Abstract
OBJECTIVE
To assess the clinical efficacy of nutritional amounts of grape polyphenols (PPs) in counteracting the metabolic alterations of high-fructose diet, including oxidative stress and insulin resistance (IR), in healthy volunteers with high metabolic risk.
RESEARCH DESIGN AND METHODS
Thirty-eight healthy overweight/obese first-degree relatives of type 2 diabetic patients (18 men and 20 women) were randomized in a double-blind controlled trial between a grape PP (2 g/day) and a placebo (PCB) group. Subjects were investigated at baseline and after 8 and 9 weeks of supplementation, the last 6 days of which they all received 3 g/kg fat-free mass/day of fructose. The primary end point was the protective effect of grape PPs on fructose-induced IR.
RESULTS
In the PCB group, fructose induced 1) a 20% decrease in hepatic insulin sensitivity index (P < 0.05) and an 11% decrease in glucose infusion rate (P < 0.05) as evaluated during a two-step hyperinsulinemic-euglycemic clamp, 2) an increase in systemic (urinary F2-isoprostanes) and muscle (thiobarbituric acid–reactive substances and protein carbonylation) oxidative stress (P < 0.05), and 3) a downregulation of mitochondrial genes and decreased mitochondrial respiration (P < 0.05). All the deleterious effects of fructose were fully blunted by grape PP supplementation. Antioxidative defenses, inflammatory markers, and main adipokines were affected neither by fructose nor by grape PPs.
CONCLUSIONS
A natural mixture of grape PPs at nutritional doses efficiently prevents fructose-induced oxidative stress and IR. The current interest in grape PP ingredients and products by the global food and nutrition industries could well make them a stepping-stone of preventive nutrition.
The Western diet, dominated by ultra-processed products rich in saturated fats and sugar, including high-fructose corn syrup, and poor in micronutrients (1), is a major contributor to the worldwide “diabesity” epidemic. In addition to contributing to calorie overconsumption, the unique metabolism of fructose (2) and its marked effect on systemic oxidative stress (3) could give it a pivotal role in the pathophysiology of insulin resistance (IR) and the metabolic syndrome (4).
The “French Paradox,” defined as a low incidence of coronary heart disease despite consumption of a diet rich in saturated fat (5), has stimulated interest in investigating whether grape polyphenols (PPs) may offer antioxidant-consequential health benefits (6–8) including improved insulin sensitivity (9), although this effect remains debated (10). If this outcome were to be confirmed in humans, then supplementation of highly processed foods with grape PPs may prove to be a promising strategy to stem the tide of chronic metabolic diseases, which, furthermore, would be quite easy to implement, since PPs are currently marketed in the form of dyes and tannins that can be used safely in relatively large amounts in sugary foods (11).
We thus designed a randomized double-blinded controlled study to assess the clinical efficacy of nutritional amounts of grape PPs in counteracting the metabolic effects of high-fructose diet (HFrD) to substantiate the hypothesis that by neutralizing oxidative stress, grape PPs can prevent fructose-induced IR.
RESEARCH DESIGN AND METHODS
Forty-three first-degree relatives of type 2 diabetic patients were recruited by advertisement in the diabetes departments of Montpellier and Lyon university hospitals and allocated to supplementations with grape PPs or placebo (PCB) (Supplementary Fig. 1). Volunteers were aged between 30 and 65 years, with BMIs between 25 and 35 kg/m2 and waist circumference >80 cm for women and >94 cm for men; consumed <30 g/day alcohol; and had a sedentary lifestyle (12). All subjects had blood pressure <140/90 mmHg and normal ferritinemia (75–300 ng/mL) and thyroid function; hepatic enzymes (γ-glutamyl transpeptidase, alanine aminotransferase [ALT], and aspartate aminotransferase [AST]) were three or less times the normal values, serum creatinine was ≤150 µmol/L, high-sensitivity C-reactive protein (hs-CRP) <8 mg/L, and fasting plasma glucose <110 mg/dL.
Five subjects were enrolled but dropped out for difficulties during blood withdrawal (one PCB) or for personal reasons (two PCB and two PP) not linked to secondary effects regarding study protocol. The study was approved by the ethics committee of Montpellier. All participants gave written informed consent.
Anthropometry and body composition measurements
Standing height was measured using a stadiometer. Body weight and hip and waist circumferences were measured in the fasting state before metabolic analysis. Body composition was evaluated by bioelectrical impedance (BodyStat). Blood pressure was measured after a 15-min rest period using an automated blood pressure device with participants in the recumbent position. A minimum of four blood pressure measurements was taken 2 min apart.
Study design
Each subject was studied on three occasions: 1) at the beginning of the study, 2) after 8 weeks of supplementation with 2 g/day grape PPs or PCB, and 3) after 9 weeks of supplementation with grape PPs or PCB while subjects also had ingested 3 g fructose/kg fat-free mass/day as a 20% fructose solution with the three main meals during the 6 days preceding the final test date (Supplementary Fig. 2). Subjects were assigned to the grape PP or PCB supplementation groups by randomization in blocks of four individuals each, sequentially allocated to grape PPs and PCB using a randomization list that was drawn up for each of the two recruiting centers. Grape PPs (Supplementary Table 1) or identical-looking PCB (microcrystalline cellulose, Avicel; UNITHER pharmaceuticals, Bordeaux, France) capsules (333.33 mg grape extract/PCB per capsule) were to be taken daily: three during breakfast and three at dinner. Evaluation of compliance (≥80% of the capsules taken) was done before starting the tests by counting the number of returned capsules after 4, 8, and 9 weeks from baseline. Volunteers were instructed to avoid polyphenol-rich foods and to follow a balanced, isoenergetic diet, which was controlled by a food diary during the 3-day period preceding each test and assessed with GENI software (Micro 6, Villers-les-Nancy, France). Intake of dietary polyphenols was further evaluated using phenol explorer (version 1.5.7; INRA, Clermont-Ferrand). Subjects were advised to avoid vigorous physical activity during the 6 days preceding each test.
