Abstract
Successful immunity to Leishmania depends on recruitment of appropriate immune effector cells to the site of infection and chemokines play a crucial role in the process. At the same time, Leishmania parasites possess the ability to modify the chemokine profiles of their host thereby facilitating establishment of progressive infection. Therapeutic and prophylactic strategies targeted at chemokines and their receptors provide a promising area for further research. This review highlights our current knowledge concerning the role of chemokines and their receptors in modulating leishmaniasis in both clinical settings and experimental disease models.
Keywords: Leishmania, chemokines, chemokine receptors, promastigotes, amastigotes, granuloma, splenomegaly
Introduction
Leishmania are obligate intracellular parasites that are transmitted by a sand fly vector and cause a wide range of diseases, such as cutaneous, mucocutaneous and visceral leishmaniasis. Over 12 million people currently suffer from leishmaniasis, and approximately 2 million are infected annually, making it a major global health problem (www.who.int/tdr). Cutaneous leishmaniasis (CL) manifests as localized skin lesions that may resolve but can become chronic, leading to severe tissue destruction and disfigurement. This disease is caused by Leishmania major (the Middle East and Mediterranean Region), Leishmania mexicana (Central America) and Leishmania amazonensis (South America). Mucocutaneous leishmaniasis (MCL) caused by Leishmania braziliensis is endemic in South America. Clinically, it is characterized by the involvement of the nasal and oropharyngeal mucosa with extensive tissue destruction due to inflammation. Visceral leishmaniasis (VL) is the most severe form of leishmaniasis caused by Leishmania donovani and Leishmania chagasi in the Old and New worlds, respectively (www.who.int/tdr, Alexander et al., 1999; Awasthi et al., 2004).
Many different aspects of the immune response to Leishmania have been studied so far, particularly cytokine interactions with cells in different cellular compartments. It is well established in mouse models that resistance to cutaneous leishmaniasis is associated with the development of T-helper type 1 (Th1) response characterized by the production of IL-12 and IFN-γ, while genetic strains that mount the Th2 response with IL-4 and IL-10 cytokine production are susceptible (Awasthi et al., 2004). Resistant mouse strains such as C57BL/6 and C3H develop a Th1 response with CD4+ cells producing IFN-γ and IL-2 during L. donovani or L. chagasi infection and susceptible mice exhibit a decrease in IFN-γ production by liver granuloma cells (Wilson et al., 1996; Melby et al., 2001b). However, this Th1/Th2 dichotomy has not been demonstrated in humans, and models of visceral leishmaniasis do not always follow this paradigm (Melby et al., 2001a). For example susceptible BALB/c mice infected with visceralizing parasite species lack Th2 antigen-specific immune responses, but the disease progresses (Kaye et al., 1991; Wilson et al., 1996; Melby et al., 2001b).
Moreover, parasite burdens are not affected in IL-4 deficient BALB/c mice (Satoskar et al., 1995; Lehmann et al., 2000). This suggests that more complex immune mechanisms govern resistance and susceptibility to Leishmania and it may well be that a combination of host immune factors and parasite strain ultimately determine disease outcome.
Chemokines and chemokine receptors have been shown to play a crucial role in determining the outcome of leishmaniasis. Chemokines are chemotactic cytokines that coordinate recruitment of leukocytes involved in homeostasis as well as in innate and adaptive immune responses. They are single polypeptides of about 67 to 127 amino acid residues in length (Moser and Willimann, 2004). The arrangement of the cysteine residues within the polypeptide chain provides the basis for their grouping and nomenclature. While CXC (α) chemokines have their first two consensus cysteines separated by an amino acid, the CC (β) chemokines have their first two cysteines adjacent to each other. The other two minor subfamilies include the CX3C chemokines (containing three amino acids between the first two cysteines) and the C chemokines (which lack two of the four canonical cysteines) (Rot and von Andrian, 2004).
Chemokines mediate their actions through binding of chemokine receptors, which are cell surface G-protein coupled receptors with seven transmembrane domains. Chemokine receptor engagement leads to numerous distinct signal transduction pathways ultimately resulting in a variety of biological functions including integrin activation and cell migration along a chemokine gradient (Viola and Luster, 2008). Some chemokines have been shown to regulate cell differentiation (Gu et al., 2000) and distinct patterns of chemokine secretion has been observed in differentiated cells (Muller et al., 2003). Approximately 50 human chemokines and 20 chemokine receptors have been identified to date (Viola and Luster, 2008). These biological molecules have been shown to play a vital role in the regulation of immunity to various diseases (Del Rio et al., 2001; Jones et al., 2003; Rodriguez-Sosa et al., 2003). Indeed distinct chemokines and chemokine receptor-integrin combinations are associated with particular diseases and control effector cell migration to their respective infected tissue sites (Rot and von Andrian, 2004).
Infection with Leishmania induces the expression of a number of chemokine genes in the host (Racoosin and Beverley, 1997; Ritter and Korner, 2002; Antoniazi et al., 2004). This could potentially be beneficial to the parasite through recruitment of host cells it can infect, survive in and proliferate (van Zandbergen et al., 2004). For example, L. major has been shown to actively modify the chemokine profile of the infection site and thus recruit cells that will favor the development of persistent infection (Katzman and Fowell, 2008). Some species of Leishmania owe their virulence partly to their ability to repress the induction of pro-inflammatory cytokines and chemokine genes, making their entry less detectable to the host (Matte and Olivier, 2002; Ji et al., 2003). A noteworthy example is lipophosphoglycan (LPG), the most abundant glycolipid on the surface of Leishmania promastigotes, which inhibits the production of CCL2 by endothelial cells thus affecting monocyte transendothelial migration (Lo et al., 1998). Furthermore, Leishmania infantum infection of human macrophages causes a down regulation of the chemokine receptor CCR1 which could potentially restrict macrophage recruitment to infected tissues thereby allowing parasite progression (Panaro et al., 2004). On the other hand, as numerous studies have shown, chemokine and chemokine receptor expression by Leishmania infected host cells could be a means of facilitating the hosts' ability to restrain the parasite to the site of inoculation and mount an effective immune response (Matte and Olivier, 2002). In addition to mediating cellular recruitment, chemokines can activate various cell populations, participate in cell mediated immunity and possess antileishmanial properties. In this review we will address these roles of chemokines in regulating immunity against cutaneous and visceral leishmaniasis.
