Abstract
OBJECTIVE
To determine whether diabetes status, including prediabetes, is associated with increased risk of peripheral neuropathy as defined by monofilament insensitivity.
RESEARCH DESIGN AND METHODS
This study used data from the 1999–2004 National Health and Nutrition Examination Survey (n = 7,818). Peripheral neuropathy was defined as one or more insensate sites detected by a Semmes-Weinstein 10-g monofilament. Generalized linear models were used to directly estimate relative risks (RRs) for the association of diabetes status and peripheral neuropathy.
RESULTS
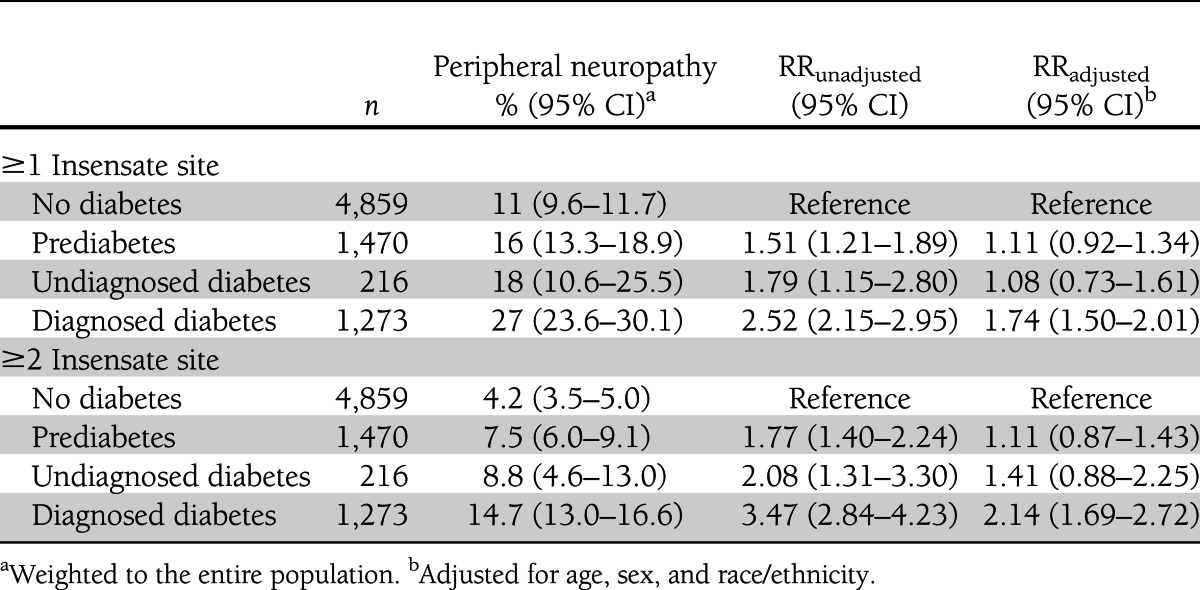
After adjustment compared with no diabetes, prediabetes [RR 1.11 (95% CI 0.92–1.34)] and undiagnosed diabetes [1.08 (0.73–1.61)] were associated with modest increases in risk of peripheral neuropathy, and diabetes was associated with a 74% higher risk of peripheral neuropathy [1.74 (1.50–2.01)].
CONCLUSIONS
Diabetes is associated with increased risk of peripheral neuropathy defined by monofilament insensitivity, but prediabetes and undiagnosed diabetes may be associated with only a modest increase in risk.
Studies suggest that prediabetes defined as impaired fasting glucose or impaired glucose tolerance may be associated with increased risk of peripheral neuropathy. Findings are influenced by the definition of prediabetes, the population studied, and the criteria for diagnosing peripheral neuropathy (1–9). It is not known whether individuals with prediabetes, based on their A1C (5.7–6.4%) (10), are at increased risk of peripheral neuropathy. The objective of this study was to determine whether diabetes status, including prediabetes (10), is associated with risk of peripheral neuropathy as defined by monofilament insensitivity.