Hyperinsulinemic-euglycemic clamp
Investigations were performed after an overnight fast. Insulin sensitivity was assessed using the hyperinsulinemic-euglycemic clamp as previously described (13), with a 1 mUI ⋅ kg−1 ⋅ min−1 insulin infusion for 120 min. Glucose infusion rate (GIR) was calculated during the final 30 min of the clamp. All subjects underwent a two-step insulin clamp (0.2 and 1 mUI ⋅ kg−1 ⋅ min−1). However, owing to regulatory issues, only the 19 subjects in Lyon (10 PP and 9 PCB) received an initial 4-h [6,6-2H2]-glucose (Eurisotop, St. Aubain, France) infusion to determine basal endogenous glucose production and its inhibition during the 0.2 mU ⋅ kg−1 ⋅ min−1 insulin infusion (14). During the clamp, blood samples were drawn every 10 min to monitor blood glucose concentration using a glucose meter (ACCU-CHEK Performa). Fasting hepatic insulin sensitivity index was calculated according to the methodology of Matsuda et al. (15).
Muscle biopsies
Before the clamp, a percutaneous biopsy of the vastus lateralis was obtained under local anesthesia (lidocaine 2%) either with Weil Blakesley pliers in Lyon (14) or using a 5-mm Bergstrom needle in Montpellier (16). A muscle sample was immediately frozen in liquid nitrogen and stored at −80°C for further analysis. For the 19 subjects in Montpellier (10 PP and 9 PCB), 80 mg muscle was permeabilized to study mitochondrial respiration and 50 mg were incubated for 15 min in PBS at 30°C in the presence or absence of human insulin (1 µmol/L) (Umuline RAPIDE; Lilly France) to study molecular mechanisms of insulin signaling.
Analytical procedures
Plasma glucose was measured by the glucose oxidase method (AU2700 Olympus; Beckman Coulter, O’Callaghan’s Mills, Co. Clare, Ireland) and nonesterified fatty acids via an enzymatic method (Wako, Neuss, Germany). Hepatic enzymes (AST, ALT, and γ-glutamyl transpeptidase), total cholesterol, HDL cholesterol, and triglycerides were determined using spectrophotometric methods (AU2700 Olympus; Beckman Coulter), and LDL cholesterol was calculated using the Friedewald formula; LDL size was determined by electrophoretic migration on polyacrylamide gel (Spirale, Dijon, France).
Plasma insulin was measured by radioimmunoassay (BI-Insulin IRMA kit; Cis Bio, Gif sur Yvette, France). Plasma concentrations of leptin, ghrelin, adiponectin, and resistin were evaluated by ELISA (R&D System), and cytokines, including interleukin (IL)-1α, IL-1β, IL-6, IL-4, tumor necrosis factor-α, and interferon-γ, were determined using multiplex Biochip Technology from Randox Laboratories (Crumlin, U.K.). The concentration of hs-CRP was determined by immuno-turbidimetry (Randox).
Western blots
Muscle samples were homogenized in a lysis buffer containing 30 mmol/L HEPES, 40 mmol/L NaCl, 5 mmol/L EDTA, 2 mmol/L EGTA, and 210 mmol/L sucrose with phosphatase and protease inhibitors and centrifuged for 10 min at 10,000g. Primary antibodies used included phospho-Akt (Ser473) (cat. no. 4060; Cell Signaling), total Akt (cat. no. 9171; Cell Signaling), and UCP3 (AB3044; Millipore). α-Tubulin (T6074; Sigma-Aldrich) was used as a loading control.
Protein carbonylation and determination of lipid oxidation in plasma, tissue, and urine
The Oxyblot Oxidized Protein Detection kit, purchased from Chemicon (Hampshire, U.K.), was used to define muscle protein carbonylation levels as previously described (17). Lipid peroxidation levels were measured as thiobarbituric acid–reactive substances (TBARS) in plasma and in tissue homogenates as previously described (18) and as urinary F2-isoprostanes determined by gas chromatography–mass spectrometry (Thermofinnigan, Courtaboeuf, France) (19). F2-isoprostanes concentrations are reported in relation to urinary creatinine (Beckman Coulter).
Determination of antioxidant activities in plasma and tissues
Blood and tissue total glutathione, tissue catalase/glutathione peroxidase, and plasma and/or tissue total and manganese superoxide dismutases (SOD and MnSOD, respectively) were measured as previously described (18).
Mitochondrial function and respiration
Mitochondrial respiratory variables were analyzed in situ on fresh permeabilized skeletal muscle fibers of subjects recruited in Montpellier as previously described (16). Basal (V0) and maximal (Vmax) respiration rates were recorded in the presence of pyruvate/malate (10 mmol/L) or palmitoyl-l-carnitine (40 μmol/L). Respiration rates were expressed in micromoles of O2 per minute per gram of muscle fibers.
Citrate synthase activity
Muscle extracts were homogenized in 10 mmol/L Tris HCl (pH 7.4). Citrate synthase activity was measured with 0.5 mmol/L oxaloacetate, 0.3 mmol/L acetyl-CoA, 0.1 mmol/L 5,5′-dithiobis 2-nitro-benzoic acid, and 100 mmol/L Tris HCl (pH = 8.0). Enzyme activity was monitored by recording the changes in absorbance at 412 nm over 2.5 min at 37°C as suggested by Srere (20), normalized to tissue weight, and expressed as micromoles per minute per gram of tissue.