Chemokines in the Regulation of Immunity against Cutaneous Leishmaniasis (CL)
Immune mechanisms governing host resistance and susceptibility to cutaneous leishmaniasis are well characterized. The development of an IL-12 driven Th1 response and IFN- γ production coupled with recruitment of effector cells comprising of macrophages, NK cells, CD4+ and CD8+ cells to the site of infection are critical to the control of CL (Heinzel et al., 1991; Reiner and Locksley, 1995). Several studies have shown that chemokines play an important role in these processes and the subsequent generation of innate and acquired immunity against CL (Vester et al., 1999; Ritter and Moll, 2000; Rosas et al., 2005; Vasquez et al., 2008). Indeed, the chemokines produced at the site of an infection are critical in determining the composition of infiltrating cells and defining the eventual outcome of the disease (Teixeira et al., 2006). This is evident in cases of localized versus diffuse American cutaneous leishmaniasis in humans caused by L. mexicana (Ritter and Korner, 2002). In localized cutaneous leishmaniasis (LCL) which is self healing, a Th1 chemokine profile is observed in the lesions consisting of CCL2, CXCL9 and CXCL10 and is associated with a concentrated dermal infiltrate comprising of macrophages and large numbers of CD4 positive cells. In contrast, the chemokine profile of lesions of chronic diffuse cutaneous leishmaniasis (DCL) is Th2 associated dominated by the expression of CCL3, and the dermal infiltrate is more diffuse with fewer CD4 positive cells (Ritter and Korner, 2002). This link between chemokine profile and disease outcome demonstrates the potential of chemokine based immuno-therapeutic strategies.
L. major possesses the ability to actively regulate chemokine gene expression enabling the establishment of infection. It has been shown that L. major can selectively inhibit the production of CXCL10 by neutrophils which could potentially prevent the activation of NK cells important in parasite containment (van Zandbergen et al., 2002). Moreover the mouse model of L. major infection demonstrates a selective upregulation of CCL7, a Th2 attracting chemokine (Katzman and Fowell, 2008). Leishmania parasites also possess a chemotactic factor that selectively attracts neutrophils which can serve as host cells in the early stages of infection (van Zandbergen et al., 2002). Among the chemokines induced in the first few hours of Leishmania infection, CXCL8 (IL-8) production amplifies the recruitment of neutrophils to the infection site (Badolato et al., 1996; van Zandbergen et al., 2002; Venuprasad et al., 2002). In mice, the chemokines MIP-2 and KC (keratinocyte-derived cytokine) which are homologs of human CXCL8 recruit neutrophils to the skin (Muller et al., 2001). Neutrophils represent the first group of leukocytes to arrive at the site of infection and they have been suggested to serve as “Trojan horses” for eventual entry into macrophages, the ultimate host for Leishmania. This is likely accomplished by the production of the chemokine MIP-1β by Leishmania infected neutrophils which is chemoattractive for macrophages (van Zandbergen et al., 2004). It has also been shown that saliva from the sand fly vector induces CCL2 expression which possesses chemotactic effects on macrophages (Zer et al., 2001; Teixeira et al., 2005). Furthermore, L. major promastigotes possess the ability to delay apoptosis of infected neutrophils, buying time for the arrival of macrophages (Aga et al., 2002; Laufs et al., 2002). In this way, the parasite utilizes the hosts' chemokine profile to facilitate its survival and establishment in the host.
CCL2 (Monocyte Chemoattractant Protein -1 or MCP-1) has been shown to play an important role in early immunity against cutaneous leishmaniasis. This chemokine mediates recruitment of macrophages, monocytes, NK cells and other CCR2 expressing leukocytes important in cellular responses to Leishmania (Allavena et al., 1994; Ritter and Moll, 2000). In patients with L. mexicana infected LCL, where lesions are self healing, high concentrations of CCL2 are observed; in contrast low concentrations of CCL2 are seen in patients with DCL (Ritter et al., 1996). In vitro studies with CCL2 demonstrate its ability to induce anti-leishmanial activity in macrophages. It induces a respiratory burst in human monocytes (Rollins et al., 1991; Moll, 1997). Indeed CCL2 synergizes with IFN-γ to activate macrophages which may potentially kill Leishmania and promote healing (Ritter and Moll, 2000). This effect is abrogated in the presence of IL-4 (Ritter and Moll, 2000). On the other hand, ccl2 knock-out mice are resistant to L. major (Gu et al., 2000). This discrepancy could be explained by the observation that CCL2 might also have a Th2 polarizing effect in adaptive immune responses or it could be that genetic background plays a role in the observed result. It has also been suggested that the absence of this chemokine is compensated for by other CCR2 ligands including CCL7 (MCP-3) and CCL12 (MCP-5) (Teixeira et al., 2006; Quinones et al., 2007). Nevertheless CCL2 does play an important role in innate immunity against cutaneous leishmaniasis.
The CCL2 receptor, CCR2 has been shown to play an important role in adaptive immunity to cutaneous leishmaniasis. Ccr2 knock-out mice are susceptible to L. major due to deficient dendritic cell migration to the lymph node and localization to T cell areas of the spleen (Sato et al., 2000). The absence of this receptor has also been shown to contribute to the development of a Th2 phenotype in genetically resistant L. major infected mice (Sato et al., 2000). Another chemokine receptor recently tested in immunity to L. major is CCR6 which has been shown to contribute to the recruitment of T cells to the site of infection but is not essential for the control of parasites during infection (Lechner et al., 2007).
