Abstract
OBJECTIVE
Development of critical limb ischemia (CLI) has been reported as an independent predictor of cardiac mortality in diabetic patients. We aimed to determine whether CLI, managed in a structured setting of close collaboration between different vascular specialists and treated with early endovascular intervention, has any impact on long-term cardiac mortality of diabetic patients initially presenting with symptomatic coronary artery disease (CAD).
RESEARCH DESIGN AND METHODS
We designed a prospective observational study of 764 consecutive diabetic patients undergoing percutaneous coronary intervention (PCI) in whom development of CLI was assessed by a dedicated diabetic foot clinic. Cardiac mortality at 4-year follow-up was the primary end point of the study.
RESULTS
Among the 764 patients, 111 (14%) developed CLI (PCI-CLI group) and underwent revascularization of 145 limbs, with procedural success in 140 (96%). PCI-CLI patients at baseline had lower left ventricular ejection fraction (51 ± 11% vs. 53 ± 10%, P = 0.008), higher prevalence of dialysis (7% vs. 0.3%, P < 0.0001), and longer diabetes duration (13 ± 8 vs. 11 ± 7 years, P = 0.02) compared with PCI-only patients. At 4-year follow-up, cardiac mortality occurred in 10 (9%) PCI-CLI patients vs. 42 (6%) PCI-only patients (P = 0.2). Time-dependent Cox regression model for cardiac death revealed that CLI was not associated with an increased risk of cardiac mortality (hazard ratio 1.08 [95% CI 0.89–3.85]; P = 0.1).
CONCLUSIONS
The development of promptly assessed and aggressively treated CLI was not significantly associated with increased risk of long-term cardiac mortality in diabetic patients initially presenting with symptomatic CAD.
Diabetes is a major risk factor for cardiovascular morbidity and mortality (1–4). This condition increases the risk of developing coronary artery disease (CAD), cerebrovascular artery disease, and peripheral artery disease (PAD) as much as fourfold and worsens the prognosis of patients with vascular disease at each stage of the disease process (3,5). Diabetes increases approximately twofold to fourfold the incidence and severity of critical limb ischemia (CLI), the end-stage clinical manifestation of peripheral arterial disease and the leading cause of nontraumatic amputation in Western countries (6). CLI, with or without lower-extremity amputation, is reportedly an independent predictor of cardiac mortality in diabetic patients, with the excess mortality also related to a high prevalence of severe CAD (7–18). Although previous studies highlighted PAD as an independent predictor of adverse events and cardiac mortality in patients initially presenting with symptomatic CAD undergoing percutaneous coronary interventions (PCIs) (19,20), diagnostic criteria, the clinical status and treatment strategy (medical treatment or limb revascularization) of PAD were not specified. For a better understanding of the clinical impact of the association of CAD with CLI in diabetic patients and of the potential effect of coronary and limb revascularization on long-term cardiac mortality, we followed all diabetic patients undergoing PCI at our institution by means of a dedicated clinical pathway and compared the outcomes of the patients who developed CLI with those of the patients who did not develop this condition.
RESEARCH DESIGN AND METHODS
The study was designed as a prospective, observational, referral center cohort study of consecutive diabetic patients who underwent PCI. All incident cases of CLI were recorded and followed within a structured, collaborative framework (diabetologist, foot care specialist, vascular surgeon, interventional cardiologist). This model of strict collaboration among different professional figures with a dedicated pathway for diabetic patients and early, aggressive attempts at endovascular revascularization, has been previously described (21) and demonstrated to result in a very low amputation rate.
Consecutive diabetic patients undergoing PCI with or without stent implantation for either acute coronary syndrome or stable coronary disease between July 2002 and May 2007 at the cardiovascular department of San Donato Hospital (Arezzo, Italy) were enrolled. This is a PCI and peripheral interventions center serving a population of 350,000 in Central Italy. Presence of diabetes, need for coronary revascularization, absence of clinical contraindications to prolonged double antiplatelet therapy, and potential long life expectancy were the only criteria for study entry. Diabetes status was ascertained during the index procedure: all patients taking any antidiabetic drug (including metformin withdrawn before the procedure) or insulin were considered patients with diabetes. All patients had to give written informed consent. The study was approved by our institutional ethics committee.