Gene expression analyses
Microarray analysis.
RNA profiling in muscle biopsies was performed using a Human GE 4×44K v2 Microarray kit (Agilent Technologies, Massy, France). Briefly, 200 ng total RNA isolated from frozen muscle samples was labeled using the Low Input Quick Amp Labeling kit (CY3) from Applied Biosystems, and microarrays were hybridized and scanned following the manufacturer’s instructions. The data were normalized using the Agilent FE one-color scenario (GeneSpring GX software). After filtration, statistical analysis was performed on 26,220 probes with the Limma package (21). The dataset is available from the GEO database (GSE35764).
RT real-time PCR.
First-strand cDNAs were synthesized from 500 ng skeletal muscle. Total RNA and real-time PCR assays were performed as previously described (22). Values were normalized using hypoxanthine guanine phosphoribosyl transferase. RT-PCR primer sequences are listed in Supplementary Table 2.
Statistical analysis
Statistical analysis was performed with JMP 9.0.0 software, with data expressed as means ± SEM. To overcome the nonnormal distribution of most parameters, we analyzed data using a repeated-measures general linear model; when significance was reached (P < 0.05), we confirmed results with Mann-Whitney U test for comparisons between the PCB and grape PP groups at each time point (baseline, post–8 weeks of PCB/grape PP supplementation, and post–6 days of fructose combined with PCB/grape PP supplementation) and with Wilcoxon signed rank sum for within-group (pre- and postinterventions) comparisons. Correlations were evaluated using Spearman rank correlation tests. Statistical significance was considered for P values <0.05 with no adjustment for multiple comparisons.
RESULTS
Subjects’ characteristics, adherence to the study protocol, and adverse events
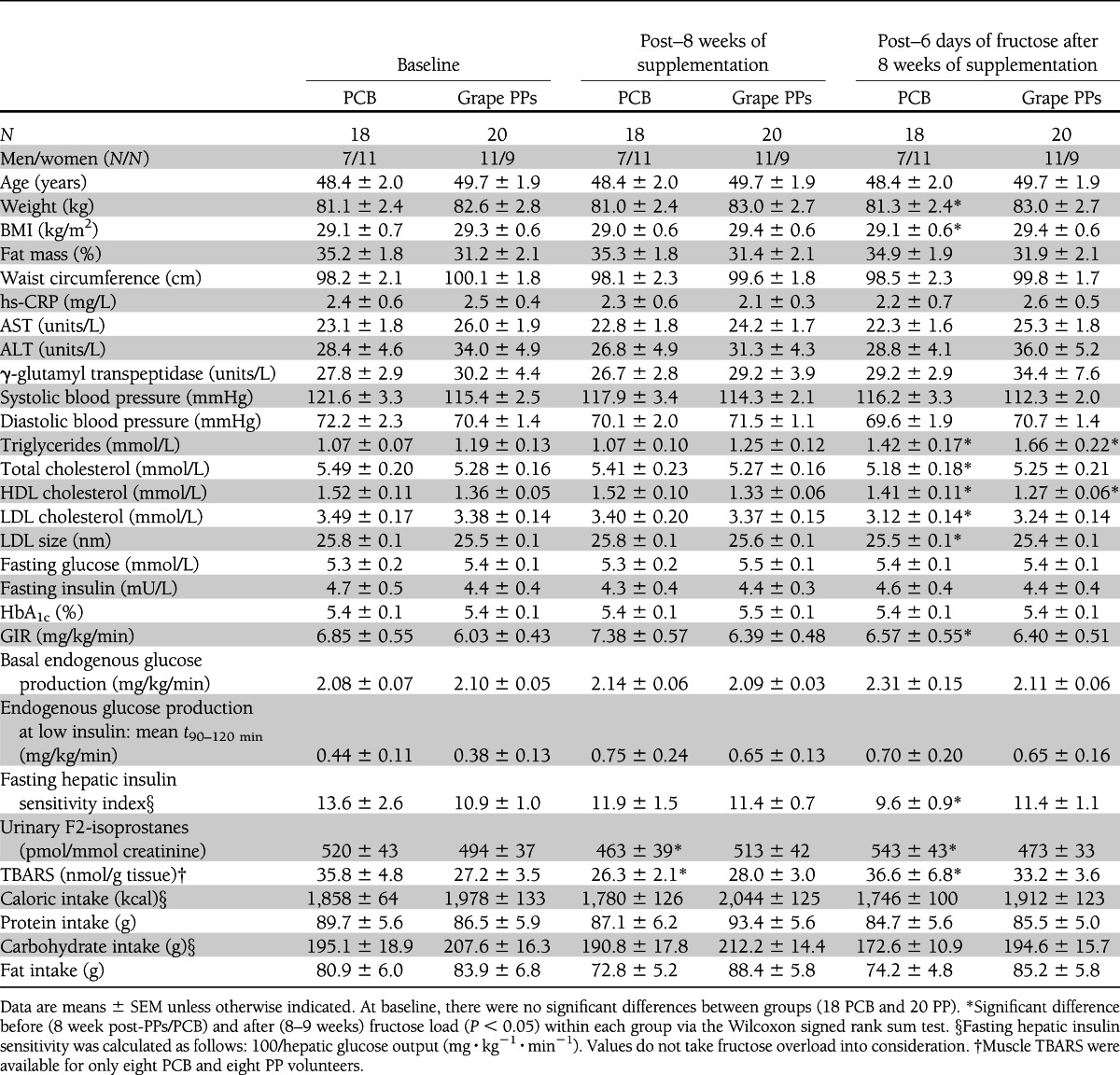
Thirty-eight subjects completed the study and were included in the per-protocol analysis. They were aged 31–65 years, with BMIs between 25.1 and 36.5 kg/m2 and waist circumference 80–118 cm for women and 94–113 cm for men. At baseline, the groups did not differ in terms of anthropometric measures; food, energy, nutrient and PP intake; or levels of plasma lipids and glucose or urinary F2-isoprostanes (Table 1). According to participants’ own reports and results of physical examination and laboratory parameters, no adverse events occurred in either supplementation group. Since decreased intestinal absorption of iron has been reported with high intakes of PPs, plasma ferritin was measured both at inclusion and at the end of the study. No significant decrease in this parameter was noted, and no subject was below the normal threshold values at the beginning or end of the study.