A correlation between resistance and the expression of the chemokine CCL5 (RANTES) in experimental L. major infection has been studied (Santiago et al., 2004). Higher levels of CCL5 expression were observed at the infection site of resistant C57BL/6 mice than in susceptible BALB/c mice. Treatment with met-RANTES (an antagonist of CCR1 and CCR5) or anti-CCL5 resulted in increased susceptibility to L. major (Santiago et al., 2004). In more recent studies involving inactivation of mast cells, increased expression of CCL2 and CCL5 mRNA correlates with a resistant phenotype in L. major infected BALB/c mice (Romao et al., 2009). On the other hand, CCL5 deficient mice do not show increased susceptibility, and ccr1 knock out mice are actually more resistant to L. major infection (Rodriguez-Sosa et al., 2003). These data demonstrate the complexities of the dynamic interactions of chemokines and chemokine receptors in the context of Leishmania infection which are yet to be completely understood. Similarly, ccr5 knock out mice are more resistant to L. major than their wild type littermates. In this case a role for regulatory T cells has been implicated since they preferentially express CCR5 and migrate efficiently to infection sites to dampen the functions of effector T cells (Yurchenko et al., 2006). Accumulation of CCR5 positive natural regulatory T cells has also been observed in the skin of patients with cutaneous leishmaniasis caused by L. braziliensis (Campanelli et al., 2006). Indeed, CCR5 presents a critical balancing role which results in the persistence of Leishmania in the infected dermis.
The chemokines CXCL9 (Monokine Induced by IFN-γ, MIG) and CXCL10 (IFN-γ Inducible Protein 10, IP-10) are critical in both innate and adaptive immune responses to Leishmania infection. These chemokines have an affinity for cells expressing the receptor CXCR3 including activated and memory CD4+ and CD8+ T cells, NK cells, macrophages, and subsets of dendritic cells (Liu et al., 2005). Since Th1 cell mediated immunity is critical against cutaneous leishmaniasis, these chemokines play a decisive role and has been well documented. CXCL10 can increase the cytotoxic activity of NK cells which might contribute to L. major resistance (Vester et al., 1999). It has also been shown to be preferentially induced in dendritic cells of L. major resistant but not susceptible mice (Steigerwald and Moll, 2005). Treatment of L. major infected susceptible BALB/c mice with recombinant CXCL10 results in increased NK cell cytotoxic activity (Muller et al., 2001). Similar treatment of susceptible mice significantly reduces parasite burdens in Leishmania amazonensis infection (Vasquez and Soong, 2006). Further studies show that CXCL10 can induce production of interleukin-12 p40 in dendritic cells and can increase the ability of CD4+ T cells to respond to IL-12 through up-regulation of IL-12Rβ2 chain. These CD4+ T cells are then able to produce large amounts of IFN-γ, generating a Th1 immune state critical for the control of L. amazonensis infection (Vasquez et al., 2008). As shown by this data, the cytokine-chemokine interplay provides a well coordinated immune response to Leishmania infection. Indeed, a previous study showed that expression of CXCL10, XCL1 and CCL2, expressed preferentially in the draining lymph nodes of L. major infected resistant mice, is dependent on IL-12 and IFN-γ (Zaph and Scott, 2003).
A number of studies have focused on the receptor CXCR3. Ligands for this receptor are CXCL9, CXCL10 and CXCL11 (Rot and von Andrian, 2004). When genetically resistant mice having the CXCR3 gene knocked out are infected with L. major, they are able to generate an effective Th1 immune response in the draining lymph nodes but are unable to control parasite growth in the lesion site. Defective CD4+ and CD8+ T cell migration to the site of infection accounts for this observed phenotype (Rosas et al., 2005). More recent studies have shown that BALB/c mice, which are genetically susceptible to L. major, are defective in their ability to induce CXCR3 on their T cells despite their ability to produce comparable amounts of IFN-γ as resistant C57BL/6 mice. It has been suggested that this deficiency might contribute to susceptibility of these mice (Barbi et al., 2008). The importance of CXCR3 in mediating resistance to L. major as shown by these studies highlights the role of its ligands in the regulation of immunity to Leishmania infection and presents a potential target for therapeutic intervention.
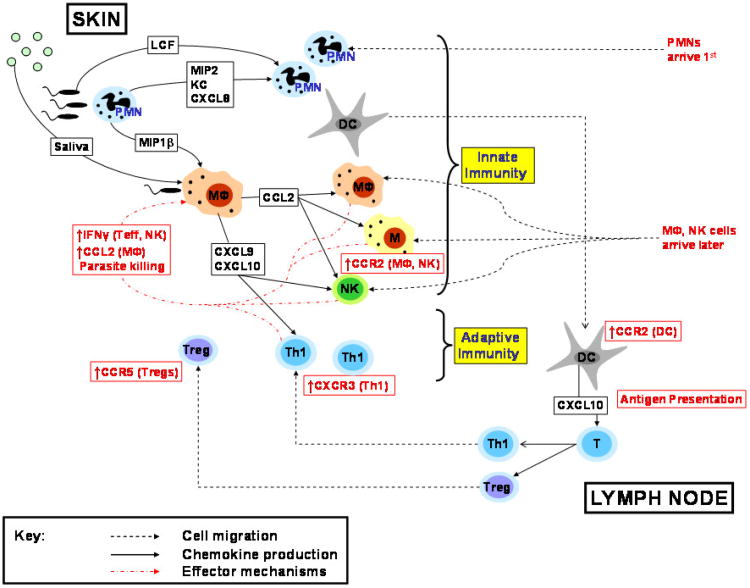
A summary of the major chemokine mediated events occurring during cutaneous leishmaniasis infection is depicted in figure 1.
Figure 1.
