Abstract
OBJECTIVE
Prescription custom-made footwear can only be effective in preventing diabetic foot ulcers if worn by the patient. Particularly, the high prevalence of recurrent foot ulcers focuses the attention on adherence, for which objective data are nonexisting. We objectively assessed adherence in patients with high risk of ulcer recurrence and evaluated what determines adherence.
RESEARCH DESIGN AND METHODS
In 107 patients with diabetes, neuropathy, a recently healed plantar foot ulcer, and custom-made footwear, footwear use was measured during 7 consecutive days using a shoe-worn, temperature-based monitor. Daily step count was measured simultaneously using an ankle-worn activity monitor. Patients logged time away from home. Adherence was calculated as the percentage of steps that prescription footwear was worn. Determinants of adherence were evaluated in multivariate linear regression analysis.
RESULTS
Mean ± SD adherence was 71 ± 25%. Adherence at home was 61 ± 32%, over 3,959 ± 2,594 steps, and away from home 87 ± 26%, over 2,604 ± 2,507 steps. In 35 patients with low adherence (<60%), adherence at home was 28 ± 24%. Lower BMI, more severe foot deformity, and more appealing footwear were significantly associated with higher adherence.
CONCLUSIONS
The results show that adherence to wearing custom-made footwear is insufficient, particularly at home where patients exhibit their largest walking activity. This low adherence is a major threat for reulceration. These objective findings provide directions for improvement in adherence, which could include prescribing specific off-loading footwear for indoors, and they set a reference for future comparative research on footwear adherence in diabetes.
Custom-made footwear is recommended and often prescribed to patients with diabetes, peripheral neuropathy, and foot deformity to prevent foot ulceration and further complications such as infection and amputation (1). Elevated plantar pressures and ill-fitting footwear are important risk factors of ulceration (2,3). Custom-made footwear aims to reduce ulcer risk by reducing foot pressures and providing proper fit (4–6). It is clear that to be effective in ulcer prevention, prescription footwear should be worn by the patient, in particular when being ambulant (7). Because annual ulcer recurrence rates are high, up to 40% found in one study (8–10), poor adherence may be a factor in this outcome. Patient self-report studies show that only 22–36% of diabetic patients with peripheral neuropathy, vascular disease, or foot deformity wear prescription footwear regularly (>80% of the day) (11,12). This is unfortunate, since it has been shown that ulcer recurrence rate can be substantially reduced when patients adhere to wearing pressure-relieving footwear (13). Nonadherence is therefore a major issue in high-risk diabetic patients that determines clinical outcome.
To date, adherence to footwear use has been assessed using subjective methods, including questionnaires, face-to-face interviews, or diaries (11–14). Subjective methods are known to have issues with accuracy and reliability and may lead to a response bias or to missing data (15–17). Furthermore, these methods do not accurately distinguish between active and nonactive periods. In removable below-the-knee walkers used for off loading diabetic foot ulcers, adherence was measured objectively (18), but the accelerometer-based sensors used were not developed to fit inside a shoe. Therefore, we use a new adherence-to-treatment monitoring system (the @monitor, developed at the Academic Medical Center in Amsterdam), which is small enough to fit inside the patients’ shoe and has been proven to be valid and reliable in determining moments of donning/doffing and feasible in use in diabetic foot patients (19).
Objective data on footwear adherence in patients who have diabetes and are at high risk for ulceration are not available. Adherence is most appropriately obtained during ambulation, when pressures on the foot are highest. Furthermore, adherence may vary according to where the patient is (at home or away from home) (14) or according to what day of the week or time of day it is. Knowledge about adherence and about what determines adherence is valuable in addressing issues of footwear effectiveness and can direct or may even reform footwear prescription practice. Therefore, the aim of this study was to objectively assess adherence to wearing prescribed custom-made footwear during ambulation in patients with diabetes at high risk for ulceration and to assess the determinants of adherence in this patient group.