Table 1.
Subjects’ characteristics at baseline and throughout study exploration visits
Effects of 8-week grape PP supplementation
Surprisingly, after 8 weeks of supplementation we noted a decrease in urinary F2-isoprostanes and in muscle TBARS in the PCB group that was not found in the grape PP group, with no effects on the other parameters measured (Table1). Muscle protein carbonylation as well as antioxidative capacities, i.e., plasmatic vitamin E, blood glutathione-to-GSSG ratio, erythrocyte SOD, and the muscle enzymatic activities of catalase, glutathion peroxydase (GPx), SOD, and MnSOD, remained unaffected (Supplementary Table 3). No significant effects of grape PP supplementation were found on plasma cytokines (IL-1α, IL-1β, IL-6, IL-4, tumor necrosis factor-α, and interferon-γ), on the main adipokines (adiponectin, leptin, and resistin), or on ghrelin (Supplementary Table 4). Importantly, insulin action, as measured by hyperinsulinemic clamps, was unaffected (Table 1).
Metabolic consequences of HFrD
Fructose load’s main effects are described in Table 1. After 6 days of HFrD, a significant increase of 300 g body wt was found in the PCB group with no alteration in body composition. HFrD increased fasting triglycerides ~30% in both groups, with a parallel decrease in fasting HDL cholesterol. A decrease in plasma LDL cholesterol associated with a decrease in LDL particle size (P < 0.01, Table 1) was noted only in the PCP group and not in the PP group. Neither in the PCB nor in the grape PP group did HFrD change plasma cytokine, plasma adipokines, or plasma ghrelin levels (Supplementary Table 4).
Effects of HFrD on oxidative stress and protective effect of grape PP supplementation
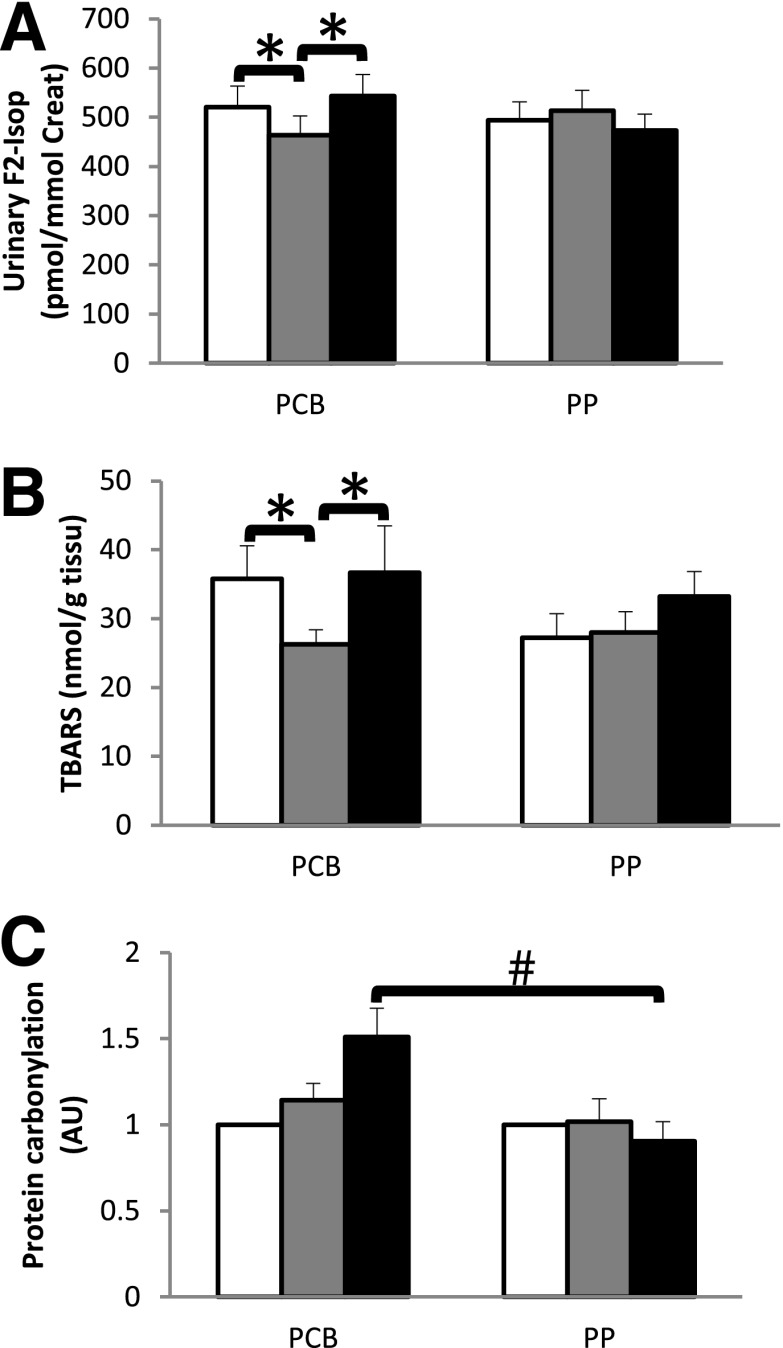
HFrD significantly increased urinary F2-isoprostanes (Fig. 1A) and muscle TBARS (Fig. 1B) in the PCB group, whereas grape PP supplementation protected against this induction of oxidative stress markers. At the end of the fructose load, the level of muscle protein carbonylation was significantly higher in the PCB group compared with the grape PP group (Fig. 1C). Antioxidative capacities, i.e., plasmatic vitamin E, blood glutathione-to-GSSG ratio, erythrocyte SOD, and the muscle enzymatic activities of catalase, GPx, SOD, and MnSOD, remained unaffected during the fructose load in both groups (Supplementary Table 3).