Major chemokine mediated events occurring in the skin and lymph node during early and late stages of cutaneous infection with Leishmania spp. PMNs are first recruited to the skin by CXCL8, MIP-2 and KC. Macrophages, monocytes and NK cells are subsequently recruited to the infection site via chemokines including CCL2. Up-regulation of CCR2 facilitates migration of antigen presenting cells to the Lymph node and priming of naïve T cells. Th1 cells express CXCR3 and eventually migrate to the infection site through the action of chemokines including CXCL9 and CXCL10.
Chemokines in the Regulation of Immunity against Visceral Leishmaniasis (VL)
The immunology and pathology of visceral leishmaniasis is a complex one. Immunity to experimental infection with L. donovani has been shown to be organ specific: the parasite persists in the spleen resulting in splenomegaly while there is control of parasite growth in the liver accompanied by formation of granulomas (Malla and Mahajan, 2006; Stanley and Engwerda, 2007). A number of genetic and cellular factors have been implicated in mediating resistance and susceptibility in humans and animal models. In the liver, monocytes, neutrophils, NKT cells, CD4+ and CD8+ cells all play an important role in granuloma formation and subsequent control of infection. Liver immunity is also dependent on a number of Th1 cytokines including IL-12, IFN-γ and TNF (Squires et al., 1989; Tumang et al., 1994; Murray, 1997). IL-4, a Th2 cytokine, has also been shown to be vital in resolution of L. donovani infection in the liver (Stager et al., 2003). As successful immunity depends on migration of appropriate cell populations to the infected sites, chemokines are critical in visceral leishmaniasis. Indeed, pathogenesis in visceral leishmaniasis is often associated with altered chemokine expression profiles and defective migration of immune cells (Stanley and Engwerda, 2007).
Patients with visceral leishmaniasis show elevated concentrations of CXCL9 and CXCL10 in their serum during active infection and it has been suggested that these chemokines along with IFN-γ play an important immunopathogenic role in the disease (Hailu et al., 2004). Analyses of chemokine profiles of target organs in infected mice do reveal an important role for CXCL10 in mediating parasite clearance and resolution of infection. In L. infantum infected mice, sustained expression of CCL2 rather than CXCL10 is detectable in the spleen. Compatible with such expression profile, Th2 cytokines dominate the splenic immune response and macrophage populations are maintained but T cell numbers are significantly reduced (Rousseau et al., 2001). Consequently parasite burdens persist and the spleen remains susceptible to infection. On the other hand, the liver of L. donovani infected mice show a rapid accumulation of MIP-1α, CCL2 and CXCL10, but only CXCL10 concentrations are elevated and maintained over a long period of the infection (Cotterell et al., 1999). Monocytes are attracted to the liver by MIP-1α and CCL2 presumably produced by infected Kupffer cells. More recently it has been shown that invariant NKT cells are major players in regulating early CXCL10 gene expression in the liver (Svensson et al., 2005). Eventually, CD4+ and CD8+ cells infiltrate the liver and are responsible for the elevated and maintained concentrations of CXCL10. Infiltrating monocytes and T cells contribute to hepatic granuloma formation and subsequent resolution of infection (Cotterell et al., 1999). Studies carried out with the CXCL10 receptor, CXCR3, demonstrate that there is a delayed development of hepatic granuloma formation in the cxcr3 knock out mice, but both groups (wild type and knock outs) eventually contain comparable numbers of granulomas and parasite loads and are able to resolve infection in the liver (Barbi et al., 2007). This would seem to indicate that other chemokines are also involved in T lymphocyte trafficking to the liver and highlights the redundancy that exists in chemokine function. This contrasts with the non redundant role played by this receptor in cutaneous infection with L. major as demonstrated in experiments with cxcr3 knock out mice (Rosas et al., 2005).
Extensive study of the spleen during the course of visceral leishmaniasis has implicated a role for the receptor CCR7 and its ligands CCL19 and CCL21 in mediating pathogenesis of the disease. During L. donovani infection, the splenic marginal zone which regulates leukocyte homing to the peri-arteriolar lymphoid sheaths (PALS, a site for parasite clearance) undergoes extensive remodeling with a selective loss of marginal zone macrophages via TNF-α mediated mechanisms (Engwerda et al., 2002). The parasites cause a reduction in the number of stromal cells in the PALS which produce the chemokines CCL19 and CCL21. More importantly, L. donovani causes TNF-α dependent IL-10 mediated inhibition of CCR7 expression on dendritic cells. This results in severely impaired dendritic cell migration to the PALS for antigen presentation to T cells, resulting in severe immunosuppression (Ato et al., 2002). Moreover, adoptive transfer of CCR7 expressing dendritic cells to L. donovani infected mice provides effective immunotherapy with a significant reduction of parasite burdens in the spleen (Ato et al., 2002). Interestingly, plt/plt mutant mice lacking both functional CCL19 and CCL21 genes are more susceptible to L. donovani infection than normal mice (Ato et al., 2004; Engwerda et al., 2004). Dendritic cell activation in these animals is defective and subsequent migration from the marginal zone to the PALS is lacking. In the liver, granuloma formation is delayed and effector CD4+ and CD8+ T cell recruitment limited. Taken together, these data indicate that chemokine-dependent encounters between dendritic cells and T cells are critical for optimal protection against L. donovani infection (Ato et al., 2006).