Once discharged, all patients were asked to return at specified intervals (at 1 month, then every 6 months) to a dedicated PCI outpatient clinic for follow-up. A diabetologist reviewed the patients during the same appointment. Relevant data were collected and entered into a computer database. For those patients who did not return at the designated time, follow-up information was collected by telephone interview. All patients developing symptoms possibly related to myocardial ischemia had a rapid-access outpatient visit for clinical, electrocardiographic, laboratory, and possible angiographic assessments.
If CLI was suspected at clinical examination, patients were also reviewed by the foot clinic specialist. The aim was to establish a definitive diagnosis of CLI, defined as a condition characterized by chronic ischemic pain at rest, ulcers, or gangrene in one or both legs attributable to objectively proven arterial occlusive disease and reported according to the University of Texas Wound Classification System (22). Neuropathic gangrene was also ruled out.
In cases of confirmed CLI, culprit limb angiography and revascularization (percutaneous transluminal angioplasty) were attempted within 1 week in all cases. CLI patients were also followed by the foot clinic specialist until complete healing of the lesions was observed and underwent control duplex scan with ankle brachial index measurement every 6 months afterward. In cases of CLI recurrence, angiography and repeat revascularization were immediately performed. In cases of target vessel occlusion on duplex scan, percutaneous transluminal angioplasty was repeated only in the presence of symptoms.
End point definitions
The primary end point of our study was the incidence of cardiac mortality at the longest possible follow-up. All deaths were considered to be of cardiac origin unless a noncardiac origin was established clinically or at autopsy. Hospital notes and autopsy reports were reviewed for patients who died in the hospital. All other possible information derived from hospital readmission, the referring physician, relatives, or municipality live registries was entered into the prospective database.
Major adverse cardiac events were also recorded and defined as death, nonfatal myocardial infarction (MI), nonfatal stroke, and ischemia-driven repeat revascularization of the target lesion. MI was defined as the presence of new Q waves in ≥2 contiguous electrocardiographic leads or an elevation of creatine kinase or its MB isoenzyme to ≥2 times the upper limit of normal. Ischemia-driven repeat revascularization of the target lesion was defined as any repeat PCI or aortocoronary bypass surgery necessitated by lumen renarrowing within the stent, or in the 5-mm segments distal or proximal to the stent, associated with symptoms or objective signs of ischemia. Coronary stent thrombosis was classified according to the Academic Research Consortium definition (23). Stroke was defined as an acute neurologic deficit lasting longer than 24 h. All events were adjudicated by an event adjudication committee (I.P., R.B., and L.R.).
Coronary and peripheral revascularization
Coronary angioplasty was performed according to commonly accepted standards. The type of stent used, the administration of glycoprotein II or IIIa inhibitors, and the interventional strategy were left to operators’ discretion. PCI was considered successful when thrombolysis in MI flow grade III and a <30% residual stenosis were obtained in the culprit vessel. Complete myocardial revascularization was considered when no stenosis >50% by visual estimation was present in the coronary vessels at the end of the procedure. Plasma concentrations of creatine kinase and its MB isoenzyme were systematically determined for 12 h after the intervention. Combined antiplatelet therapy with aspirin (≥100 mg daily) and clopidogrel (75 mg daily) was started at least 24 h before procedure and continued for at least 1 month in patients receiving bare metal stents and 12 months in patients receiving drug-eluting stents.
Limb revascularization was performed with an antegrade femoral approach in most cases. Retrograde contralateral femoral and retrograde ipsilateral popliteal approaches were performed in cases of ostial occlusion of the superficial femoral artery or stenosis of the common femoral artery of the culprit limb. Retrograde tibial approach was performed in case of failure of the antegrade recanalization. Balloon angioplasty was followed by nitinol stent implantation only in case of suboptimal angiographic results in the superficial femoral artery or popliteal artery. No stents were used in the infrapopliteal arteries. Limb revascularization was considered successful if it reestablished continuous in-line flow to the pedal arch. Antegrade femoral sheaths were removed by operators at the end of the procedure after heparin reversion with protamine.
Angiographic analysis
Coronary angiograms were analyzed by a semiautomated edge contour detection computer analysis system (MEDIS QCA CMS, version 4). Reference diameter, minimal lumen diameter, percentage diameter stenosis, and lesion length were measured before and at the end of the procedure.