Figure 1.
Protective effects of grape PP supplementation on HFrD-induced oxidative stress. Linking oxidative stress to IR, from baseline (white bars) to post–8 weeks of PCB or grape PP supplementation (gray bars) and after 6 days of HFrD with PCB/grape PP supplementations (black bars) with urinary F2-isiprostanes (Isop) (picomoles per millimole of creatinine [creat]) (18 PCB and 20 PP) (A), TBARS (nanomoles per gram of tissue) (8 PCB and 8 PP) (B), and muscle protein carbonylation as fold induction relative to baseline (arbitrary units [AU]) (8 PCB and 13 PP) (C). *P < 0.05 for statistical pre- and postintervention comparisons within each group via the Wilcoxon signed rank sum test. #P < 0.05 for intergroup comparisons by Mann-Whitney U tests. Data are means ± SEM.
Effects of HFrD on IR and protective effect of grape PP supplementation
A 20% reduction of the fasting hepatic insulin sensitivity index was noted after HFrD (Table 1), associated with an 11% reduction in GIR in the PCB group (Table 1). Grape PP supplementation fully blunted this fructose effect. Digging deeper in the insulin-signaling pathway, we evaluated the levels of phosphorylation of skeletal muscle Akt at serine 473, a critical residue in response to insulin, in the presence and absence of human insulin in a subset of 12 subjects (5 PCB and 7 PP). We found a 53% decrease in the phosphorylated Akt–to–Akt ratio under insulin-stimulated conditions that nevertheless did not reach the level of statistical significance (P = 0.07). This trend was not observed in the grape PP group (Supplementary Fig. 3). The fructose-induced changes of GIR during the clamp were negatively correlated with changes in urinary F2-isoprostanes (r = −0.39, P = 0.02), suggesting a link between IR and oxidative stress in response to fructose (Supplementary Fig. 4).
Effects of grape PP supplementation and of HFrD on transcriptomic profile and gene expression
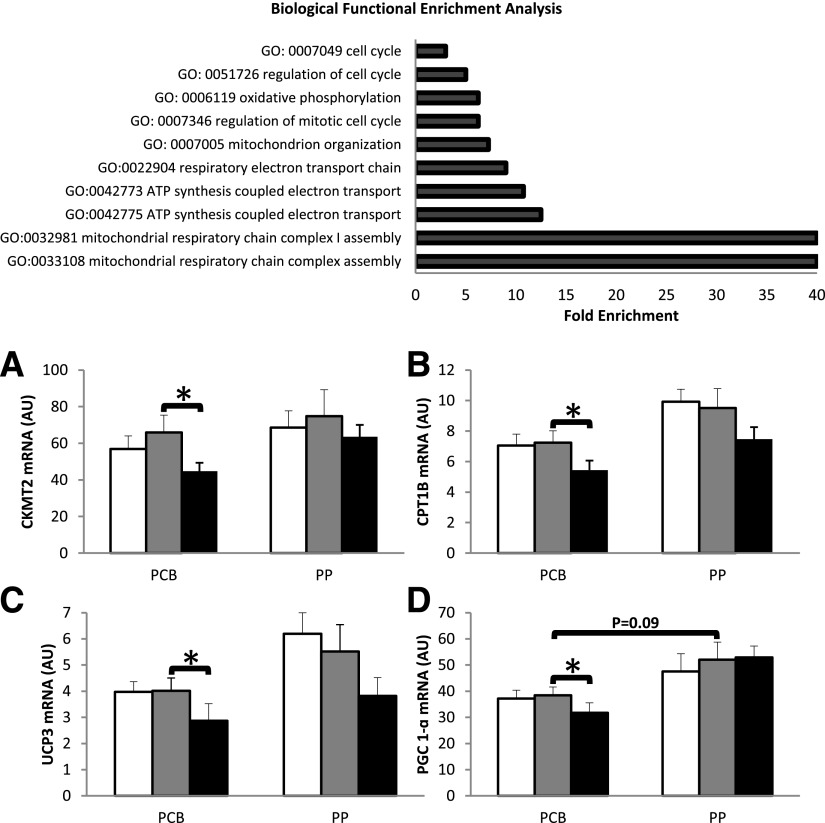
Skeletal muscle transcriptomic profile was not significantly modified by the 8-week grape PP supplementation, with only 66 probes differentially regulated between both groups (P < 0.01 with moderated t test) (Supplementary Table 5). We did not reveal function or pathway enrichment in this small set of genes using functional profiling tools such as Babelomics or DAVID. In contrast, HFrD was accompanied by a differential regulation of 277 genes in skeletal muscle between the two groups (Supplementary Table 6). Functional profiling analysis enlightened a specific enrichment in mitochondrial genes including respiratory chain electron transport and oxidative phosphorylation (OXPHOS) (Fig. 2A). Importantly, most of these differentially regulated genes were downregulated in response to HFrD in the PCB group, while grape PP supplementation counteracted this effect (Supplementary Table 6).