RESEARCH DESIGN AND METHODS
Patients were selected from the database of a randomized controlled trial on custom footwear effectiveness (DIAbetic Foot Orthopedic Shoe [DIAFOS], clinical trial reg. no. NTR1091), in which patients were consecutively recruited from the outpatient multidisciplinary foot clinics of 10 Dutch hospitals. The first 120 patients in this trial who were assessed for adherence were included in the current study. Inclusion criteria were diagnosed diabetes, loss of protective sensation as confirmed by 10-g Semmes-Weinstein monofilament and vibration perception threshold testing (20), a prior plantar forefoot or mid-foot ulcer that healed in the 18 months before inclusion in the trial, and prescription custom-made footwear. Exclusion criteria were bilateral amputation proximal to the tarso-metatarsal joint, nonambulatory status, unlikelihood to survive 18 months’ follow-up, and inability to follow the study instructions. Written informed consent was obtained from each patient prior to inclusion in the trial, which was approved by all involved local research ethics committees.
Footwear
Patients wore fully custom-made footwear (i.e., custom insoles in custom shoes) or semi–custom-made footwear (i.e., custom insoles in off-the-shelf extra-depth shoes). The footwear was prescribed by a rehabilitation medicine specialist and manufactured by a shoe technician, both of whom were experienced in treating diabetic foot patients. Shoes were mostly ankle high or boot style and were in some cases tibia high. The footwear generally had a stiffened rubber outsole with roller configuration and multidensity insoles.
Instrumentation
Data on footwear use were collected using a temperature-based adherence-to-treatment monitor, the @monitor, which has previously been described in detail (19). In short, the @monitor measures 35 × 15 × 5 mm (length × width × height) and integrates two digital-to-digital temperature sensors (one on each flat side of the monitor), a battery, and a data logger. The @monitor samples temperatures at a maximum 1-min interval giving a 14-day collection period. The @monitor is placed in a plastazote foam pad and taped to the inner lateral shoe border just below ankle level. Only thin adhesive tape (covering the @monitor) and the patient’s sock separate the @monitor from the leg. Because the temperature difference across the @monitor when wearing the footwear is unequal to the temperature difference when not wearing the footwear, footwear use can be determined. Response of the @monitor to donning and doffing footwear is immediate, with temperature change present at the next measurement sample. The @monitor has been shown to be accurate in determining moments of donning and doffing of footwear (mean 0.4-min difference with an accurately kept log [95% CI 0.2–0.6]) and feasible for use by high-risk diabetic patients (19). Using a docking station and custom software, start date and time, number of days of data collection, and sample frequency are defined. Temperature readouts are exported to a text file after data collection.
Ambulatory activity was recorded using a step activity monitor (StepWatch; Orthocare Innovations), which was strapped to the lateral side of the leg above the ankle. The step activity monitor stores the number of steps per minute over a maximum period of 14 days. Measurement accuracy is optimized by personalizing body height and type of gait of the patient (normal, fast, and slow) in the settings of the monitor and verified by a light on the monitor that blinks at each of the first 40 steps taken by the patient after initialization. The error between counted steps and measured steps with the StepWatch is 0.3% (21).
Procedures
At baseline, demographic, socioeconomic, disease-related, and foot complication history data were collected and a foot examination was performed. Each patient received brochures and standard verbal information from the researcher on diabetic foot care and the need to wear prescribed footwear as much as possible, preferably with each step taken. Because of the break-in period of footwear, data on adherence were collected a minimum of 3 months after footwear delivery. Three months after footwear delivery, perceived footwear aesthetics and comfort were scored on a visual analog scale using the Questionnaire of Usability Evaluation (22).
To avoid change in behavior of the patient during the measurement, patients were informed that foot temperature (not adherence) would be measured. The sample frequency of the @monitor was set at the maximum one sample per minute. Both the @monitor and the step activity monitor were synchronized to local time on the same personal computer before each measurement. Shoes were equipped with the @monitor, and the step activity monitor was strapped to the ankle. If a patient had more than one pair of prescription custom-made shoes, a second pair was also equipped with the @monitor. If a patient had more than two pairs of prescription shoes, the patient was asked to wear only those two pair equipped with the @monitor. Each patient was asked to wear the step activity monitor for seven consecutive full days, at all times, except when taking a shower or bath or when discomfort was felt. Patients were also instructed not to remove the @monitor from the shoes. Additionally, they were asked to write down in a daily log the time periods (from [hh:mm] to [hh:mm], where h is hour and m is minute) that they were away from home, were cycling, or did not wear the step activity monitor. Patients returned the monitors and log after the measurement through postal mail.