Statistical analysis
Values are reported as number of patients with relative percentage or mean ± SD. Nominal variables were compared with the Fisher exact test; continuous variables were compared with t test. To assess whether CLI was an independent predictor of cardiac mortality, a time-sensitive Cox proportional hazard regression model was used for estimating the hazard ratios (HR) and corresponding 95% CIs. First, a univariate exploratory analysis was performed that included CLI as well as the baseline clinical variables. Next, a multivariable model was constructed that included CLI along with the variables with a probability value <0.05 at univariate analysis, which were entered en bloc into the multivariate model, with age and sex as background variables. Analyses were performed with SPSS software, version 19 (IBM Corporation, Armonk, NY).
RESULTS
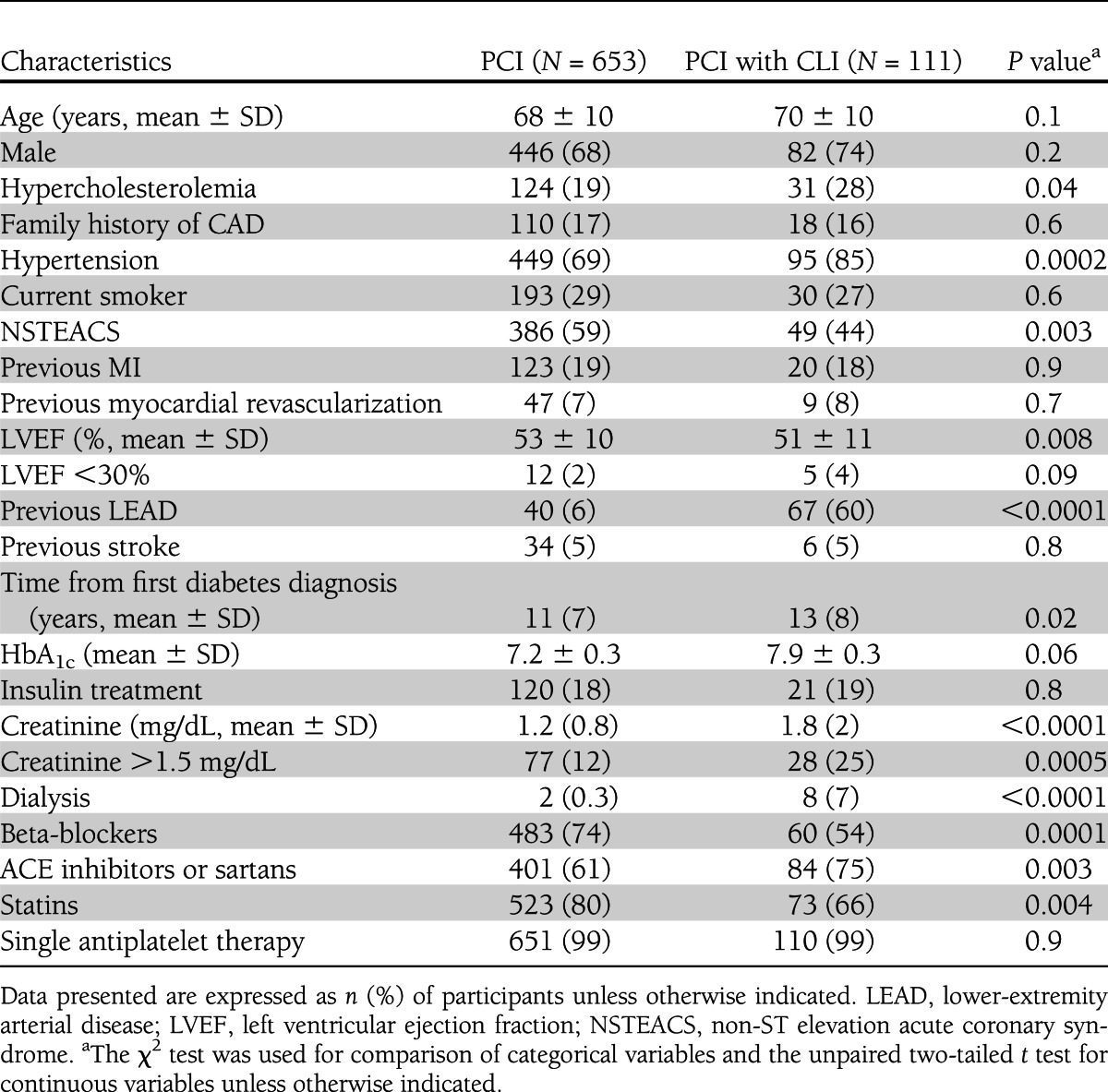
During the study period, 917 diabetic patients underwent PCI at the San Donato Hospital (Supplementary Fig. 1). Among these patients, 764 were enrolled in the study, whereas 153 were excluded for the following reasons: lack of informed consent (47 patients), refusal to participate (41 patients), contraindications to prolonged dual antiplatelet therapy (47 patients), and short life expectancy (18 patients). Twenty patients underwent PCI and limb revascularization in the same index hospitalization and 91 developed CLI and underwent limb revascularization during follow-up, for a total of 111 (14%) patients with both PCI and CLI (PCI-CLI group). Baseline clinical characteristics of PCI-only and PCI-CLI patients are reported in Table 1. Patients with CLI were more hypertensive, were more likely to present with acute coronary syndrome, had lower left ventricular ejection fraction and more impaired renal function, and were more often on dialysis. They also had more frequently a previous diagnosis of peripheral arterial disease and were less frequently treated with beta-blockers and statins. No significant differences were noted between the study groups in terms of coronary angiographic and procedural characteristics (Supplementary Table 1). Clinical and procedural data related to peripheral intervention in the 145 limbs of PCI-CLI patients are reported in Supplementary Table 2.
