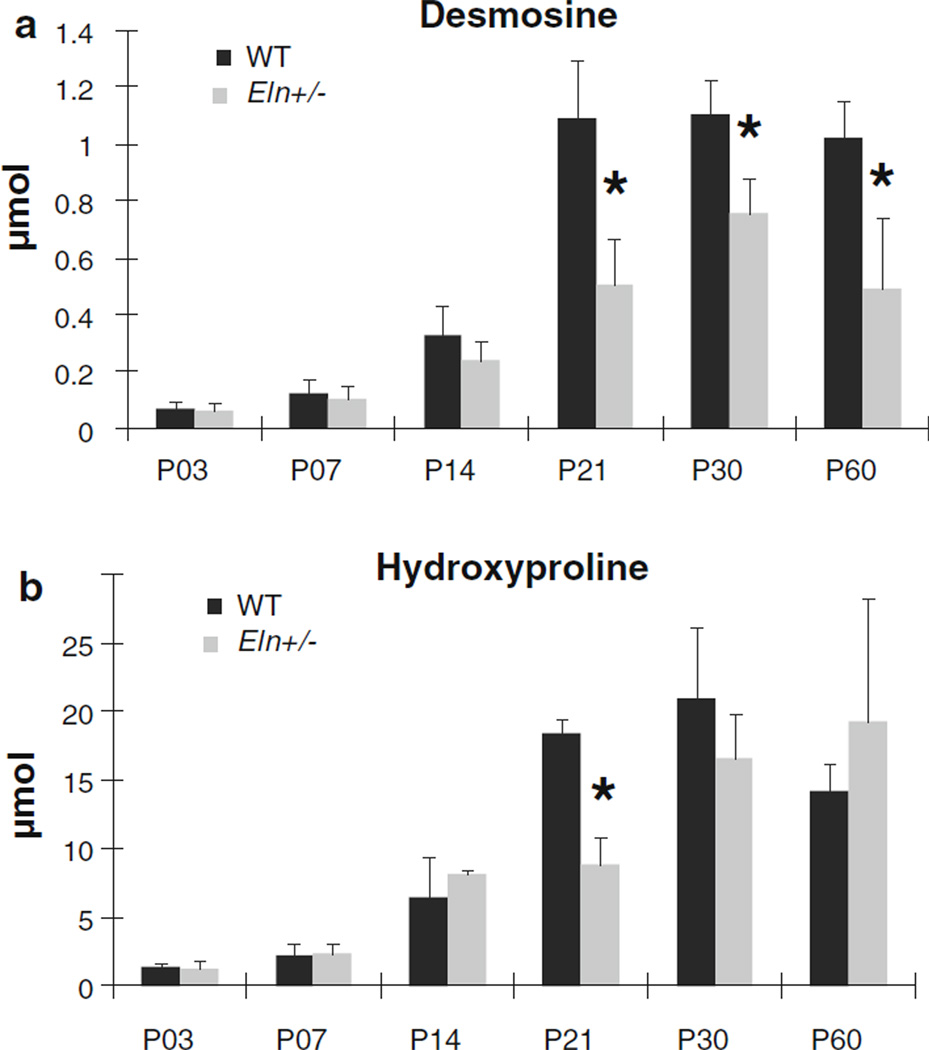
Quantification of total desmosine, a cross-link specific to mature elastin, is presented in Fig. 7a. Desmosine amounts are significantly affected by age in both genotypes (P < .001). There are increases in desmosine amounts for each successive age from P3 to P21 in WT aorta (P < .03) and between P7 and P30 in Eln+/− aorta (P < .05). Desmosine amounts increase 16-fold in WT aorta and 12-fold in Eln+/− aorta between P3 and P30. Desmosine amounts are reduced 30–50 % in Eln+/− aorta compared with WT at P21 and older (P < .05). P21 coincides with the age where elastin gene expression begins declining in the WT mouse aorta (Kelleher et al. 2004). Quantification of total hydroxyproline, an amino acid abundant in collagen, is shown in Fig. 7b. Hydroxyproline amounts are significantly affected by age in both genotypes (P < .001). There are increases in hydroxyproline amounts between successive ages from P7 to P30 in WT aorta (P < .05) and from P3 to P14 and P21 to P30 in Eln+/− aorta (P < .05). Hydroxyproline amounts increase 14-fold in both genotypes from P3 to P30. Between P30 and P60, hydroxyproline amounts decrease 30 % in the WT aorta (P < .01). At P21, there is 50 % less hydroxyproline in Eln+/− aorta compared with WT (P < .001), but there are no significant differences between genotypes at any other age. We found significant interactions between age and genotype for desmosine and hydroxyproline amounts (P < .001). Along with desmosine and hydroxyproline amounts, the size of the aorta will increase with age. Using the in vivo length, unloaded diameter, and unloaded thickness of the aorta for each age and genotype (Le et al. 2011), we calculated the approximate wall volume of the aorta. Normalizing the desmosine and hydroxyproline values by the wall volume does not change the observed trends in Fig. 7.