Data analysis
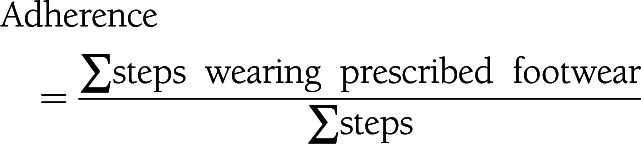
Recordings with <4 days of step activity or without a weekend day included were considered incomplete and were excluded from analysis. The @monitor and step activity monitor data were analyzed using Matlab R2011a software (MathWorks, Natick, MA) (19). For patients with two pairs of custom-made shoes in the study, data were accumulated. For each measurement day, step count and total wearing time were calculated. Adherence was calculated from the cumulative number of steps over the full measurement period as follows:
 |
When step activity was recorded during periods that the @monitor did not record footwear use, it was assumed that the patient walked either barefoot or in nonprescription footwear. The reported time periods in the daily log for cycling were used to filter the step count data to keep walking-only data. Reported time in the daily log for being away from home was used to separate step count, wearing time, and adherence data for periods at home and for periods away from home. Subgroup analyses were done for patients with adherence ≥80% (adherencehigh) and adherence <60% (adherencelow). To compare outcomes with previous studies that used subjective methods, we also calculated adherence as percentage of daytime that the prescription footwear was worn. We assumed out-of-bed daytime to be 16 h.
Determinants of adherence
As potential determinants of adherence, the following factors were taken into account: age, sex, education level (low, medium, and high), living status, employement, diabetes type, diabetes duration, cumulative months of past ulceration, history of amputation, presence of peripheral arterial disease, BMI, HbA1c, severity of foot deformity, daily step count, variation in daily step count over the measurement period, type of footwear, and perceived footwear aesthetics and footwear comfort.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 19 (SPSS, Chicago, IL). Mann-Whitney U tests assessed baseline differences between included and excluded patients. Descriptive analyses were done on baseline patient characteristics and on outcomes for wearing time, adherence, and step activity. Paired t tests assessed differences in adherence between being at home and away from home and between weekdays and weekend days. One-way ANOVA tested for differences in adherence between participating centers and between patient subgroups (adherencehigh vs. adherencelow). Pearson correlation coefficients were calculated between adherence and wearing time and between adherence and daily step count. For all of the above tests, a significance level of P < 0.05 was used. Univariate regression analysis (P < 0.10) was used to assess the association between variation in step count and time away from home and to assess factors significantly associated with adherence. Significant univariate factors were entered in a multivariate regression analysis of adherence (with backward selection, P < 0.10).
RESULTS
Thirteen of the 120 included patients were excluded from analysis because of incomplete (<4 days) step activity data (N = 10), technical failure (N = 2), or refusal to wear the step activity monitor (N = 1). Baseline characteristics did not differ significantly between excluded and analyzed patients, except for sex (relatively more women were excluded, P = 0.018).
Of the remaining 107 patients, 93 (87%) were men, 103 (96%) were Caucasian, 76 (71%) had type 2 diabetes, and 89 (83%) wore fully custom-made footwear. Mean ± SD age was 63.8 ± 9.6 years and diabetes duration 17.3 ± 11.9 years. Thirty-five patients had one pair of prescription custom-made shoes, and 72 had two pairs. Footwear age at assessment of adherence was 1.4 ± 0.9 years. We had 6.5 ± 0.9 days of analyzed data per patient. Seventy-nine patients (74% of total group) had complete reports of time spent away from home. The step activity monitor was not worn during 3.5 ± 9.6% of the day, and nonuse occurred mostly at night. Patients donned and doffed their footwear 1.3 ± 0.9 times/day.
