Abstract
OBJECTIVE
Transcutaneous oxygen tension (TcPO2) measures tissue perfusion and is important in the management of peripheral artery disease (PAD). Ankle brachial index (ABI) is used for the diagnosis of PAD and represents a predictor of major adverse cardiovascular events (MACE), even if in diabetes its diagnostic and predictive value seems to be reduced. No study has evaluated TcPO2 as a predictor of cardiovascular events. Aim of this longitudinal study was to assess whether TcPO2 is better than ABI at predicting MACE in type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
Among 361 consecutive patients with apparently uncomplicated diabetes, 67 MACE occurred during a follow-up period of 45.8 ± 23.2 months.
RESULTS
The percentage of both subjects with low ABI (≤0.9) and subjects with low TcPO2 (≤46 mmHg as measured by a receiver operating characteristic curve) was significantly (<0.001) greater among patients with than among those without MACEs (ABI 64.2 vs. 40.8; TcPO2 58.2 vs. 34%). The Kaplan-Meier method showed that both low ABI (Mantel log-rank test, 4.087; P = 0.043) and low TcPO2 (Mantel log-rank test, 33.748; P > 0.0001) were associated with a higher rate of MACEs. Cox regression analysis showed that low TcPO2 (hazard ratio 1.78 [95% CI 1.44–2.23]; P < 0.001) was a significant predictor of MACE, while ABI did not enter the model.
CONCLUSIONS
This longitudinal study showed that TcPO2 may be a potential predictor of MACE among patients with uncomplicated type 2 diabetes and that its predictive value seems to be greater than that of ABI.
Diabetic patients have an increased cardiovascular risk (1,2). Indeed, cardiovascular disease is the main cause of mortality and morbidity related to diabetes, and approximately two-thirds or more of diabetic patients die of cardiovascular disease (1,2). Cardiovascular death rate of diabetic patients without prior myocardial infarction even seems to be similar to that of nondiababetic patients with prior myocardial infarction (3).
Ankle brachial index (ABI), commonly used as a diagnostic test for peripheral arterial disease (PAD) (4), is considered an independent powerful marker of cardiovascular morbidity and mortality in the general population (5,6). An ABI of ≤0.90 is universally recognized as the cutoff for the diagnosis of PAD (4). However, in diabetic people the use of ABI has some important diagnostic limitations: indeed, the presence of typical medial artery calcifications causes arterial wall stiffness and a high prevalence of false-negative values (7–9). Transcutaneous oxygen tension (TcPO2) is a noninvasive method to measure tissue perfusion: it reflects very well the metabolic state of lower limbs (10,11). TcPO2 is currently used in clinical practice in the management of the vascular diabetic foot (11,12); in particular, it is important in determining amputation level, wound healing evaluation, and revascularization procedures (11,12). TcPO2 is not affected by arterial calcification and is particularly useful in evaluating PAD in diabetic patients (13); in addition, it has a good reproducibility (10–12). Nevertheless, there is not thus far a universally recognized specific cutoff of TcPO2 for the diagnosis of PAD (11). Finally, it is unknown whether TcPO2 may be a reliable marker as well of cardiovascular morbidity and mortality in diabetes. Aim of the current study was to assess whether TcPO2 is better than ABI in predicting major adverse cardiovascular events (MACEs) among diabetic patients.
RESEARCH DESIGN AND METHODS
For this study, we enrolled 377 consecutive patients with uncomplicated type 2 diabetes who attended the diabetic foot clinic for the routine screening visit for the prevention or detection of diabetic foot. Exclusion criteria were as previously reported (14): age <41 or >75 years, symptoms of coronary events as defined by Rose questionnaire, history of coronary events, coronary artery revascularization, heart failure, uncontrolled hypertension (>180/100 mmHg), significant valvular diseases, cardiomyopathy, chronic or acute diseases, pregnancy, liver or kidney disease (creatinine >130 μmol/L), diabetic proliferative retinopathy or previous photocoagulation, therapy with digitalis, neoplasia, duration of diabetes <12 months. Additional exclusion criteria were presence of current or previous foot ulcers, history of stroke or transient ischemic attack, and claudicatio intermittens.
We hypothesized that the total rate of cardiac, cerebral, and peripheral vascular complications over a 4-year follow-up would be 20% in the whole population. We estimated a prevalence of PAD of ~25% among patients without future occurrence vascular complications and an approximately double prevalence of PAD among subjects with vascular complications. Considering an α-type I error <0.05 and a β-type II error of 90%, we estimated a sample size of 370 patients.
The study was approved by an ethics committee. All patients gave informed consent for both performing each test and participating in the study.