Fig. 7.
Mean values for total desmosine (a), an elastin specific cross-link, and hydroxyproline (b), an amino acid abundant in collagen, per ascending aortic segment for each age and genotype. For clarity, significant differences between ages are not shown, but are discussed in the text. Desmosine and hydroxyproline amounts are significantly affected by age (P < .001). * = P < .05 between WT and Eln+/−. N = 5−6 for each age and genotype. Mean ± SD
4 Discussion
A fiber-based constitutive model was used to fit mechanical data from WT and Eln+/− aortas throughout postnatal development. As modeled, passive SMCs contribute minimally to the total circumferential stress in the aortic wall, and the major contributors are elastin and collagen. The amount of elastin and collagen in each aorta was measured by quantifying desmosine and hydroxyproline. The model fits the mechanical data from older ages (P14–P60) better than from earlier ages (P3–P7), suggesting that the mechanical properties or relative contributions of elastin, collagen, and SMCs may be different in early postnatal development. The elastic lamellae between cell layers are not fully connected and presumably not fully functional mechanically, until about P7 in the mouse aorta (Davis 1995). However, the mechanical model presented here for all time points predicts changes in elastin and collagen stress contributions that are consistent with measured changes in protein amounts with age and disease.
In examining the best fit values of the model parameters over postnatal development, multiple trends are observed. First, there is an increase in the parameters c1 (elastin) and c2 (collagen) in WT and Eln+/− aortas up to P21 or P30. This trend coincides with desmosine and hydroxyproline amounts in WT and Eln+/− aortas that increase with age up to P21 or P30. Desmosine and hydroxyproline amounts are directly proportional to elastin and collagen amounts, respectively. The changes in model parameters and elastin and collagen amounts with age match elastin and collagen gene expression data for the WT mouse aorta, where expression begins declining around P21 (Kelleher et al. 2004). Interestingly, c1 in the Eln+/− aorta continues to increase up to P60, while Eln+/− desmosine levels continue to increase up to P30. In contrast, there is a clear peak in c1 magnitude and desmosine content at P21 for the WT aorta. Although we do not have gene expression data for Eln+/− aorta, it is possible that high elastin expression is prolonged past P21 in these mice in an attempt to increase total elastin amounts. The collagen parameter, c2, increases significantly up to P21 and decreases between P30 and P60 in both genotypes, approximately mirroring the changes in hydroxyproline amounts in the aortas with age. The changes in hydroxyproline amounts with age in Eln+/− aorta are different than in WT with no significant changes between P14 and P21 or P30 and P60, which may represent an altered timeline of collagen expression to compensate for reduced elastin levels. The interactions between age and genotype for c1, α, desmosine and hydroxyproline amounts suggest an altered timeline of elastin and collagen organization, mechanical contribution, and accumulation in the two genotypes. By P60, when the mice are young adults, Eln+/− mice have approximately half the desmosine and similar hydroxyproline to WT mice, consistent with previous data (Faury et al. 2003).
At P60, the average elastin parameter, c1, for WT aorta is in the same range as those in previous studies (8–26 kPa) that modeled the mechanical behavior of adult mouse carotid arteries with a four-fiber-family model (Eberth et al. 2009; Gleason et al. 2008; Hansen et al. 2009; Wan et al. 2010). In all of the studies on mouse arteries, the elastin material parameter is lower than that found from fitting neo-Hookean constitutive models to isolated elastin from pig arteries (70–140 kPa) (Gundiah et al. 2009; Watton et al 2009). The parameter differences may be due to variations between species or to changes in the elastin mechanical properties upon isolation from the arterial wall (Gundiah et al. 2007). The collagen parameters are harder to compare between studies due to the different number of collagen fiber families in various studies and the nonlinear mechanical behavior, but our results show that in the physiologic range of stretch ratios (1.5–1.8) (Wagenseil and Mecham 2009), collagen is significantly more stiff than elastin, as expected (Greenwald 2007).