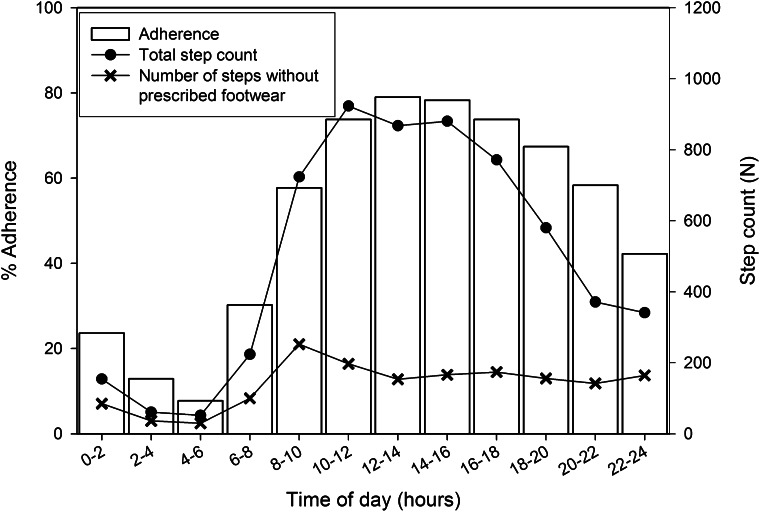
Outcome data for step count and adherence are shown in Table 1. Footwear adherence was 71 ± 25% (range 10–100%). When patients were at home, adherence was significantly lower than when away from home (P < 0.001), while daily step count was significantly higher at home (P < 0.001). Adherence was <60% between 8 p.m. and 10 a.m. and below 40% between midnight and 8 a.m. (Fig. 1). Both adherence and step count were significantly lower during weekend days than weekdays (P < 0.001). Adherence and daily step count were not significantly correlated (r = 0.14, P = 0.16). Correcting for cycling had a negligible effect on adherence.
Table 1.
Data on daily step count and adherence
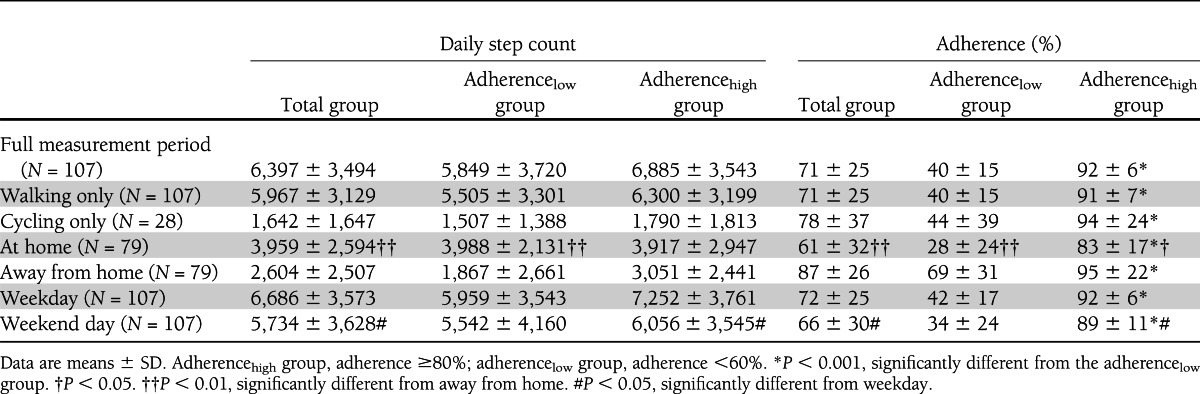
Figure 1.
Mean adherence, total step count, and the number of steps taken without wearing prescribed footwear during 2-h time slots over the day.
Patients wore their prescribed footwear 9.4 ± 4.4 h/day, at home 6.4 ± 4.6 h, and away from home 3.5 ± 2.7 h. Wearing time was 59 ± 27% of daytime. Twenty-nine percent of patients wore their prescription footwear >80% of daytime. Wearing time was significantly correlated with adherence (r = 0.87, P < 0.001). Adherence was not significantly different between participating centers (P = 0.16), and neither was daily step count (P = 0.35) or wearing time (P = 0.59). Day-to-day variation in step count increased significantly when patients were more away from home (β = 181 steps/h [95% CI 80–282], P < 0.001).
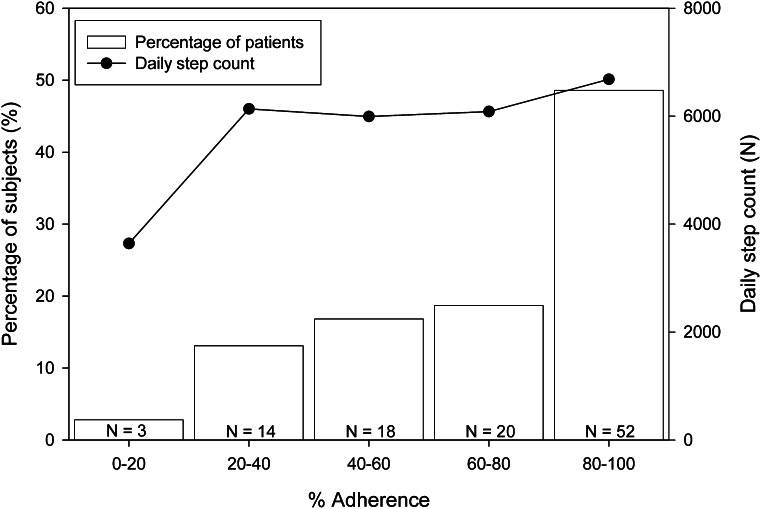
Thirty-three percent of the patients had adherence <60% (Fig. 2). In this adherencelow group, adherence was 40 ± 15% and was 2.5 times higher for away from home than for at home (Table 1). In the adherencehigh group, adherence was 85 ± 12% and was 1.1 times higher for away from home than for at home. Daily step count was not significantly different between the adherence subgroups (P = 0.19) (Table 1).