RESEARCH DESIGN AND METHODS
We included all respondents from National Health and Nutrition Examination Survey (NHANES) 1999–2004 (11) aged ≥40 years; who completed the lower-extremity disease exam; had measured A1C; answered the question, “Other than during pregnancy have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?”; and were not pregnant. This study was deemed exempt from human subjects review by the Department of Veterans Affairs, Puget Sound Health Care System.
Variables and measures
Diabetes status was determined by questionnaire and A1C data (10). Those with no prior physician diagnosis of diabetes were classified as having no diabetes if their A1C was <5.7%, prediabetes if their A1C was 5.7–6.49%, and undiagnosed diabetes if their A1C was ≥6.5%. Peripheral neuropathy was defined as one or more insensate sites of three sites tested per foot based on the Semmes-Weinstein 10-g monofilament (1,12–15). The unit of analysis was the individual. Covariates included age, sex, and self-reported race/ethnicity.
Statistical methods
Generalized linear models were used to directly calculate the relative risks (RRs) for the association between diabetes status and peripheral neuropathy. Final models included covariates that were thought 1) a priori to be associated with diabetes status and risk factors for peripheral neuropathy and 2) unlikely to be a consequence of diabetes status or peripheral neuropathy. All analyses were weighted to account for the survey design, and statistical tests were at a two-sided α-level of 0.05. Analyses were completed using SAS software and STATA 12.
RESULTS
Between 1999 and 2004, 9,145 adults aged ≥40 years completed the NHANES survey and physical exam. We excluded 1,131 who did not complete a lower-extremity exam, 190 with unknown diabetes status, and 6 who were pregnant. The prevalence of prediabetes was 16% (95% CI 14–17), of undiagnosed diabetes 2% (1.6–2.3), and of diagnosed diabetes was 12% (11–13). After adjustment for age, sex, and race/ethnicity compared with individuals with no diabetes, those with prediabetes had 11% higher risk of peripheral neuropathy [RR 1.11 (95% CI 0.92–1.34)], those with undiagnosed diabetes had 8% higher risk of peripheral neuropathy [1.08 (0.73–1.61)], and those with diagnosed diabetes had 74% higher risk of peripheral neuropathy [1.74 (1.50–2.01)] (Table 1). Results were unchanged when we defined peripheral neuropathy as two or more insensate sites. Further adjustment for height, alcohol use, and BMI did not appreciably change the results.
Table 1.
Association of diabetes status and peripheral neuropathy, defined by monofilament insensitivity, among U.S. adults aged ≥40 years: NHANES 1999–2004
CONCLUSIONS
This study found that after adjustment, prediabetes, defined as A1C 5.7–6.5%, was associated with a modest increase in risk of peripheral neuropathy. Use of A1C for defining prediabetes may identify individuals with more severe, chronic hyperglycemia, which might explain the higher-than-expected prevalence of peripheral neuropathy among those with prediabetes in our study (1,3). Recently, Dyck et al. (8) reported no detectable association between impaired glucose (impaired fasting glucose or impaired glucose tolerance) and peripheral neuropathy, as defined by nerve conduction scores. While this study included detailed neurophysical measures, the sample size was small (n = 542) with low prevalence of peripheral neuropathy among those with prediabetes (12%) or diabetes (17%) (8).
Strengths of this study include the large nationally representative sample and separation of those with prediabetes, undiagnosed diabetes, and diagnosed diabetes. Limitations include classification of peripheral neuropathy and the cross-sectional study design.