The second trend that is observed from the model predictions is that the unloaded collagen fiber angle, α, decreases with postnatal development. This corresponds to the fibers shifting from a more axial orientation toward a more circumferential orientation with age, which increases the ability of the fibers to resist the higher blood pressure and bear the increased circumferential wall stress found in adult arteries. The reorientation is more pronounced in Eln+/− than WT aorta, which indicates remodeling of the collagen fiber orientation over time to adapt to altered mechanical behavior caused by reduced elastin levels. The predicted unloaded orientation of collagen fibers at 43° in the adult mouse aorta by the model agrees well with previous measurements of 40° in the unloaded medial layer of human aorta (Holzapfel 2006). To our knowledge, the reorientation of fibers over postnatal development has not been studied, but the model predictions can be checked with careful histology or confocal microscopy. While traditional histological studies of ECM fiber orientation have provided limited information, new methods in multiphoton confocal microscopy can provide greater detail to investigate this in the unloaded and loaded configurations (Ferruzzi et al. 2011; Wan et al. 2010). The combination of decreasing c2 after P30 in both genotypes and the continuous changes in unloaded collagen fiber angle suggest that the continued increase in total stress in the artery wall throughout development may be caused by rearrangement and remodeling of the collagen fibers, rather than changes in collagen amount. This is consistent with the hydroxyproline data for both genotypes and gene array data for WT aorta (Kelleher et al. 2004) that show limited additional expression or accumulation of collagen after P30 in the mouse.
In comparing the model parameters between genotypes, the elastin parameter, c1, for Eln+/− aorta is significantly reduced compared with WT beginning at P7, the age when the elastic lamellae are complete (Davis 1995), and continuing through P21, the age when elastin gene expression begins decreasing in the WT aorta (Kelleher et al. 2004). The desmosine data do not show significant differences in elastin amount between genotypes until P21. Small differences in elastin amounts at the younger ages may have large effects on the mechanical properties and hence significant effects on the elastin parameter, c1. After P30, the desmosine amounts for Eln+/− mice are approximately half those of WT mice, but the differences in c1 are no longer significant. There is still a trend toward reduced c1 in Eln+/− aorta compared with WT (P = .06 and .09 for P30 and P60, respectively), and increased samples at these ages may improve the statistical significance. It is possible that varying the elastin contribution to the total circumferential stress with age may have resulted in better agreement between c1 and the desmosine data. However, the changes in c1 and desmosine amounts with time and between genotypes show that the current, simple model is capable of predicting mechanical differences in the aortic wall caused by altered elastin amounts with postnatal development and disease. Another notable difference between genotypes is the trend in the reduction in collagen parameter, c2, in Eln+/− mice compared with WT at the older ages (P14–P60). While this may seem contradictory to the similar hydroxyproline amounts between genotypes at most ages, the change in c2 is accompanied by an increase in the exponential term, c3, at most ages and slight differences in α, which also characterize collagen in the model. The nonlinear behavior of the collagen fibers in the model, compared with the linear behavior of the elastin matrix, makes it more difficult to equate the collagen parameters to protein amounts. The changes in all of the collagen parameters suggest that the mechanical properties and organization of the collagen fibers may differ between WT and Eln+/− aorta.
When the predicted circumferential stress is separated into the contributions of each constituent in the model, reduced predicted contributions from elastin in Eln+/− aorta are compensated for by increased predicted contributions from collagen that result in a similar total circumferential stress at physiologic pressure at most ages. It is perhaps the need for the arteries to achieve a homeostatic stress state that is driving the remodeling process and altering the relative contribution of each component. Previous investigators have noted a consistency at physiologic pressures in the tension per lamellar unit inmammals (Wolinsky and Glagov 1967), elastic modulus in vertebrates (Gibbons and Shadwick 1989), and elastic modulus in jawless vertebrates and invertebrates (Davison et al. 1995). Mathematical modeling including turnover of the major wall constituents also observed a remodeling response that returned wall stress toward a homeostatic value (Gleason and Humphrey 2004). Thus, circumferential wall stress at each age may be an important target for developmental remodeling.