Table 1.
Baseline clinical characteristics
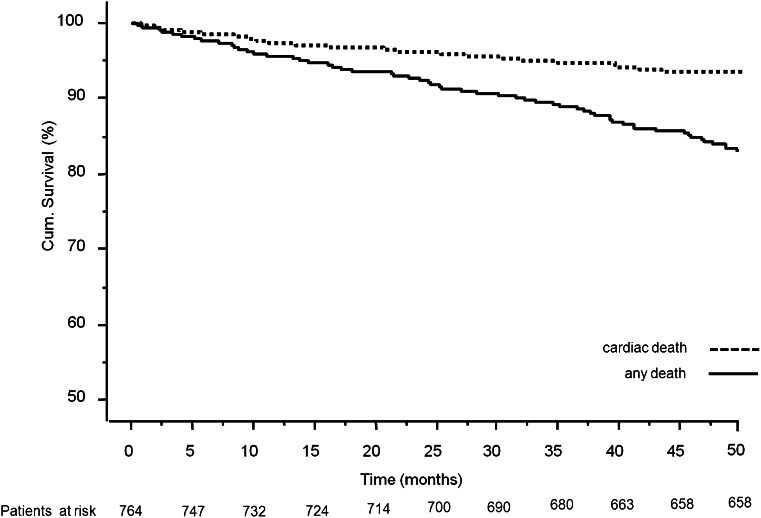
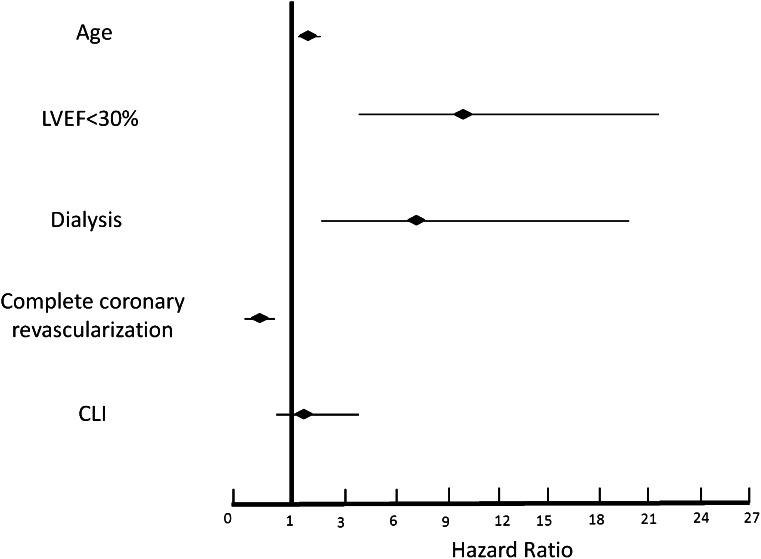
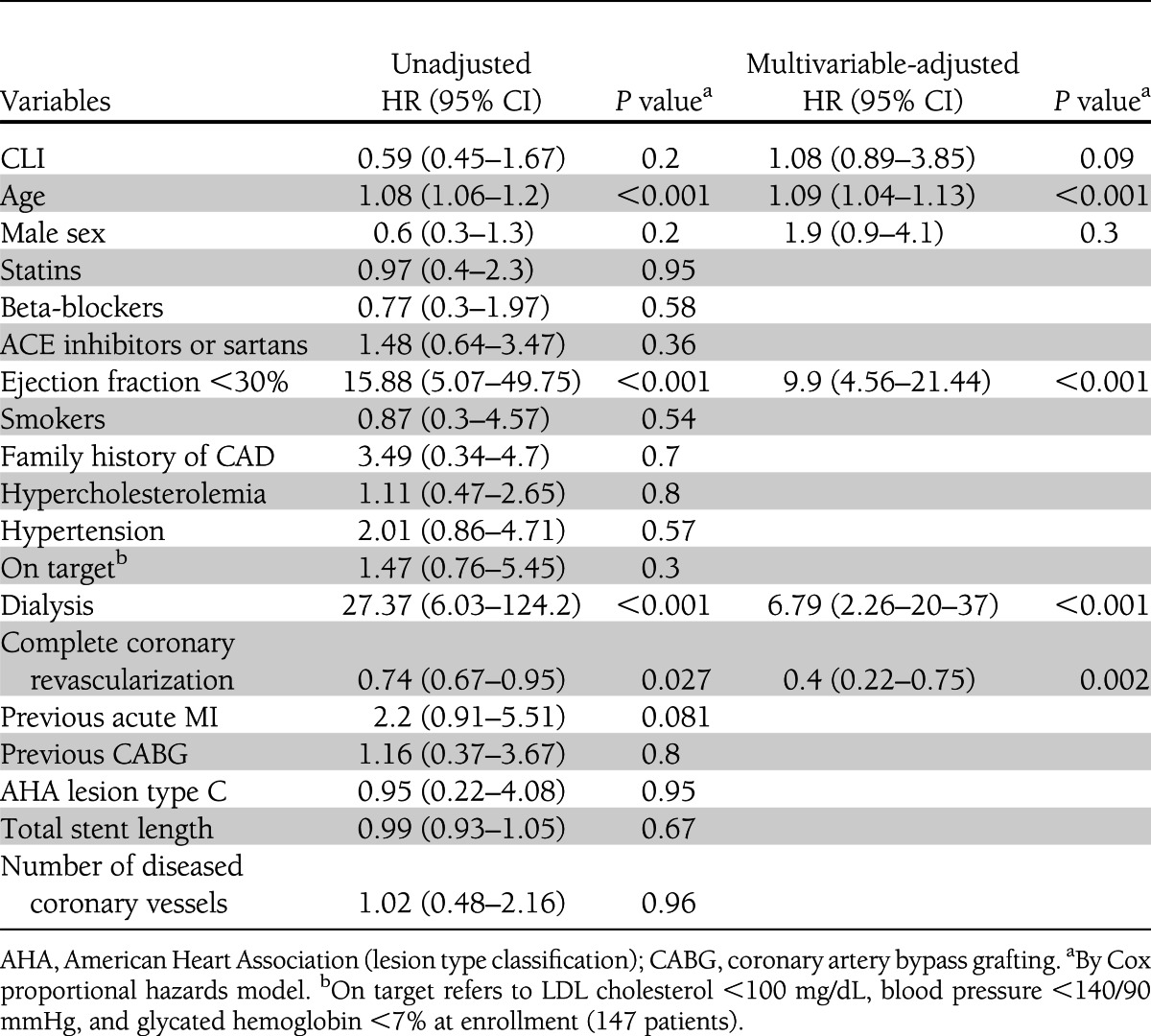
Major adverse cardiac event occurrences at 4-year follow-up for both study groups are reported in Supplementary Table 3. Overall mortality was 17% (132 patients) (Fig. 1), 16% among PCI-only patients and 24% among PCI-CLI patients (P = 0.02). Cardiac death occurred in 52 (6.8%) patients, 42 (6%) PCI-only patients and 10 (9%) PCI-CLI patients (P = 0.2). Supplementary Table 4 reports the detailed causes of death. Time-sensitive Cox proportional hazard regression model for cardiac death revealed age (HR 1.09 [95% CI 1.05–1.13]; P < 0.001), left ventricular ejection fraction ≤30% (9.90 [4.56–21.44]; P < 0.001) dialysis (6.79 [2.26–20.37]; P < 0.001) and a complete coronary revascularization (0.40 [0.22–0.75]; P = 0.002) to be independent predictors of cardiac death. Development of CLI was not independently associated with an increased risk of cardiac mortality (1.08 [0.89–3.85]; P = 0.09) (Fig. 2 and Table 2).