Figure 2.
Differentially regulated biological pathways in response to HFrD and protective effects of grape PP supplementation on fructose-induced alterations of mitochondrial genes and a major transcription factor of mitochondrial biogenesis. A: Analysis of the 277 differentially regulated probes in response to fructose load between both groups was performed with Babelomics (http://babelomics.bioinfo.cipf.es/index.html). Only biological processes with adjusted P value <0.05 and fold enrichment >2 are displayed. Change in mRNA levels of skeletal muscle CKMT2 (B), CPT1B (C), and UCP3 (D) and regulation of PGC-1α mRNA (E), expressed by reference to hypoxanthine guanine phosphoribosyl transferase mRNA abundance, at baseline (white bars), after 8 weeks of PCB or grape PP supplementation (gray bars), and after 6 days of HFrD with PCB/PP supplementations (black bars). Transcript levels were measured by reverse transcriptase–quantitative PCR as described in research design and methods (11 PCB and 12 PP). *P < 0.05 for statistical pre- and postintervention comparisons within each group via the Wilcoxon signed rank sum test; Mann-Whitney U test was used for intergroup comparisons. Data are means ± SEM. AU, arbitrary units.
Reverse transcriptase–quantitative PCR and Western blots further confirmed grape PPs’ protective effect against the aftermath of HFrD on key skeletal muscle mitochondrial genes and proteins, as shown in Fig. 2 for CKMT2 (Fig. 2B), CPT1B (Fig. 2C), and UCP3 mRNA expression (Fig. 2D). A significant decrease was also noted for the mRNA of peroxisome proliferator–activated receptor γ coactivator (PGC)-1α, a central regulator of mitochondrial biogenesis and function (−23% [Fig. 2E]). Regarding UCP3, the fructose-induced reduction in mRNA levels in the PCB group was associated with a trend for a decrease in UCP3 (59% reduction, P = 0.08) (Supplementary Fig. 5). All of these effects were prevented by grape PP supplementation.
Effects of HFrD on skeletal muscle mitochondrial function and protective effect of grape PP supplementation
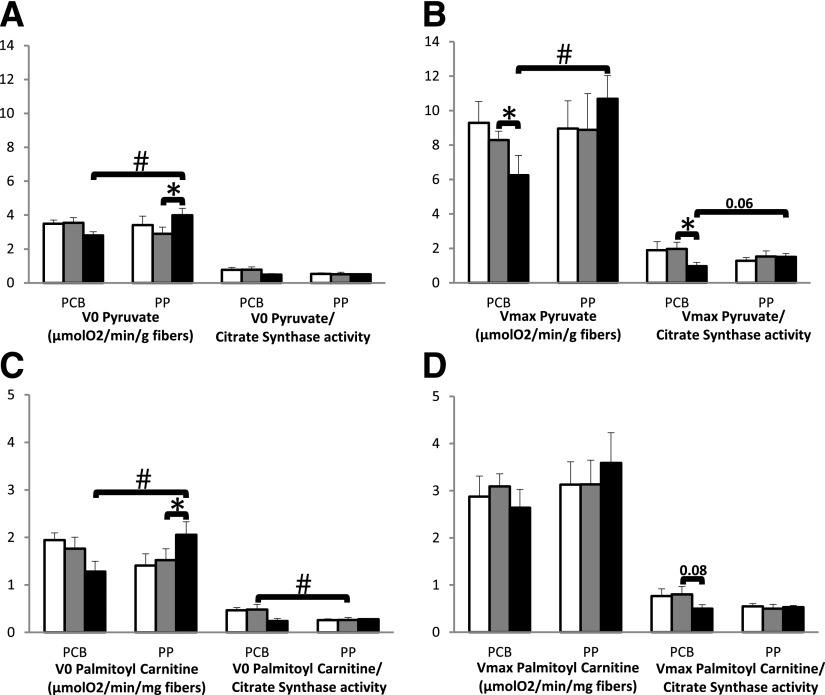
Mitochondrial function was evaluated by oximetry on permeabilized muscle fibers. Both V0 and Vmax rates for pyruvate (Fig. 3A and B) and palmitoyl-l-carnitine (Fig. 3C and D) were decreased in the PCB group by HFrD, while they were significantly increased in the grape PP group. Furthermore, in the PCB group mitochondrial function variation was associated with insulin response, as evidenced by a strong negative correlation between Vmax palmitoyl-l-carnitine change during fructose load and GIR fluctuation (r = −0.97, P < 0.001); this relationship was not found in the grape PP group (Supplementary Fig. 6A and B). When results were corrected for mitochondrial content (citrate synthase activity), fructose had no effect on basal respiration rate (Fig. 3A and C), whereas Vmax remained decreased (Fig. 3B and D), indicating a joint effect on mitochondrial density and function.
Figure 3.
Protective effects of grape PP supplementation against HFrD-induced mitochondrial dysfunction. Mitochondrial functional adaptations at baseline (white bars), after 8 weeks of PCB or grape PP supplementation (gray bars), and after 6 days of HFrD with PCB or PP supplementation (black bars). A and C: Mitochondrial V0 without ADP in the presence of pyruvate or palmitoyl-l-carnitine (V0) (micromoles O2 per minute per gram of fibers) (seven PCB and nine PP) on the left, and on the right, basal measures of respiration are divided by citrate synthase activity to correct for mitochondrial content. B and D: ADP-stimulated mitochondrial Vmax rate (micromoles O2 per minute per gram of fibers) (seven PCB and nine PP) in the presence of pyruvate or palmitoyl-l-carnitine on the left, and on the right, Vmax is divided by citrate synthase activity to correct for mitochondrial content. *P < 0.05 for statistical pre- and postintervention comparisons within each group via the Wilcoxon signed rank sum test. #P < 0.05 for intergroup comparisons by Mann-Whitney U tests. Correlations were established with Spearman rank correlations tests; data are means ± SEM.