Constraints were imposed on the model parameters to ensure physiologically relevant contributions from the elastin and collagen proteins. The contributions were determined from previous reports that elastin contributes mostly in the low to mid-pressure range (Dobrin and Canfield 1984; Ferruzzi et al. 2011; Fonck et al. 2007) and that collagen contributes mostly in the high pressure range as the fibers are relatively unengaged at physiologic pressures (Greenwald et al. 1997). The constraint values were chosen from prior modeling of adult mouse carotid arteries (Hansen et al. 2009). While the selection of the particular constraint values was admittedly arbitrary, we found that varying the elastin contribution from 10 to 40%and collagen contribution from 55 to 85 % of the total circumferential stress did not change the observed trends in the parameters. Constraints were not applied to the SMCs or to the axial stress behavior, because there are more limited data available to determine the appropriate constraints, and we wished to impose the least amount of constraints necessary. Inclusion of similar constraints in the axial direction with 30 % elastin contribution toward the total axial stress between PL < P < PH and 75 % collagen contribution toward the total axial stress at P > PH, also did not change the observed parameter trends. In addition, an alternate method of constraining the circumferential stress contributions of elastin and collagen by completely separating their contributions to different operating pressure ranges produced similar trends in the parameters, indicating that separate mechanical contributions of elastin and collagen can describe the total stress behavior of the developing mouse aorta. The model predicts minimal contributions to the total stress from the SMCs, which is consistent with previous data for large, elastic arteries. Using Cytochalasin D to dissolve the SMC actin cytoskeleton or Triton X to remove the cells from the arterial wall has minimal effects on the passive pressure–diameter behavior of adult mouse carotid arteries (Corley et al. 2010).
This study is not without limitations. To minimize the number of mice required, male mice were used for mechanical characterization and female mice were used for protein quantification. Differences between the sexes in the relationship between aortic mechanical behavior and wall constituents may account for some of the inconsistencies in correlating model parameters with elastin and collagen amounts. The current model does not explicitly include volume or mass fractions of the wall constituents. While a case can be made for changes in the material parameters to represent changes in constituent amounts, they are not directly relatable to protein amounts. Future studies may involve the use of volume or mass fraction terms to more accurately capture the differences in relative protein amounts; however, this increases the number of fitted parameters and the chance of obtaining non-unique parameter values. Also, the aortic wall was considered a single homogeneous layer. Histology results clearly indicate that the aortic wall consists of three distinct layers with different structures and compositions. This spatial organization is a key difference between large elastic arteries, muscular arterioles, and thinner capillaries, and future modeling efforts should consider the spatial arrangement of proteins. Despite having less total elastin, the Eln+/− mouse aorta has increased lamellar units (Li et al. 1998). This difference in spatial organization of elastin may contribute to the observed differences in the age trends of the elastin parameter, c1, between the two genotypes. The transmural wall stress distribution was not included in the model. Previous work has shown that the residual stresses normalize the transmural wall stress, so that the mean wall stress is a reasonable approximation (Chuong and Fung 1986). Similar models without residual stress have proven effective at comparing mechanical behavior in mouse arteries with altered ECM amounts (Eberth et al. 2009; Ferruzzi et al. 2011). Unloaded orientation of the collagen fiber families is included, but the orientation distribution, as well as any considerations about the waviness or unloaded configuration of individual collagen fibers, is not included (Fonck et al. 2007). Additionally, there is evidence that the elastin matrix is not completely isotropic in the arterial wall (Rezakhaniha et al. 2011). Work is ongoing to determine the best strain energy functions to describe the ECM proteins. It is also important to note that all of the parameters were determined from fits to ex vivo test results. In vivo mechanical behavior may be slightly different due to interactions with surrounding tissue (Liu et al. 2007), but the ex vivo tests allow the application of a range of loading protocols for robust error minimization of the best fit parameters.