As in our previous studies (14–16), diabetes was diagnosed according to American Diabetes Association criteria and hypertension according to European Society of Hypertension/European Society of Cardiology criteria. Patients with an albumin excretion rate (AER) <30 mg/day were considered normoalbuminuric, while patients with AER between 30 and 299 mg/day were considered microalbuminuric (14–16). Macroalbuminuria was defined as AER ≥300 mg/day or based on a dipstick-positive proteinuria (14–16). Patients were considered smokers if they were current smokers or former smokers (14–16). A family history of coronary artery disease (CAD) was considered positive in the presence of a documented myocardial ischemia or infarction in a first-degree relative (14–16). BMI was calculated as weight in kilograms divided by the square of height in meters (14–16). Diabetic autonomic neuropathy was identified by an abnormal finding in at least one of the following five standard repeatable tests as previously reported (17): the heart rate response to a Valsalva maneuver, the heart rate variation during deep breathing, the blood pressure response to sustained handgrip, the immediate heart rate response to standing, and the blood pressure response to standing (17).
In all of the patients, ABI, i.e., the ratio of systolic blood pressure in the ankle over the pressure in the brachial artery, and TcPO2 were evaluated as previously reported (15). In particular, TcPO2 measurements were performed at the dorsum of the foot, ~2 cm proximal to the basis of the third toe, avoiding areas overlying bone or superficial veins. A TCM4 Radiometer (Medical Aps, Bronshoj, Denmark) device was used. The patient was in supine position after 20 min of rest in a room with a temperature between 22 and 24°C. The measuring site was carefully cleaned with saline solution. The electrochemical transducer was fixed to the skin by using double-sized adhesive rings and contact liquid supplied by the manufacturer. Calibration of the device was carried out before each measurement. The calibration period was 10 min, and the TcPO2 signal was recorded for 30 min. In every patient, ABI and TcPO2 were evaluated in both legs; the lowest value both for ABI and for TcPO2 was reported in the results and used for the analyses. For assessment of the presence of distal polyneuropathy, nerve conduction testing were performed at a stable skin temperature of 31°C and a room temperature of 24°C as previously reported (15).
Venous blood sample was taken from subjects after fasting for 12 h. Cholesterol, HDL, and triglycerides were measured by an automatic analyzer HITACHI 737. LDL was calculated by the Friedewald formula (18). HbA1c was measured by high-performance liquid chromatography (Biorad, Richmond, California). AER was measured by nephelometry (Beckmann, Milan, Italy).
Follow-up
Among 377 patients, 16 (4.2%) were lost at follow-up. So, a total of 361 patients with complete follow up data were included in the current study.
The end point of this study was the occurrence of MACEs, which included, as previously described (14,16), CAD death, sudden death, nonfatal myocardial infarction, death due to congestive heart failure, unstable angina, need for repeat revascularization (aside from restenosis), stroke or transient ischemic attack, and symptomatic PAD documented by angiography. Criteria for the diagnosis of MACEs and collection of any information regarding MACEs have previously been described (14,16).
Statistical analysis
Differences in normally distributed variables were evaluated by the Student t test, while differences in nonnormal variables were assessed by the Mann-Whitney U test. The Pearson χ2 was used for frequency comparison. To identify the best cutoff of TcPO2 for MACE, a receiver operating characteristic (ROC) curve with the area under the curve was evaluated. Survival curves were estimated by the Kaplan-Meier test and compared by the Mantel log-rank test. The influence of each variable on the occurrence of MACEs was assessed by stepwise Cox regression analysis. Hazard ratios (HRs) (95% CI) were computed to identify significant predictors of MACEs. We considered statistically significant a P value <0.05.
RESULTS
Occurrence of MACEs
Follow-up period duration was defined as the period of time up to the occurrence of the first MACE or up to the last information obtained. Mean follow-up period was 45.8 ± 23.2 months. During the follow-up period, 67 patients had MACE.
Table 1 shows the features of the whole study population at baseline and of the patients stratified by presence/absence of MACEs. Male sex, family history of CAD, percentage of smokers, microalbuminuria, autonomic neuropathy, and serum levels of total and LDL cholesterol were significantly higher in the MACE than in the non-MACE group.
Table 1.