Figure 1.
Cox survival model for total mortality and cardiac mortality at 4-year follow-up in the overall study population.
Figure 2.
Time-sensitive Cox proportional hazard regression model for independent predictors of cardiac death at 4-year follow-up in the overall study population. HR is reported with relative 95% CI. LVEF, left ventricular ejection fraction.
Table 2.
Univariable- and multivariable-adjusted predictors of primary end point (cardiac death)
CONCLUSIONS
Although previous studies have shown high cardiac mortality rates in diabetic patients with CLI, major design flaws did not allow inferences regarding the prognostic impact of the association of coronary and acute PAD and the role of revascularization. We therefore evaluated the impact of CLI on cardiac mortality in a large population of consecutive diabetic patients with known CAD successfully treated with PCI who were closely followed up and aggressively revascularized (if CLI developed) in a dedicated outpatient cardiologic and diabetic foot clinic. Our aim was to ascertain the independent prognostic value of CLI in a combined care setting of close collaboration between different vascular specialists.
The major findings of the current study are as follows: 1) Development of CLI was not an independent predictor of cardiac mortality at a mean follow-up of 4 years. Although this finding is seemingly in contrast with the trend toward increased cardiac mortality in CLI patients, such a phenomenon could be mainly explained by the higher risk factor burden in this cohort and thus by the associated variables. 2) Age, left ventricular ejection fraction, and dialysis were the main independent predictors of cardiac mortality in patients with diabetes with or without CLI. 3) A strategy of close observation for development of CLI and early percutaneous revascularization of the ischemic limbs was highly effective, with a major amputation rate of only 4% at 4 years.
The cardiac mortality of 6% at 4 years observed among PCI patients was similar to that reported for stable CAD patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial (24), whereas the rate of 9% among PCI-CLI patients was substantially lower than previously reported rates. Indeed, a total mortality of 50%, a cardiac mortality of 31%, and a major amputation rate of 13% at a mean follow-up of 5.9 years were reported by Faglia et al. (15) in 564 consecutive CLI patients. Similar results have been reported by other authors (8,25). The low cardiac mortality rate observed in PCI-CLI patients in this study was probably due to a combination of cardiovascular risk management, coronary revascularization, and low major amputation rate.
With regard to risk factor management, it should be noted that our patients had a first diagnosis of CAD and that their care team consistently included a cardiologist. Patients with PAD as first diagnosis were found to be less intensively managed for hypertension and hyperlipidemia and less often receiving antiplatelet therapy than patients with CAD with or without PAD in primary care (26,27). In our study, although medical therapy was still not optimal, all patients with or without CLI were receiving ≥1 antiplatelet medication indefinitely, >65% of patients were receiving statins, and all patients with hypertension were receiving either beta-blockers or angiotensin converting enzyme inhibitors. Although Cox regression did not show a significant interaction between cardiac mortality and any of these drugs, several reports have demonstrated a mortality reduction in diabetic CAD and diabetic CLI patients with an aggressive cardiovascular risk management policy (26,28–30).
With respect to coronary revascularization, we enrolled patients only after PCI to avoid a potential bias from underlying undiagnosed CAD. In a study by Faglia et al. (31), 4-year cardiac mortalities in CLI patients hospitalized for limb revascularization were 10% among diabetic patients without a history of CAD, 38% among patients with history of CAD without previous myocardial revascularization, 15% in CAD patients with previous myocardial revascularization, and only 2% in patients in whom myocardial revascularization was performed immediately after revascularization of the ischemic limb. The indications for coronary angiography in this study, however, were based on ventricular function, and no functional data were reported.
In our series, the major amputation rate was 4% at 4 years, and this may have contributed to the low cardiac mortality observed (9,10,13,16). The low amputation rate was probably a consequence of a dedicated clinical pathway for PCI-CLI patients, with continuous clinical observation of the foot lesion healing process and strict monitoring of vessel patency.