5 Conclusion
In summary, a fiber-based constitutive model for the mouse aorta is presented that predicts changes in ECM mechanical properties and organization in postnatal development and disease. Material parameters were fitted from mechanical data of WT and Eln+/− aorta from P3 to P60 using constraints based on prior literature. The fitted material parameters predict changes in elastin and collagen stress contributions that are consistent with protein quantification data for both genotypes and previous gene expression data in developing WT aorta. The model also predicts changes in collagen fiber orientation that must be confirmed in future studies. Relating changes in constituent amounts and organization to the mechanical behavior of the composite aortic wall may help understand developmental remodeling and guide treatments for human disease.
Supplementary Material
Acknowledgments
This work was supported, in part, by the NIH [GrantsRO1HL074138 (RPM), R00HL087563 (JEW), R01HL105314 (RPM and JEW), and T32 HL007275 (JKC)].
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s10237-012-0420-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jeffrey K. Cheng, Department of Biomedical Engineering, Washington University, St. Louis, MO, USA
Ivan Stoilov, Department of Cell Biology and Physiology, Washington University School of Medicine, St. Louis, MO, USA.
Robert P. Mecham, Department of Cell Biology and Physiology, Washington University School of Medicine, St. Louis, MO, USA
Jessica E. Wagenseil, Department of Biomedical Engineering, Saint Louis University, 3507 Lindell Blvd., St. Louis 63103, MO, USA, jwagense@slu.edu
References
- Amin M, Kunkel AG, Le VP, Wagenseil JE. Effect of storage duration on the mechanical behavior of mouse carotid artery. J Biomech Eng. 2011;133(7):071007. doi: 10.1115/1.4004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S, Valentin A, Humphrey JD. Biochemomechanics of cerebral vasospasm and its resolution: II. Constitutive relations and model simulations. Ann Biomed Eng. 2007;35(9):1498–1509. doi: 10.1007/s10439-007-9322-x. [DOI] [PubMed] [Google Scholar]
- Chuong CJ, Fung YC. On residual stresses in arteries. J Biomech Eng. 1986;108(2):189–192. doi: 10.1115/1.3138600. [DOI] [PubMed] [Google Scholar]
- Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985;5(1):19–34. doi: 10.1161/01.atv.5.1.19. [DOI] [PubMed] [Google Scholar]
- Corley B, Le VP, Wagenseil JE. Mechanical contribution of smooth muscle cells in large elastic arteries. Paper presented at the Biomedical Engineering Society Annual Meeting; Austin, TX. 2010. [Google Scholar]
- Davis EC. Elastic lamina growth in the developing mouse aorta. J Histochem Cytochem. 1995;43(11):1115–1123. doi: 10.1177/43.11.7560894. [DOI] [PubMed] [Google Scholar]
- Davison IG, Wright GM, DeMont ME. The structure and physical properties of invertebrate and primitive vertebrate arteries. J Exp Biol. 1995;198(Pt 10):2185–2196. doi: 10.1242/jeb.198.10.2185. [DOI] [PubMed] [Google Scholar]
- Dobrin PB. Chapter 3: physiology and pathophysiology of blood vessels. In: Sidawy AN, Sumpio BE, DePalma RG, editors. The basic science of vascular disease. New York: Futura Publishing; 1997. pp. 69–105. [Google Scholar]
- Dobrin PB, Canfield TR. Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. Am J Physiol. 1984;247(1 Pt 2):H124–H131. doi: 10.1152/ajpheart.1984.247.1.H124. [DOI] [PubMed] [Google Scholar]
- Eberth JF, Taucer AI, Wilson E, Humphrey JD. Mechanics of carotid arteries in a mouse model of Marfan Syndrome. Ann Biomed Eng. 2009;37(6):1093–1104. doi: 10.1007/s10439-009-9686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faury G, Maher GM, Li DY, Keating MT, Mecham RP, Boyle WA. Relation between outer and luminal diameter in cannulated arteries. Am J Physiol. 1999;277(5 Pt 2):H1745–H1753. doi: 10.1152/ajpheart.1999.277.5.H1745. [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112(9):1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi J, Collins MJ, Yeh AT, Humphrey JD. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc Res. 2011;92(2):287–295. doi: 10.1093/cvr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonck E, Prod’hom G, Roy S, Augsburger L, Rufenacht DA, Stergiopulos N. Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol. 2007;292(6):H2754–H2763. doi: 10.1152/ajpheart.01108.2006. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: mechanical properties of living tissues. 2nd edn. New York: Springer; 1993. [Google Scholar]