In vitro studies have shown that L. donovani infection results in the upregulation of the chemokines MIP-1α, MIP-1β and CCL5 along with the chemokine receptor CCR5 in human macrophages in a time dependent manner (Dasgupta et al., 2003; Bhattacharyya et al., 2008). This selective induction of chemokines is significant as L. donovani has been known to cause general suppression of macrophage gene expression in macrophages and this might seem to indicate the parasites ability to induce genes that will favor parasite survival and growth within the host cell (Buates and Matlashewski, 2001). In other studies evaluating the role of CCR5 and its ligand MIP-1α during L. donovani infection, ccr5 knock out mice and mip-1α knock out mice had increased levels of antigen specific IFN-γ production accompanied by lower parasite burdens in their spleens and livers after 8 weeks following infection compared to wild type and ccr2 knock out mice (Sato et al., 1999). These data suggests a deleterious role played by CCR5 and MIP-1α in mediating susceptibility to L. donovani infection. More recently, some researchers have successfully used small interfering RNAs (siRNA) to target and shut down the expression of the CCR5 gene (Bhattacharyya et al., 2008). Interestingly, in vitro administration of CCR5 siRNA not only enhanced the proinflammatory response of macrophages but also significantly restricted parasite burden early on during infection. This was accompanied by an increased level of Th1 cytokines and nitric oxide, both of which favor parasite clearance and are selectively inhibited in L. donovani infections (Bhattacharyya et al., 2002; Awasthi et al., 2004).
Antileishmanial activity mediated by chemokines has been demonstrated in both in vitro and in vivo infections with L. donovani. In macrophages primed with the CC chemokines MIP-1α and CCL2, multiplication of L. donovani amastigotes is inhibited through the induction of the respiratory burst and nitric oxide (Mannheimer et al., 1996; Bhattacharyya et al., 2002; Dey et al., 2005). This is also observed in L. infantum infected human macrophages via the same mechanism (Brandonisio et al., 2002). Furthermore, in vivo treatment of L. donovani infected BALB/c mice with MIP-1α or CCL2 significantly suppressed parasite burdens in the liver and spleen (Dey et al., 2005). Interestingly, these chemokines have been shown to induce Th1 immune responses through upregulation of IL-12 and suppress Th2 responses through inhibition of IL-10 in the liver and spleen of BALB/c mice infected with L. donovani. Moreover, the antigen presenting ability of infected macrophages, which is usually suppressed, is restored upon administration of these chemokines (Dey et al., 2007). These data clearly demonstrate the immuno-therapeutic potential of these chemokines in visceral leishmaniasis.
It should be noted that while MIP-1α has been shown to be beneficial in generating an antileishmanial microenvironment that favors parasite clearance, and therapeutic administration in vivo reduces parasite burdens (Bhattacharyya et al., 2002; Brandonisio et al., 2002; Dey et al., 2005), conflicting results have been observed using mip-1α knock out mice where they appear more resistant than wild type mice (Sato et al., 1999). The strains of mice used in these experiments might partly account for this discrepancy. BALB/c mice which are generally more susceptible were used for the treatment studies while a more resistant outbred strain (B6 × 129) was used for the knock out experiments. It is of interest that mip-1α knock out mice showed comparable parasite burdens in the spleens and livers early on in the course of infection, and antigen specific IFN-γ and IL-2 cytokine production was significantly lower 4 days post infection than wild type mice (Sato et al., 1999). Taken together, it would seem that MIP-1α is important in early containment of parasite burdens and the generation of an antileishmanial cytokine environment, but may be deleterious in the latter stages of chronic L. donovani infection. Further studies will be required to establish this dynamic relationship.
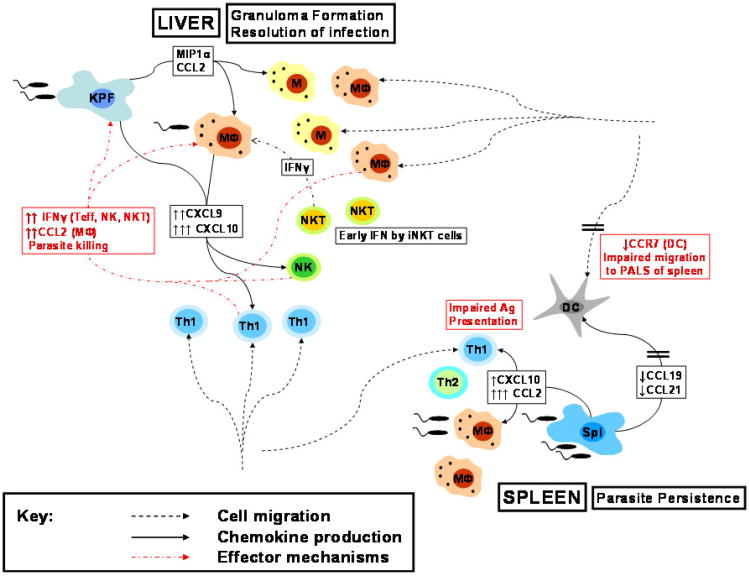
The major chemokine mediated events occurring in the spleen and liver during visceral leishmaniasis is shown in figure 2.
Figure 2.
Major chemokine mediated events occurring in the spleen and liver during visceral leishmaniasis. Production of CCL2 and CXCL10 followed by accumulation of macrophages and Th1 cells in the infected liver results in formation of granulomas and eventual resolution of infection. In contrast, impaired chemokine driven cellular encounters between DCs and T cells due to down-regulation of CCL19, CCL21 and CCR7, coupled with a lack of persistent production of CXCL10 results in parasite persistence in the spleen.
Conclusion
The complex immunological responses mounted against the various forms of Leishmania infection includes a role for chemokines and chemokine receptors in the process (Table 1). The contributions of individual chemokines to the overall immune response are yet to be completely understood. Yet, it is quite evident that the biological effects of chemokine-chemokine receptor interactions extend beyond cell migration and trafficking to tissues and secondary lymphoid organs. What has been discovered thus far demonstrates the potential of harnessing this arm of the hosts' defenses in successful prophylactic and therapeutic strategies against cutaneous and visceral leishmaniasis.