The excess cardiac mortality among PCI-CLI patients, according to multivariate analysis, was related to age, dialysis, severe left ventricular systolic dysfunction, and incomplete coronary revascularization (all factors associated with CLI) and not to CLI itself. This concept is also strengthened by the consistent ratio of cardiac to noncardiac mortality of 2:3 in both CLI and non-CLI patients. These findings are in agreement with those reported by previous studies either in diabetic CAD or diabetic CAD plus CLI patients (15,32–34). Heart failure was responsible for most cardiac deaths in both groups, and this may be a consequence of postinfarction remodeling, which is more frequent in patients with diabetes, or of diabetic cardiomyopathy (35,36). Dialysis represents the end stage of diabetic nephropathy and was a crucial determinant of the development of CLI and of the difference in cardiac mortality between PCI and PCI-CLI patients. As reported in a previous study, CLI represents a main cause of death among patients with end-stage renal disease (37).
Another important concern is the relatively high incidence of noncardiac mortality in our sample, mainly of stroke, sepsis (especially among patients who developed CLI), and malignancy, which underlines the importance of a close, interdisciplinary follow-up of these frail, elderly diabetic patients (38).
Study limitations
First, as a real-world single-center registry, the study is limited by a lack of a valid control group. One should also recognize, however, that a randomized study would be very difficult to implement in this particular subset of patients. Second, our findings stem from a tight cooperation of multidisciplinary professionals aimed at arranging a dedicated pathway for the treatment of diabetic patients with systemic atherosclerosis and therefore might not be reproduced in different settings. Third, although we believe that strict control of risk factors could in part explain our results, we were not able to find a correlation between clinical outcome and being on-target at baseline on variables such as LDL cholesterol, blood pressure, and glycemic control. Although this could be because many of our patients were enrolled during their first episode of manifest CAD, the lack of consistent reporting of such variables is an inherent limitation of our result. Finally, non-CLI symptomatic or asymptomatic PAD status was also not consistently reported in our database, limiting possible inferences on such patients. Reporting the survival data on such patients, however, might have shifted the main focus of our study, the prognosis of CLI patients.
Conclusion
CLI in diabetic patients with CAD may have no influence on clinical outcome and cardiac death in the context of a global cardiovascular program including clinical and interventional diagnostic and therapeutic activities with both peripheral and coronary percutaneous revascularization.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
F.L. wrote the manuscript and researched data. P.A., S.G., I.P., K.D., and G.F. contributed to the discussion and reviewed and edited the manuscript. R.B. researched data. L.R. and D.T. contributed to the discussion. G.B. and L.B. reviewed and edited the manuscript. F.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01676519, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1603/-/DC1.
References
- 1.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003;108:1527–1532 [DOI] [PubMed] [Google Scholar]
- 2.Lüscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003;108:1655–1661 [DOI] [PubMed] [Google Scholar]
- 3.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007;167:1145–1151 [DOI] [PubMed] [Google Scholar]
- 4.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 5.Hackam DG, Tan MK, Honos GN, Leiter LA, Langer A, Goodman SG, Vascular Protection registry investigators How does the prognosis of diabetes compare with that of established vascular disease? Insights from the Canadian Vascular Protection (VP) Registry. Am Heart J 2004;148:1028–1033 [DOI] [PubMed] [Google Scholar]
- 6.Resnick HE, Valsania P, Phillips CL. Diabetes mellitus and nontraumatic lower extremity amputation in black and white Americans: the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study, 1971-1992. Arch Intern Med 1999;159:2470–2475 [DOI] [PubMed] [Google Scholar]
- 7.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–386 [DOI] [PubMed] [Google Scholar]
- 8.Uccioli L, Gandini R, Giurato L, et al. Long-term outcomes of diabetic patients with critical limb ischemia followed in a tertiary referral diabetic foot clinic. Diabetes Care 2010;33:977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, Leese GP, DARTS/MEMO Collaboration Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care 2006;29:2252–2256 [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Carter EA, Lindsay R, et al. Relation of lower-extremity amputation to all-cause and cardiovascular disease mortality in American Indians: the Strong Heart Study. Diabetes Care 2004;27:1286–1293 [DOI] [PubMed] [Google Scholar]