Figure 2.
Distribution of patients across five subgroups of adherence. Also shown is the mean daily step count for each subgroup.
In the univariate regression analysis, a lower BMI, a history of amputation, more severe foot deformity, more variation in daily step count, and a better perception of footwear aesthetics were significantly associated with higher adherence (Table 2). In the multivariate analysis, all of these factors except history of amputation remained significant (R2 = 0.18, P < 0.10) (Table 2).
Table 2.
Outcomes for the univariate and multivariate regression analysis on determinants of adherence

CONCLUSIONS
Adherence to wearing prescription custom-made footwear is important to prevent ulceration in high-risk patients with diabetes. With use of objective methods to measure adherence, the study results showed that 71% of the steps taken were in prescription custom-made footwear, while, among individuals, differences in adherence were large (10–100%). Adherence was much lower at home than away from home, which substantiates earlier studies that use subjective data (14). In particular, in the patient group with low adherence (<60%), adherence at home was poor (28% compared with 69% away from home). Patients were significantly more active at home than away from home, which corresponds with previous data (23). This further amplifies the problem of footwear use at home, increasing the cumulative stress on an inadequately protected foot. Therefore, interventions aimed to increase adherence should primarily target the home situation, e.g., through the prescription of special off-loading footwear for indoors.
When calculating adherence in similar units to what most previous studies did, our results show that 29% of the patients wore their prescribed footwear >80% of the daytime. This is comparable with earlier studies that used mailed questionnaires (with a less-than-optimal response rate) and face-to-face interviews and that showed that 22–36% of diabetic patients at risk for ulceration wear their prescription footwear all day (11,12) or for at least >80% of daytime (14). These consistent outcomes across studies reinforce the problem of nonadherence in this patient group. Furthermore, they show that interpretations may vary based on which method is chosen to report adherence (percentage of daytime versus percentage of steps). A major disadvantage of subjective methods is that they lack the sensitivity, accuracy, and reliability to measure adherence during ambulant and nonambulant periods added with the risk of reporting bias. Therefore, these methods lack the ability to accurately assess adherence when it is most important, namely, when the foot is loaded most. This strongly supports the use of objective methods to assess true adherence in a patient. Using these methods, our study still showed that on average, 29% of steps were taken without wearing custom-made footwear. Nonadherence was largest during the late-evening, night, and early-morning hours, when patients may walk more on a hard bathroom or kitchen floor. This further increases the risk for ulcer recurrence.
The multivariate regression analysis showed that patients with more severe deformity had higher adherence to prescribed footwear—maybe because these patients have no other choice than to wear custom-made footwear or because they are more aware of its benefits. Patients with higher BMI were less adherent, which may reflect overall difficulty with adhering to a healthy lifestyle in overweight or obese patients. More day-to-day variation in activity was positively associated with adherence, probably because patients who spent more time away from home (higher adherence) were the ones more variable in activity. Finally, patients who perceived their footwear as more attractive were more adherent, which seems intuitive, although previous studies are inconclusive on this association (14,24). Despite these significant associations, overall explained variance in adherence was only 18%, which implies that optimizing any of these predicting factors may have a limited effect on adherence. More research is needed to further elucidate why patients are adherent or not.