CONCLUSIONS
The main finding of the current study is the demonstration that 9 weeks of supplementation with nutritional doses of grape PPs protects against fructose-induced oxidative stress and IR. Microarray analysis demonstrated that expression of many oxidative and mitochondrial genes, including OXPHOS genes and CPT1, was reduced in response to HFrD in skeletal muscle. A potential contribution to this observation in IR may be inferred from other studies reporting that expression of OXPHOS genes is decreased and multiple energy metabolism genes’ expression is altered in the context of type 2 diabetes in humans (23–25) and that a decrease in CPT activity, which transports long-chain acyl-CoA into the mitochondria, may lead to intracellular lipid accumulation triggering IR (26). We further demonstrated that fructose has detrimental effects on mitochondrial respiration when either pyruvate or palmitoyl-l-carnitine was used as a substrate. This effect appears to be related both to variations in mitochondrial density as reflected by the lack of reduction in mitochondrial basal respiration by fructose when data were corrected for mitochondrial content (citrate synthase activity) and to altered function as exemplified by the decreased Vmax rate corrected for mitochondrial density in response to fructose. Fructose impairment of mitochondrial gene expression and mitochondrial function could result from altered expression of PGC-1α, a critical transcriptional regulator of nuclear-encoded mitochondrial genes whose reduced expression and activity has been related to IR and was suggested to be a primary feature of prediabetes pathophysiology (24).
As oxidative stress participates in skeletal muscle mitochondrial dysfunction, including altered biogenesis (27), it is tempting to speculate that fructose-induced mitochondrial alterations were related to oxidative stress. Our data demonstrating that grape PP supplementation prevents both oxidative stress in the presence of fructose and the downfall of mitochondrial function with maintenance of mitochondrial gene expression at normal levels in skeletal muscle support this hypothesis.
The protective effect of grape PPs on muscle oxidative stress and mitochondria was associated with maintenance of insulin sensitivity in the face of fructose overload. We found a negative correlation between fructose-induced changes of urinary F2-isoprostanes and GIR during the clamp, suggesting a link between oxidative stress and fructose-induced IR. Similarly, there was a strong negative correlation between the decrease in GIR and Vmax palmitoyl-l-carnitine variations during fructose overload (r = −0.97), powerfully supporting a direct association between fructose-induced decreased skeletal muscle mitochondrial function and IR. Therefore, by promoting mitochondrial biogenesis, reducing oxidative stress, and facilitating the uncoupling of oxidative metabolism, grape PPs may help maintain high levels of substrate oxidation while limiting free radical production, explaining their protective effect with respect to fructose-induced IR.
Major adipokines and hormones were not affected by grape PP supplementation, which is somewhat different from what is seen in animal models with grape seed procyanidin modulating CRP and adiponectin plasma levels in rats fed a hyperlipidic diet (28). This may suggest that, in the current study, grape PPs acted directly on metabolic tissues (skeletal muscle and liver) without systemic changes in regulating factors.
Whatever the mechanism, the protective effect of grape PPs on IR and oxidative stress was not associated with a reduction of fasting plasma triglycerides, indicating that it failed to impact the metabolic steps involved in hepatic de novo lipogenesis. Surprisingly, HFrD was associated with a decrease in LDL cholesterol in the PCB group only. Nevertheless, LDL particles in this group were more atherogenic as testified by a decrease in small-dense LDL size that was counteracted by grape PPs (29).
Even though resveratrol accounts for a very small portion of the grape PPs used in the present work, our results present similarities with the metabolic effects of this compound. Indeed, as for the latter, grape PPs were able to prevent diet-induced IR and potentially weight gain—effects that could be mediated by a control of mitochondrial biogenesis and respiration secondary to PGC-1α activation (30,31). We also obtained results similar to those of the clinical study of Timmers et al. (32) using resveratrol, including the upregulation of mitochondrial genes with an improved function and increased PGC-1α level favoring mitochondrial biogenesis; using the hyperinsulinemic-euglycemic clamp, we further complement the results of these authors showing an 11% improvement in homeostasis model assessment index after resveratrol (32).
Grape in itself is a panoply of polyphenols consisting mostly of anthocyanins and flavonols, including quercetin (33), much of which has also demonstrated multiple metabolic health benefits (6–8). Even though the dose of 2 g/day polyphenols used in the current study can be regarded as a high dose, it remains nevertheless compatible with the supply of a diet rich in red grapes, other fruits and vegetables, and eventually red wine.
By nature, the use of a polyphenol mixture does not enable us to identify clearly what components of the grape extract are causing any of the observed effects.
We would further like to recall that grape PPs are already used by the food industry in the form of dyes and tannins, notably in sugary foods (11). Our results constitute a basis for considering further work studying the effects of direct addition of PPs to foods containing fructose.
In conclusion, 9 weeks of grape PP supplementation secures an unwavering metabolic state in healthy overweight/obese first-degree relatives of type 2 diabetic subjects faced with a 6-day fructose overload, preventing liver and muscle IR while bearing no adverse effects. Future studies should investigate the effects of grape PP coadministration with processed food rich in fructose and their potential role in counteracting the metabolic syndrome.
Acknowledgments
This study was funded by the French National Research Agency Agence Nationale de la Recherche (PolyOxResist-2007-A01375-48).
No potential conflicts of interest relevant to this article were reported.