Clinical and anthropometric features of the whole population and of patients stratified by presence/absence of MACE
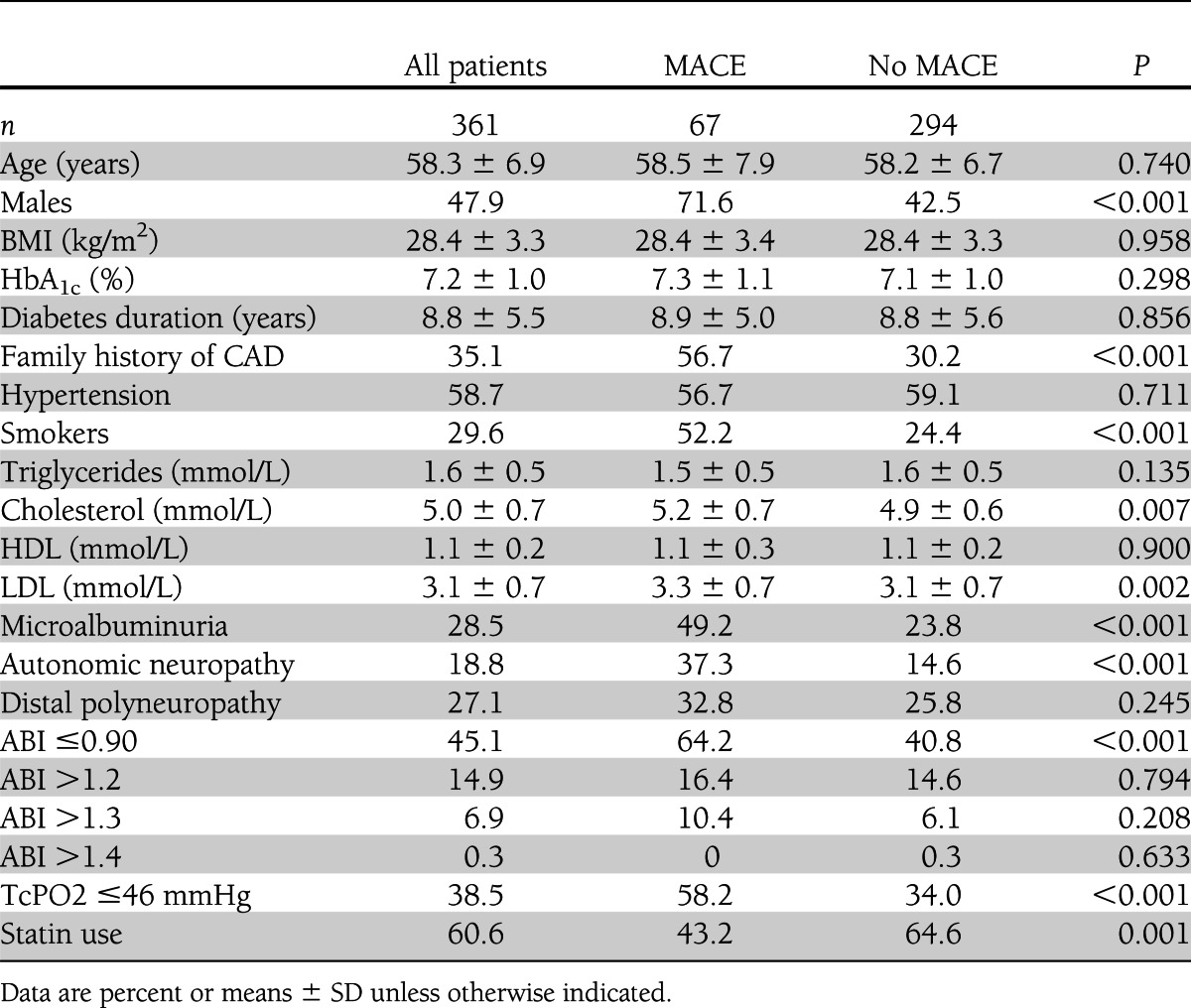
An ABI of ≤0.90 was used as the cutoff for the diagnosis of PAD (11,15). The percentage of patients with ABI values ≤0.90 was significantly higher in the MACE than in the non-MACE group.