The objective data collected on adherence provide an excellent basis for further exploration of predictors of adherence and have great value in guiding footwear prescription practice and diabetic foot treatment. In many chronic diseases, adherence to treatment is a major problem and influenced by social and economic factors, the health care team, disease characteristics, therapy, and patient-related factors (25). Therefore, objective footwear adherence data could be used to assess patient groups with different social-economic or cultural backgrounds, ethnicity, or past experiences with foot complications. Assessment of adherence in different regions or at different centers may provide information on more or less successful prescription and health care practices. Effects of patient education and other interventions can be accurately determined. Finally, objective adherence data could be used to explore reasons for nonadherence and to individualize type and frequency of footwear prescription (19). Effectively, these analyses could shape and potentially reform the prescription and use of specialist diabetic footwear.
Some limitations apply. First, we did not measure adherence while standing, even though patients spend twice as much time standing than walking (26) and forces equal to body weight are applied to the foot. Custom-made footwear was worn 9.4 h/day and was strongly associated with adherence. We therefore suggest that adherence may be as high in standing as in walking. Second, we measured adherence objectively, but we were still dependent on daily kept logs to determine periods of cycling, being away from home, and nonuse of the step activity monitor. This increases the chance for missing data or unreliable data. More objective ways to evaluate these events should be further explored, as well as methods to assure that patients do not take off the step activity monitor during measurement. Nonuse of the step activity monitor may under- or overestimate adherence. We verified from the daily kept logs that nonuse occurred only during 3.5% of the day, suggesting a negligible impact on the adherence values. Third, we attempted to avoid a conscious change in behavior by blinding the patient for the goal of the measurement, but we have no confirmation of whether we succeeded. Finally, we did not measure adherence to wearing nonprescription footwear (e.g., off-the-shelf shoes, sandals, slippers), and therefore we lack information on the amount of barefoot walking, which is the most hazardous walking condition.
In conclusion, the results show that adherence to wearing prescribed custom-made footwear is insufficient in neuropathic diabetic patients with prior foot ulceration, in particular at home where they exhibit their largest walking activity. This low adherence is a major threat for reulceration in this high-risk patient group. Improvement of adherence could therefore include the prescription of specific protective footwear for indoors, while the importance of wearing prescription footwear should be further promoted. The objective data collected on adherence have great value in guiding clinical practice and provide an excellent basis to further explore predictive factors of adherence, to perform comparative research, and to investigate interventions that aim to improve adherence.
Acknowledgments
The DIAFOS trial was supported by project grants from the Dutch Diabetes Research Foundation (project no. 2007.00.067), the Dutch Foundation for the Development of Orthopedic Footwear, and the Dutch Organization for Health Research and Development (project no. 14350054).
No potential conflicts of interest relevant to this article were reported.
R.W., R.K., and W.P.P. researched data. R.W., R.K., M.d.H., F.N., and S.A.B. contributed to discussion. R.K., M.d.H., W.P.P., F.N., and S.A.B. reviewed and edited the manuscript. R.W. and S.A.B. wrote the manuscript. S.A.B. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 11th Diabetic Foot Study Group Meeting, Potsdam, Germany, 28–30 September 2012.
The Academic Medical Center (Amsterdam, the Netherlands) collaborates with nine other multidisciplinary diabetic foot centers and nine orthopedic footwear companies in the Netherlands in the DIAFOS trial on the effectiveness of custom-made footwear to prevent foot ulcer recurrence. The authors gratefully acknowledge the contribution of Mark Arts (Academic Medical Center) in collecting data for the study and also acknowledge the following persons for their contribution in patient recruitment and footwear prescription: T.E. Busch-Westbroek, MD; P.J.A. Mooren (Academic Medical Center); J.W.E. Verlouw, MD; I. Ruijs and H. van Wessel (Maxima Medical Center, Veldhoven, the Netherlands); J.P.J. Bakker, MD, PhD; C. van den Eijnde (Medical Center Alkmaar); D. Wever, MD; H. Wessendorf (Medisch Spectrum Twente, Enschede, the Netherlands); R. Dahmen, MD; B. Koomen (Slotervaart Hospital, Amsterdam, the Netherlands); J.G. van Baal, MD, PhD; R. Haspels (Ziekenhuisgroep Twente, Almelo, the Netherlands); J. Harlaar, PhD; V. de Groot, MD, PhD; J. Pulles (VU Medical Center, Amsterdam, the Netherlands); R. Lever; G. du Mont (Spaarne Hospital, Hoofddorp, the Netherlands); H.G.A. Hacking, MD; J. de Bruin (St. Antonius Hospital, Nieuwegein, the Netherlands); H. Berendsen, MD; and W. Custers (Reinier de Graaf Gasthuis, Delft, the Netherlands).