The authors thank the two major distilleries of the Languedoc-Roussillon area, Société Française de Distilleries and GRAP’SUD, for provision of the grape extracts.
M.H., E.B., H.V., K.L., M.L., and A.A. researched data, contributed to discussion, and reviewed, edited, and wrote the manuscript. E.M. researched data. C.F.-C. and C.C. contributed to discussion and reviewed and edited the manuscript. S.P., C.L., S.L.-P., V.S., C.F., J.-F.B., and J.R. researched data. C.B., A.S., J.M., J.G., A.-M.D., and J.-P.C. contributed to discussion and reviewed and edited the manuscript. A.A is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank G. Fourret (INRA UMR 866, Unité Dynamique Musculaire et Métabolisme), M.-C. Granat (University Hospital of Montpellier), and C. Bezes (University Hospital of Montpellier) for technical support as well as A. Coma (University Hospital of Montpellier) and A. Cadène (University Hospital of Montpellier) for administrative work relating to the implementation of good clinical practice throughout the study. The authors also express deep gratitude to all of the recruited subjects and the members of the clinical and research teams of both investigation centers (Department of Nutrition-Diabetes, University Hospital of Montpellier, and the Rhône-Alpes Research Center for Human Nutrition, University Hospital of Lyon).
Footnotes
Clinical trial reg. no. NCT01478841, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1652/-/DC1.
References
- 1.Monteiro CA, Cannon G. The impact of transnational “big food” companies on the South: a view from Brazil. PLoS Med 2012;9:e1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tappy L, Lê KA, Tran C, Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition 2010;26:1044–1049 [DOI] [PubMed] [Google Scholar]
- 3.Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y. High fructose feeding of magnesium deficient rats is associated with increased plasma triglyceride concentration and increased oxidative stress. Magnes Res 2003;16:7–12 [PubMed] [Google Scholar]
- 4.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E685–E694 [DOI] [PubMed] [Google Scholar]
- 5.De Lorgeril M, Salen P, Martin JL, et al. Effect of a mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the cardioprotective effect of certain nutriments. J Am Coll Cardiol 1996;28:1103–1108 [DOI] [PubMed] [Google Scholar]
- 6.Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr 2005;135:2291–2294 [DOI] [PubMed] [Google Scholar]
- 7.Terra X, Montagut G, Bustos M, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 2009;20:210–218 [DOI] [PubMed] [Google Scholar]
- 8.Pinent M, Blay M, Bladé MC, Salvadó MJ, Arola L, Ardévol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004;145:4985–4990 [DOI] [PubMed] [Google Scholar]
- 9.Napoli R, Cozzolino D, Guardasole V, et al. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism 2005;54:306–313 [DOI] [PubMed] [Google Scholar]
- 10.Cordain L, Melby CL, Hamamoto AE, et al. Influence of moderate chronic wine consumption on insulin sensitivity and other correlates of syndrome X in moderately obese women. Metabolism 2000;49:1473–1478 [DOI] [PubMed] [Google Scholar]
- 11.Gollücke APB. Recent applications of grape polyphenols in foods, beverages and supplements. Recent Pat Food Nutr Agric 2010;2:105–109 [PubMed] [Google Scholar]
- 12.Voorrips LE, Ravelli AC, Dongelmans PC, Deurenberg P, Van Staveren WA. A physical activity questionnaire for the elderly. Med Sci Sports Exerc 1991;23:974–979 [PubMed] [Google Scholar]
- 13.Laville M, Auboeuf D, Khalfallah Y, Vega N, Riou JP, Vidal H. Acute regulation by insulin of phosphatidylinositol-3-kinase, Rad, Glut 4, and lipoprotein lipase mRNA levels in human muscle. J Clin Invest 1996;98:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducluzeau PH, Perretti N, Laville M, et al. Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes 2001;50:1134–1142 [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Sirvent P, Perrey S, Raynaud E, Mercier J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J Appl Physiol 2004;97:2132–2138 [DOI] [PubMed] [Google Scholar]
- 17.Bravard A, Bonnard C, Durand A, et al. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab 2011;300:E581–E591 [DOI] [PubMed] [Google Scholar]
- 18.Feillet-Coudray C, Sutra T, Fouret G, et al. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic Biol Med 2009;46:624–632 [DOI] [PubMed] [Google Scholar]
- 19.Mas E, Michel F, Guy A, et al. Quantification of urinary F2-isoprostanes with 4(RS)-F4t-neuroprostane as an internal standard using gas chromatography-mass spectrometry Application to polytraumatized patients. J Chromatogr B Analyt Technol Biomed Life Sci 2008;872:133–140 [DOI] [PubMed] [Google Scholar]
- 20.Srere PA. An eclectic view of metabolic regulation: control of citrate synthase activity. Adv Enzyme Regul 1970;9:221–233 [DOI] [PubMed] [Google Scholar]
- 21.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004;3:Article3 [DOI] [PubMed]
- 22.Bravard A, Lefai E, Meugnier E, et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 2011;60:258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 2002;51:1913–1920 [DOI] [PubMed] [Google Scholar]
- 24.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debard C, Laville M, Berbe V, et al. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia 2004;47:917–925 [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Liu Z-X, Choi CS, et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 2007;104:17075–17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terra X, Pallarés V, Ardèvol A, et al. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem 2011;22:380–387 [DOI] [PubMed] [Google Scholar]
- 29.Rizzo M, Berneis K. Small, dense low-density-lipoproteins and the metabolic syndrome. Diabetes Metab Res Rev 2007;23:14–20 [DOI] [PubMed] [Google Scholar]
- 30.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 32.Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang C-C, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr 2011;31:155–176 [DOI] [PubMed] [Google Scholar]