- Gibbons CA, Shadwick RE. Functional similarities in the mechanical design of the aorta in lower vertebrates and mammals. Experientia. 1989;45(11–12):1083–1088. doi: 10.1007/BF01950164. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Dye WW, Wilson E. Quantification of the mechanical behavior of carotid arteries from wild-type, dystrophin-deficient, and sarcoglycan-δ knockout mice. J Biomech. 2008;41(15):3213–3218. doi: 10.1016/j.jbiomech.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason RL, Humphrey JD. A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J Vasc Res. 2004;41(4):352–363. doi: 10.1159/000080699. [DOI] [PubMed] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211(2):157–172. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- Greenwald SE, Moore JE Jr, Rachev A, Kane TP, Meister JJ. Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng. 1997;119(4):438–444. doi: 10.1115/1.2798291. [DOI] [PubMed] [Google Scholar]
- Gundiah N, Ratcliffe MB, Pruitt LA. Determination of strain energy function for arterial elastin: Experiments using histology and mechanical tests. J Biomech. 2007;40(3):586–594. doi: 10.1016/j.jbiomech.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Gundiah N, Ratcliffe MB, Pruitt LA. The biomechanics of arterial elastin. J Mech Behav Biomed Mater. 2009;2(3):288–296. doi: 10.1016/j.jmbbm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Hansen L, Wan W, Gleason RL. Microstructurally motivated constitutive modeling of mouse arteries cultured under altered axial stretch. J Biomech Eng. 2009;131(10):101015. doi: 10.1115/1.3207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res. 2007;101(5):523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA. Determination of material models for arterial walls from uniaxial extension tests and histological structure. J Theor Biol. 2006;238(2):290–302. doi: 10.1016/j.jtbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Gasser TC, Ogden RW. A new constitutive frame-work for arterial wall mechanics and a comparative study of material models. J Elast. 2000;61(1–3):1–48. [Google Scholar]
- Holzapfel GA, Ogden RW. Constitutive modelling of arteries. P R Soc A Math Phy. 2010;466(2118):1551–1596. [Google Scholar]
- Huang Y, Guo X, Kassab GS. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am J Physiol Heart Circ Physiol. 2006;290(2):H657–H664. doi: 10.1152/ajpheart.00803.2005. [DOI] [PubMed] [Google Scholar]
- Ishii T, Kuwaki T, Masuda Y, Fukuda Y. Postnatal development of blood pressure and baroreflex in mice. Auton Neurosci. 2001;94(1–2):34–41. doi: 10.1016/S1566-0702(01)00339-3. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res. 2003;93(9):857–865. doi: 10.1161/01.RES.0000100389.57520.1A. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301(1):H221–H229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102(10):1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6(7):1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dang C, Garcia M, Gregersen H, Kassab GS. Surrounding tissues affect the passive mechanics of the vessel wall: theory and experiment. Am J Physiol Heart Circ Physiol. 2007;293(6):H3290–H3300. doi: 10.1152/ajpheart.00666.2007. [DOI] [PubMed] [Google Scholar]
- Rahman M. In: An enzyme-linked immunosorbent assay (ELISA) to quantitate desmosine and isodesmosine. Stephen F, editor. Texas, United States: Austin State University; 2009. [Google Scholar]
- Rezakhaniha R, Fonck E, Genoud C, Stergiopulos N. Role of elastin anisotropy in structural strain energy functions of arterial tissue. Biomech Model Mechanobiol. 2011;10(4):599–611. doi: 10.1007/s10237-010-0259-x. [DOI] [PubMed] [Google Scholar]
- Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202(Pt 23):3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- Vito RP, Dixon SA. Blood vessel constitutive models-1995–2002. Annu Rev Biomed Eng. 2003;5:413–439. doi: 10.1146/annurev.bioeng.5.011303.120719. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289(3):H1209–H1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- Wan W, Yanagisawa H, Gleason RL Jr. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann Biomed Eng. 2010;38(12):3605–3617. doi: 10.1007/s10439-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watton PN, Ventikos Y, Holzapfel GA. Modelling the mechanical response of elastin for arterial tissue. J Biomech. 2009;42(9):1320–1325. doi: 10.1016/j.jbiomech.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A, Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol. 2000;278(2):H652–657. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20(1):99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.