References
- 1.Bakker K, Apelqvist J, Schaper NC, International Working Group on Diabetic Foot Editorial Board Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(Suppl. 1):225–231 [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane RM, Jeffcoate WJ. Factors contributing to the presentation of diabetic foot ulcers. Diabet Med 1997;14:867–870 [DOI] [PubMed] [Google Scholar]
- 3.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 1992;35:660–663 [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh PR. Therapeutic footwear for people with diabetes. Diabetes Metab Res Rev 2004;20(Suppl. 1):S51–S55 [DOI] [PubMed] [Google Scholar]
- 5.Praet SF, Louwerens JW. The influence of shoe design on plantar pressures in neuropathic feet. Diabetes Care 2003;26:441–445 [DOI] [PubMed] [Google Scholar]
- 6.Waaijman R, Arts ML, Haspels R, Busch-Westbroek TE, Nollet F, Bus SA. Pressure-reduction and preservation in custom-made footwear of patients with diabetes and a history of plantar ulceration. Diabet Med 2012;29:1542–1549 [DOI] [PubMed] [Google Scholar]
- 7.Connor H, Mahdi OZ. Repetitive ulceration in neuropathic patients. Diabetes Metab Res Rev 2004;20(Suppl. 1):S23–S28 [DOI] [PubMed] [Google Scholar]
- 8.Chantelau E, Kushner T, Spraul M. How effective is cushioned therapeutic footwear in protecting diabetic feet? A clinical study. Diabet Med 1990;7:355–359 [DOI] [PubMed] [Google Scholar]
- 9.Uccioli L, Faglia E, Monticone G, et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care 1995;18:1376–1378 [DOI] [PubMed] [Google Scholar]
- 10.Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med 2005;22:1306–1309 [DOI] [PubMed] [Google Scholar]
- 11.Knowles EA, Boulton AJ. Do people with diabetes wear their prescribed footwear? Diabet Med 1996;13:1064–1068 [DOI] [PubMed] [Google Scholar]
- 12.McCabe CJ, Stevenson RC, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med 1998;15:80–84 [DOI] [PubMed] [Google Scholar]
- 13.Chantelau E, Haage P. An audit of cushioned diabetic footwear: relation to patient compliance. Diabet Med 1994;11:114–116 [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane DJ, Jensen JL. Factors in diabetic footwear compliance. J Am Podiatr Med Assoc 2003;93:485–491 [DOI] [PubMed] [Google Scholar]
- 15.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2009;15:589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-report bias in assessing adherence to guidelines. Int J Qual Health Care 1999;11:187–192 [DOI] [PubMed] [Google Scholar]
- 17.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 2004;42:649–652 [DOI] [PubMed] [Google Scholar]
- 18.Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJ. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care 2003;26:2595–2597 [DOI] [PubMed] [Google Scholar]
- 19.Bus SA, Waaijman R, Nollet F. New monitoring technology to objectively assess adherence to prescribed footwear and assistive devices during ambulatory activity. Arch Phys Med Rehabil 2012;93:2075–2079 [DOI] [PubMed] [Google Scholar]
- 20.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 21.Coleman KL, Smith DG, Boone DA, Joseph AW, del Aguila MA. Step activity monitor: long-term, continuous recording of ambulatory function. J Rehabil Res Dev 1999;36:8–18 [PubMed] [Google Scholar]
- 22.Jannink MJ, de Vries J, Stewart RE, Groothoff JW, Lankhorst GJ. Questionnaire for usability evaluation of orthopaedic shoes: construction and reliability in patients with degenerative disorders of the foot. J Rehabil Med 2004;36:242–248 [DOI] [PubMed] [Google Scholar]
- 23.Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc 2001;91:451–455 [DOI] [PubMed] [Google Scholar]
- 24.Williams AE, Nester CJ. Patient perceptions of stock footwear design features. Prosthet Orthot Int 2006;30:61–71 [DOI] [PubMed] [Google Scholar]
- 25.Yach D. WHO Report. Adherence to Long-term Therapies. Evidence for Action Geneva, World Health Org., 2003 [Google Scholar]
- 26.Najafi B, Crews RT, Wrobel JS. Importance of time spent standing for those at risk of diabetic foot ulceration. Diabetes Care 2010;33:2448–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20(Suppl. 1):S90–S95 [DOI] [PubMed] [Google Scholar]