Table 1. Major Chemokines and Chemokine Receptors and their Biological Effects in Leishmaniasis.
| Chemokine | Parasite | Biological Effect |
|---|---|---|
| CCL2 | L. mexicana | Attract macrophages to CL* lesions Ritter and Korner, 2002 |
| CXCL9,10 | L. mexicana | Attract CD4+ cells (Th1) to CL* lesions Ritter and Korner, 2002 |
| CCL3 | L. mexicana | Attract macrophages and few CD4+ cells (Th2) to DCL* lesions Ritter and Korner, 2002 |
| CCL7 | L. major | Attract Th2 cells to lesion site Katzman and Fowell, 2008 |
| CXCL8 | L. major | Attract neutrophils to lesion Badolato et al., 1996; Venuprasad et al., 2002 |
| CCL2 | L. major | Attract T cells, NK cells, induce antileishmanial activity in Macs Romao et al., 2009 |
| CCR2 | L. major | Migration of DCs to lymph node and T cell areas of spleen Sato et al., 2000 |
| CCL5 | L. major | Contributes to resistance to parasite Santiago et al., 2004 |
| CCR5 | L. major | Treg migration to dermal sites and parasite persistence Yurchenko et al, 2006 |
| CCL4 | L. major | Attract macrophages to lesion site van Zandbergen et al., 2004 |
| CXCL9, 10 | L. amazonensis | Attract CD8+, CD4+ T cells, macrophages and dendritic cells Vasquez et al., 2008 |
| CXCL10 | L. major | Attracts Th1 cells and activates them to release IFN-γ Zaph and Scott, 2003 |
| CXCR3 | L. major | Th1 migration to skin lesions Rosas et al., 2005 |
| CCL2 | L. infantum | Induction of Th2 cells and parasite persistence in the spleen Rousseau et al., 2001 |
| MIP1α | L. donovani | Attract monocytes to liver Cotterell et al., 1999 |
| CXCL10 | L. donovani | Attract CD4+ and CD8+ cells and prolonged expression contributes to granuloma formation and parasite elimination in liver Cotterell et al., 1999 |
| CCL19, 21 | L. donovani | Downregulation inhibits dendritic cell migration to PALS Engwerda et al., 2002 |
| CCR7 | L. donovani | Downregulation in DCs impairs migration to T cell areas of spleen Ato et al., 2002 |
| CCR5 | L. donovani | Enhances susceptibility to infection Sato et al., 1999; Bhattacharyya et al., 2008 |
Infection in humans
Therapeutic approaches currently studied including the use of recombinant chemokines and siRNA for the treatment of the various forms of leishmaniasis seems to be promising fields of research. Of course, the redundancy and pleiotropy observed in the action of chemokines demonstrates the need for more extensive research in these areas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Muller K, Solbach W, Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. Journal of Immunology (Baltimore, Md: 1950) 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. Journal of Cell Science. 1999;112 Pt 18:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. European Journal of Immunology. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- Antoniazi S, Price HP, Kropf P, Freudenberg MA, Galanos C, Smith DF, Muller I. Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infection and Immunity. 2004;72:5168–5174. doi: 10.1128/IAI.72.9.5168-5174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nature Immunology. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- Ato M, Nakano H, Kakiuchi T, Kaye PM. Localization of marginal zone macrophages is regulated by C-C chemokine ligands 21/19. Journal of Immunology. 2004;173:4815–4820. doi: 10.4049/jimmunol.173.8.4815. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. Journal of Immunology. 2006;176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. Baltimore, Md.: 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Mathur RK, Saha B. Immune response to Leishmania infection. The Indian Journal of Medical Research. 2004;119:238–258. [PubMed] [Google Scholar]
- Badolato R, Sacks DL, Savoia D, Musso T. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Experimental Parasitology. 1996;82:21–26. doi: 10.1006/expr.1996.0003. [DOI] [PubMed] [Google Scholar]
- Barbi J, Brombacher F, Satoskar AR. T cells from Leishmania major-susceptible BALB/c mice have a defect in efficiently up-regulating CXCR3 upon activation. Journal of Immunology. 2008;181:4613–4620. doi: 10.4049/jimmunol.181.7.4613. Baltimore, Md.: 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbi J, Oghumu S, Rosas LE, Carlson T, Lu B, Gerard C, Lezama-Davila CM, Satoskar AR. Lack of CXCR3 delays the development of hepatic inflammation but does not impair resistance to Leishmania donovani. The Journal of Infectious Diseases. 2007;195:1713–1717. doi: 10.1086/516787. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Dey R, Majumder N, Bhattacharjee S, Majumdar S. A novel approach to regulate experimental visceral leishmaniasis in murine macrophages using CCR5 siRNA. Scandinavian Journal of Immunology. 2008;67:345–353. doi: 10.1111/j.1365-3083.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. The Journal of Infectious Diseases. 2002;185:1704–1708. doi: 10.1086/340820. [DOI] [PubMed] [Google Scholar]
- Brandonisio O, Panaro MA, Fumarola I, Sisto M, Leogrande D, Acquafredda A, Spinelli R, Mitolo V. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1 alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clinical and Experimental Medicine. 2002;2:125–129. doi: 10.1007/s102380200017. [DOI] [PubMed] [Google Scholar]
- Buates S, Matlashewski G. General suppression of macrophage gene expression during Leishmania donovani infection. Journal of Immunology. 2001;166:3416–3422. doi: 10.4049/jimmunol.166.5.3416. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, Goncalves HS, Belkaid Y, Barral-Netto M, Barral A, Silva JS. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. The Journal of Infectious Diseases. 2006;193:1313–1322. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- Cotterell SE, Engwerda CR, Kaye PM. Leishmania donovani infection initiates T cell-independent chemokine responses, which are subsequently amplified in a T cell-dependent manner. European Journal of Immunology. 1999;29:203–214. doi: 10.1002/(SICI)1521-4141(199901)29:01<203::AID-IMMU203>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Roychoudhury K, Ganguly S, Akbar MA, Das P, Roy S. Infection of human mononuclear phagocytes and macrophage-like THP1 cells with Leishmania donovani results in modulation of expression of a subset of chemokines and a chemokine receptor. Scandinavian Journal of Immunology. 2003;57:366–374. doi: 10.1046/j.1365-3083.2003.01227.x. [DOI] [PubMed] [Google Scholar]
- Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. Journal of Immunology. 2001;167:6503–6509. doi: 10.4049/jimmunol.167.11.6503. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Dey R, Majumder N, Bhattacharyya Majumdar S, Bhattacharjee S, Banerjee S, Roy S, Majumdar S. Induction of host protective Th1 immune response by chemokines in Leishmania donovani-infected BALB/c mice. Scandinavian Journal of Immunology. 2007;66:671–683. doi: 10.1111/j.1365-3083.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- Dey R, Sarkar A, Majumder N, Bhattacharyya Majumdar S, Roychoudhury K, Bhattacharyya S, Roy S, Majumdar S. Regulation of impaired protein kinase C signaling by chemokines in murine macrophages during visceral leishmaniasis. Infection and Immunity. 2005;73:8334–8344. doi: 10.1128/IAI.73.12.8334-8344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Kaye PM. Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends in Parasitology. 2004;20:524–530. doi: 10.1016/j.pt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Engwerda CR, Ato M, Cotterell SE, Mynott TL, Tschannerl A, Gorak-Stolinska PM, Kaye PM. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. The American Journal of Pathology. 2002;161:429–437. doi: 10.1016/s0002-9440(10)64199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Hailu A, van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-gamma, IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. The American Journal of Tropical Medicine and Hygiene. 2004;71:561–567. [PubMed] [Google Scholar]
- Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infection and Immunity. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Price DA, Dahm-Vicker M, Cerundolo V, Klenerman P, Gallimore A. The influence of macrophage inflammatory protein-1 alpha on protective immunity mediated by antiviral cytotoxic T cells. Immunology. 2003;109:68–75. doi: 10.1046/j.1365-2567.2003.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman SD, Fowell DJ. Pathogen-imposed skewing of mouse chemokine and cytokine expression at the infected tissue site. The Journal of Clinical Investigation. 2008;118:801–811. doi: 10.1172/JCI33174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye PM, Curry AJ, Blackwell JM. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. Journal of Immunology. 1991;146:2763–2770. Baltimore, Md.: 1950. [PubMed] [Google Scholar]
- Laufs H, Muller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, Solbach W, Laskay T. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infection and Immunity. 2002;70:826–835. doi: 10.1128/iai.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A, Ritter U, Varona R, Marquez G, Bogdan C, Korner H. Protective immunity and delayed type hypersensitivity reaction are uncoupled in experimental Leishmania major infection of CCR6-negative mice. Microbes and Infection/Institut Pasteur. 2007;9:291–299. doi: 10.1016/j.micinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Enssle KH, Lehmann I, Emmendorfer A, Lohmann-Matthes ML. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research. 2000;20:63–77. doi: 10.1089/107999000312748. [DOI] [PubMed] [Google Scholar]
- Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Current Topics in Developmental Biology. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- Lo SK, Bovis L, Matura R, Zhu B, He S, Lum H, Turco SJ, Ho JL. Leishmania lipophosphoglycan reduces monocyte transendothelial migration: modulation of cell adhesion molecules, intercellular junctional proteins, and chemoattractants. Journal of Immunology. 1998;160:1857–1865. Baltimore, Md.: 1950. [PubMed] [Google Scholar]
- Malla N, Mahajan RC. Pathophysiology of visceral leishmaniasis - some recent concepts. The Indian Journal of Medical Research. 2006;123:267–274. [PubMed] [Google Scholar]
- Mannheimer SB, Hariprashad J, Stoeckle MY, Murray HW. Induction of macrophage antiprotozoal activity by monocyte chemotactic and activating factor. FEMS Immunology and Medical Microbiology. 1996;14:59–61. doi: 10.1111/j.1574-695X.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Matte C, Olivier M. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of proinflammatory mediators. The Journal of Infectious Diseases. 2002;185:673–681. doi: 10.1086/339260. [DOI] [PubMed] [Google Scholar]
- Melby PC, Chandrasekar B, Zhao W, Coe JE. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1 -like cytokine response. Journal of Immunology. 2001a;166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Melby PC, Tabares A, Restrepo BI, Cardona AE, McGuff HS, Teale JM. Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Experimental Parasitology. 2001b;99:17–25. doi: 10.1006/expr.2001.4640. [DOI] [PubMed] [Google Scholar]
- Moll H. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Institute Mitteilungen. 1997;(99):73–78. [PubMed] [Google Scholar]
- Moser B, Willimann K. Chemokines: role in inflammation and immune surveillance. Annals of the Rheumatic Diseases. 2004;63(2):ii84–ii89. doi: 10.1136/ard.2004.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Bischof S, Sommer F, Lohoff M, Solbach W, Laskay T. Differential production of macrophage inflammatory protein 1gamma (MIP-1gamma), lymphotactin, and MIP-2 by CD4(+) Th subsets polarized in vitro and in vivo. Infection and Immunity. 2003;71:6178–6183. doi: 10.1128/IAI.71.11.6178-6183.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, van Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, Laskay T. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Medical Microbiology and Immunology. 2001;190:73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- Murray HW. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. The Journal of Infectious Diseases. 1997;175:1477–1479. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Spinelli R, Lisi S, Sisto M, Acquafredda A, Fumarola L, Mitolo V, Brandonisio O. Reduced expression of the chemokine receptor CCR1 in human macrophages and U-937 cells in vitro infected with Leishmania infantum. Clinical and Experimental Medicine. 2004;3:225–230. doi: 10.1007/s10238-004-0029-0. [DOI] [PubMed] [Google Scholar]