We defined peripheral neuropathy as one or more insensate sites for optimum sensitivity and specificity (12,16) to ensure comparability with earlier published studies (1) and so that our results were relevant to current standards in primary care of diabetes (17). Sensitivity of the monofilament test ranges from 30 to 100%, and specificity ranges from 63 to 100% (16). If the low specificity of the monofilament test was independent of diabetes status, this would have led to an underestimation of the association of diabetes status and peripheral neuropathy. In research and clinical practice, the Semmes-Weinstein monofilament test is widely used and recommended to screen for peripheral neuropathy, since it is a rapid, comfortable, and inexpensive means of assessment and highly correlated with risk of injury, ulceration, and amputation (16–18). However, the monofilament test may primarily identify individuals at the highest risk for ulceration with large-fiber nerve damage. Therefore, our results may best be interpreted as measuring the association of diabetes status with risk of ulceration rather than peripheral neuropathy.
NHANES is a cross-sectional survey. Variables such as BMI, physical activity, or alcohol use may be influenced by diabetes status or presence of peripheral neuropathy. Therefore, these three variables were not included in our final models.
With a shift toward use of A1C for diagnosis of diabetes, it is important to determine whether diabetes status, based on A1C, is associated with risk for peripheral neuropathy. Our study is the first to examine this question, and the findings suggest that while diabetes is associated with a nearly twofold increased risk of peripheral neuropathy, prediabetes and undiagnosed diabetes are associated with only a modest increase in risk of peripheral neuropathy.
Acknowledgments
This material is based on work supported by the Office of Research and Development, Health Services Research and Development, U.S. Department of Veterans Affairs, including a postdoctoral fellowship to J.G.K. (TPP 61-026) and a Senior Career Scientist Award to G.E.R. (grant RCS 98-353).
No potential conflicts of interest relevant to this article were reported.
J.G.K. researched the data and drafted the manuscript. G.E.R. contributed to the discussion and reviewed the manuscript. K.M.N. conceived of the original research idea, contributed to the discussion, and reviewed the manuscript. J.G.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Jeff Rodenbaugh of Health Services Research and Development, Veterans Affairs Puget Sound Health Care System, for managing the data.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 2007;77:485–488 [DOI] [PubMed] [Google Scholar]
- 2.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 3.Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol 1990;131:633–643 [DOI] [PubMed] [Google Scholar]
- 4.de Neeling JN, Beks PJ, Bertelsmann FW, Heine RJ, Bouter LM. Peripheral somatic nerve function in relation to glucose tolerance in an elderly Caucasian population: the Hoorn study. Diabet Med 1996;13:960–966 [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes 1987;36:730–739 [DOI] [PubMed] [Google Scholar]
- 6.Eriksson KF, Nilsson H, Lindgärde F, et al. Diabetes mellitus but not impaired glucose tolerance is associated with dysfunction in peripheral nerves. Diabet Med 1994;11:279–285 [DOI] [PubMed] [Google Scholar]
- 7.Sosenko JM, Kato M, Soto R, Goldberg RB. Sensory function at diagnosis and in early stages of NIDDM in patients detected through screening. Diabetes Care 1992;15:847–852 [DOI] [PubMed] [Google Scholar]
- 8.Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care 2012;35:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24:1448–1453 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey data [article online]. Available from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm Accessed November 2, 2011
- 12.McGill M, Molyneaux L, Spencer R, Heng LF, Yue DK. Possible sources of discrepancies in the use of the Semmes-Weinstein monofilament. Impact on prevalence of insensate foot and workload requirements. Diabetes Care 1999;22:598–602 [DOI] [PubMed] [Google Scholar]
- 13.Mayfield JA, Sugarman JR. The use of the Semmes-Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract 2000;49(Suppl.):S17–S29 [PubMed] [Google Scholar]
- 14.Abbott CA, Carrington AL, Ashe H, et al. North-West Diabetes Foot Care Study The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377–384 [DOI] [PubMed] [Google Scholar]
- 15.Gregg EW, Sorlie P, Paulose-Ram R, et al. 1999-2000 national health and nutrition examination survey Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care 2004;27:1591–1597 [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;50:675–682 [DOI] [PubMed]
- 17.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1.):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dros J, Wewerinke A, Bindels PJ, van Weert HC. Accuracy of monofilament testing to diagnose peripheral neuropathy: a systematic review. Ann Fam Med 2009;7:555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]