- 11.Norman PE, Davis WA, Bruce DG, Davis TM. Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2006;29:575–580 [DOI] [PubMed] [Google Scholar]
- 12.Leibson CL, Ransom JE, Olson W, Zimmerman BR, O’fallon WM, Palumbo PJ. Peripheral arterial disease, diabetes, and mortality. Diabetes Care 2004;27:2843–2849 [DOI] [PubMed] [Google Scholar]
- 13.Hambleton IR, Jonnalagadda R, Davis CR, Fraser HS, Chaturvedi N, Hennis AJ. All-cause mortality after diabetes-related amputation in Barbados: a prospective case-control study. Diabetes Care 2009;32:306–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flu HC, Lardenoye JH, Veen EJ, Aquarius AE, Van Berge Henegouwen DP, Hamming JF. Morbidity and mortality caused by cardiac adverse events after revascularization for critical limb ischemia. Ann Vasc Surg 2009;23:583–597 [DOI] [PubMed] [Google Scholar]
- 15.Faglia E, Clerici G, Clerissi J, et al. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: data of a cohort study of 564 patients. Eur J Vasc Endovasc Surg 2006;32:484–490 [DOI] [PubMed] [Google Scholar]
- 16.de Virgilio C, Toosie K, Lewis RJ, et al. Cardiac morbidity and operative mortality following lower-extremity amputation: the significance of multiple Eagle criteria. Ann Vasc Surg 1999;13:204–208 [DOI] [PubMed] [Google Scholar]
- 17.Belmont PJ, Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg 2011;213:370–378 [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med 1998;3:241–245 [DOI] [PubMed] [Google Scholar]
- 19.Saw J, Bhatt DL, Moliterno DJ, et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol 2006;48:1567–1572 [DOI] [PubMed] [Google Scholar]
- 20.Eagle KA, Rihal CS, Foster ED, Mickel MC, Gersh BJ, The Coronary Artery Surgery Study (CASS) Investigators Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. J Am Coll Cardiol 1994;23:1091–1095 [DOI] [PubMed] [Google Scholar]
- 21.Liistro F, Grotti S, Venturuzzo G, et al. [Clinical outcome of percutaneous revascularization by stent-assisted balloon angioplasty of femoro-popliteal and tibial vessels in diabetic patients with critical limb ischemia]. Italian. G Ital Cardiol (Rome) 2009;10:713–717 [PubMed] [Google Scholar]
- 22.Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 2001;24:84–88 [DOI] [PubMed] [Google Scholar]
- 23.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007;356:1020–1029 [DOI] [PubMed] [Google Scholar]
- 24.Goldfine AB, Fonseca V. Management of diabetes mellitus in patients with cardiovascular disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 2010;121:2447–2449 [DOI] [PubMed] [Google Scholar]
- 25.Ghanassia E, Villon L, Thuan Dit Dieudonné JF, Boegner C, Avignon A, Sultan A. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5-year follow-up study. Diabetes Care 2008;31:1288–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med 2002;17:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med 1997;12:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286:1317–1324 [DOI] [PubMed] [Google Scholar]
- 29.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Namini H, Seely L. Risk factors, medical therapies and perioperative events in limb salvage surgery: observations from the PREVENT III multicenter trial. J Vasc Surg 2005;42:456–464; discussion 464–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg 2008;47:774–781 [DOI] [PubMed] [Google Scholar]
- 31.Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Morabito A. Advantages of myocardial revascularization after admission for critical limb ischemia in diabetic patients with coronary artery disease: data of a cohort of 564 consecutive patients. J Cardiovasc Med (Hagerstown) 2008;9:1030–1036 [DOI] [PubMed] [Google Scholar]
- 32.Bundó Vidiella M, Pérez Pérez C, Montero Alia JJ, Cobos Solórzano MD, Aubà Llambrich J, Cabezas Peña C. [Peripheral artery disease of the lower limbs and morbidity/mortality in type 2 diabetics]. Aten Primaria 2006;38:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cassar A, Poldermans D, Rihal CS, Gersh BJ. The management of combined coronary artery disease and peripheral vascular disease. Eur Heart J 2010;31:1565–1572 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz L, Bertolet M, Feit F, et al. Impact of completeness of revascularization on long-term cardiovascular outcomes in patients with type 2 diabetes mellitus: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D). Circ Cardiovasc Interv 2012;5:166–173 [DOI] [PubMed] [Google Scholar]
- 35.Murcia AM, Hennekens CH, Lamas GA, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 2004;164:2273–2279 [DOI] [PubMed] [Google Scholar]
- 36.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]
- 37.Koch M, Trapp R, Kulas W, Grabensee B. Critical limb ischaemia as a main cause of death in patients with end-stage renal disease: a single-centre study. Nephrol Dial Transplant 2004;19:2547–2552 [DOI] [PubMed] [Google Scholar]
- 38.Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011;34:1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]