- Quinones MP, Estrada CA, Jimenez F, Martinez H, Willmon O, Kuziel WA, Ahuja SK, Ahuja SS. CCL2-independent role of CCR2 in immune responses against Leishmania major. Parasite Immunology. 2007;29:211–217. doi: 10.1111/j.1365-3024.2006.00935.x. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Beverley SM. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Experimental Parasitology. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annual Review of Immunology. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Ritter U, Moll H. Monocyte chemotactic protein-1 stimulates the killing of leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. European Journal of Immunology. 2000;30:3111–3120. doi: 10.1002/1521-4141(200011)30:11<3111::AID-IMMU3111>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ritter U, Korner H. Divergent expression of inflammatory dermal chemokines in cutaneous leishmaniasis. Parasite Immunology. 2002;24:295–301. doi: 10.1046/j.1365-3024.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- Ritter U, Moll H, Laskay T, Brocker E, Velazco O, Becker I, Gillitzer R. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. The Journal of Infectious Diseases. 1996;173:699–709. doi: 10.1093/infdis/173.3.699. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa M, Rosas LE, Terrazas LI, Lu B, Gerard C, Satoskar AR. CC chemokine receptor 1 enhances susceptibility to Leishmania major during early phase of infection. Immunology and Cell Biology. 2003;81:114–120. doi: 10.1046/j.0818-9641.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–1116. [PubMed] [Google Scholar]
- Romao PR, Da Costa Santiago H, Ramos CD, De Oliveira CF, Monteiro MC, De Queiroz Cunha F, Vieira LQ. Mast cell degranulation contributes to susceptibility to Leishmania major. Parasite Immunology. 2009;31:140–146. doi: 10.1111/j.1365-3024.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- Rosas LE, Barbi J, Lu B, Fujiwara Y, Gerard C, Sanders VM, Satoskar AR. CXCR3-/- mice mount an efficient Th1 response but fail to control Leishmania major infection. European Journal of Immunology. 2005;35:515–523. doi: 10.1002/eji.200425422. [DOI] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual Review of Immunology. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Demartino S, Anjuere F, Ferrua B, Fragaki K, Le Fichoux Y, Kubar J. Sustained parasite burden in the spleen of Leishmania infantum-infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. European Cytokine Network. 2001;12:340–347. [PubMed] [Google Scholar]
- Santiago HC, Oliveira CF, Santiago L, Ferraz FO, de Souza DG, de-Freitas LA, Afonso LC, Teixeira MM, Gazzinelli RT, Vieira LQ. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infection and Immunity. 2004;72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. The Journal of Experimental Medicine. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja SK, Ahuja SS. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. Journal of Immunology (Baltimore, Md: 1950) 1999;163:5519–5525. [PubMed] [Google Scholar]
- Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infection and Immunity. 1995;63:4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. Journal of Immunology (Baltimore, Md: 1950) 1989;143:4244–4249. [PubMed] [Google Scholar]
- Stager S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infection and Immunity. 2003;71:4804–4807. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunology and Cell Biology. 2007;85:138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- Steigerwald M, Moll H. Leishmania major modulates chemokine and chemokine receptor expression by dendritic cells and affects their migratory capacity. Infection and Immunity. 2005;73:2564–2567. doi: 10.1128/IAI.73.4.2564-2567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Zubairi S, Maroof A, Kazi F, Taniguchi M, Kaye PM. Invariant NKT cells are essential for the regulation of hepatic CXCL10 gene expression during Leishmania donovani infection. Infection and Immunity. 2005;73:7541–7547. doi: 10.1128/IAI.73.11.7541-7547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CR, Teixeira MJ, Gomes RB, Santos CS, Andrade BB, Raffaele-Netto I, Silva JS, Guglielmotti A, Miranda JC, Barral A, Brodskyn C, Barral-Netto M. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. Journal of Immunology. 2005;175:8346–8353. doi: 10.4049/jimmunol.175.12.8346. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Teixeira MJ, Teixeira CR, Andrade BB, Barral-Netto M, Barral A. Chemokines in host-parasite interactions in leishmaniasis. Trends in Parasitology. 2006;22:32–40. doi: 10.1016/j.pt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Tumang MC, Keogh C, Moldawer LL, Helfgott DC, Teitelbaum R, Hariprashad J, Murray HW. Role and effect of TNF-alpha in experimental visceral leishmaniasis. Journal of Immunology. 1994;153:768–775. Baltimore, Md.: 1950. [PubMed] [Google Scholar]
- van Zandbergen G, Hermann N, Laufs H, Solbach W, Laskay T. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infection and Immunity. 2002;70:4177–4184. doi: 10.1128/IAI.70.8.4177-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. Journal of Immunology. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Vasquez RE, Soong L. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infection and Immunity. 2006;74:6769–6777. doi: 10.1128/IAI.01073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RE, Xin L, Soong L. Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection. Infection and Immunity. 2008;76:161–169. doi: 10.1128/IAI.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuprasad K, Banerjee PP, Chattopadhyay S, Sharma S, Pal S, Parab PB, Mitra D, Saha B. Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-gamma secretion and restriction of Leishmania growth. Journal of Immunology. 2002;169:920–928. doi: 10.4049/jimmunol.169.2.920. Baltimore, Md.: 1950. [DOI] [PubMed] [Google Scholar]
- Vester B, Muller K, Solbach W, Laskay T. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infection and Immunity. 1999;67:3155–3159. doi: 10.1128/iai.67.6.3155-3159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annual Review of Pharmacology and Toxicology. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Sandor M, Blum AM, Young BM, Metwali A, Elliott D, Lynch RG, Weinstock JV. Local suppression of IFN-gamma in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi. Journal of Immunology. 1996;156:2231–2239. Baltimore, Md.: 1950. [PubMed] [Google Scholar]
- Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. The Journal of Experimental Medicine. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Scott P. Interleukin-12 regulates chemokine gene expression during the early immune response to Leishmania major. Infection and Immunity. 2003;71:1587–1589. doi: 10.1128/IAI.71.3.1587-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zer R, Yaroslavski I, Rosen L, Warburg A. Effect of sand fly saliva on Leishmania uptake by murine macrophages. International Journal for Parasitology. 2001;31:810–814. doi: 10.1016/s0020-7519(01)00190-4. [DOI] [PubMed] [Google Scholar]