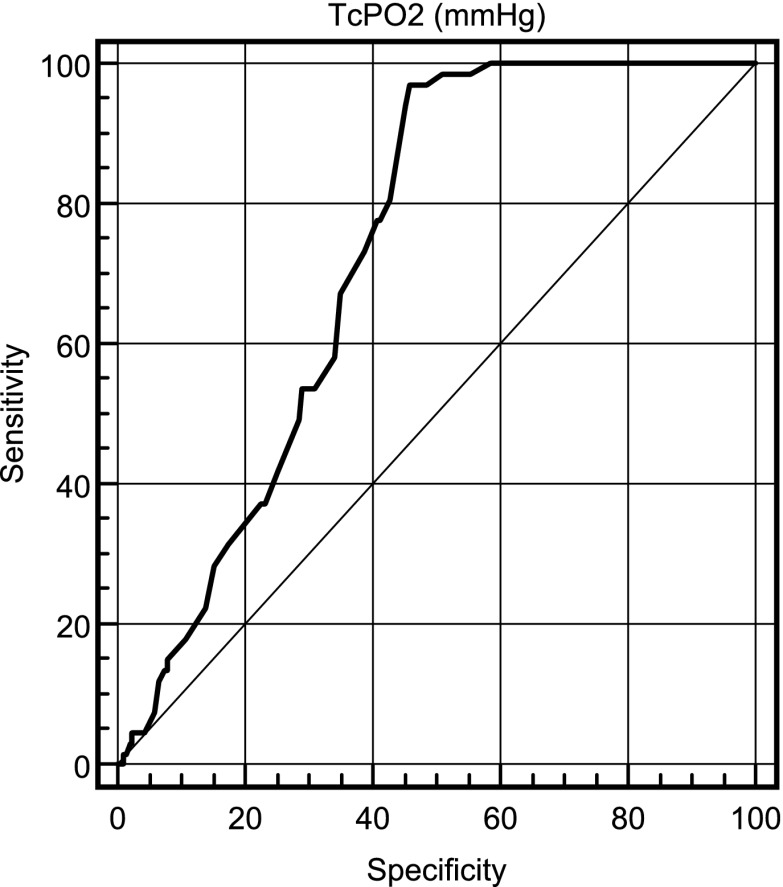
TcPO2, measured by an ROC curve, showed a value 46 mmHg as the best threshold for identifying MACE, with an area under the curve of 0.73 (95% CI 0.68–0.77). The ROC curve is reported in Fig. 1. The analysis showed the following results for that cutoff: sensitivity 0.97, specificity 0.54, positive predictive value 0.32, and negative predictive value 0.99. By using this cutoff, the percentage of patients with low TcPO2 was significantly higher in the MACE than in the non-MACE group.
Figure 1.
ROC curve of TcPO2 in detection of MACE in diabetic patients.
No significant differences in diabetes treatment or other medications (antihypertensive drugs, aspirin, and so on) were observed between the two study groups. The percentage of patients treated with statins was significantly higher in the non-MACE than in the MACE group (64.6 vs. 43.2%; P = 0.001).
Multivariate analysis
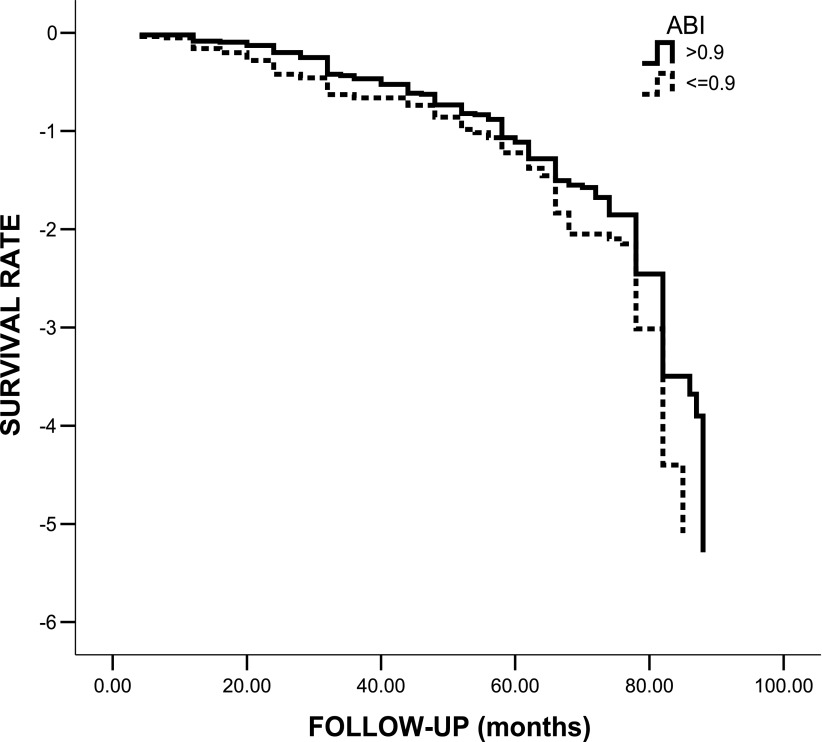
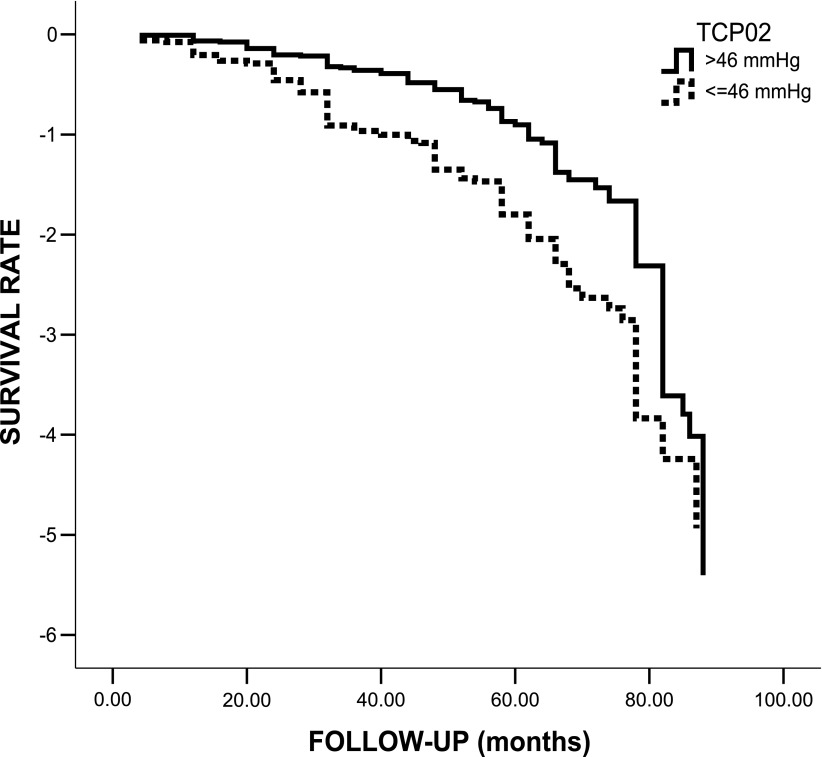
The Kaplan-Meier method showed that ABI ≤0.90 (Mantel log-rank test 4.087; P = 0.043 [Fig. 2]) and TcPO2 ≤46 mmHg (Mantel log-rank test 33.748; P < 0.0001; Fig. 3) at baseline were both significantly associated with a higher incidence of MACE. For assessment of the impact of several variables, including ABI, as independent predictors of MACE, a multivariate Cox regression analysis was performed. The following variables were tested as potential predictors: sex, age, diabetes duration, hypertension, family history of CAD, smoking, microalbuminuria, HbA1c, BMI, cholesterol, triglycerides, LDL, HDL, autonomic dysfunction, distal polyneuropathy, and ABI. Variables were dichotomized as previously reported (14–17,19). The only independent predictor of MACE was microalbuminuria (HR 1.3 [95% CI 1.03–1.64]; P = 0.023). ABI did not enter the model. When TcPO2 (≤46 mmHg) was added to the list of potential predictors, Cox regression analysis showed that the only independent predictor of MACE was TcPO2 (1.78 [1.44–2.23]; P < 0.001) and that microalbuminuria and ABI did not enter the model. When a different cutoff for TcPO2 was used (<40 mmHg), Cox regression analysis showed similar predictive values for TcPO2 (1.69 [1.41–2.29]; P < 0.001). ABI did not enter the model, even when other cutoffs (>1.2, >1.3, and >1.4) were tested.
Figure 2.
Kaplan-Meier survival curve according to the presence of normal (>0.9) or pathological (≤0.9) ABI in 361 patients with apparently uncomplicated type 2 diabetes.
Figure 3.
Kaplan-Meier survival curve according to the presence of normal (>46 mmHg) or pathological (≤46 mmHg) TcPO2 in 361 patients with apparently uncomplicated type 2 diabetes.
CONCLUSIONS
Our study shows for the first time that 1) TcPO2 is a simple, reproducible, and reliable tool to identify subjects at very high risk for MACE among patients with uncomplicated type 2 diabetes and 2) TcPO2 has a predictive power for MACE higher than that of ABI. It is well-known that type 2 diabetic patients suffering from PAD have a very high cardiovascular risk and an increased mortality (20,21). However, PAD is often asymptomatic, and therefore it should be assessed in every diabetic patient (11). At the moment, ABI represents the parameter commonly used for the diagnosis of PAD and is also considered to be a strong predictor of cardiovascular morbidity and mortality in the general population (22,23). This prognostic value was also suggested in diabetic patients (9). Nevertheless, in diabetic patients both the diagnostic and the predictive value of ABI may be limited because of a high prevalence of false-negative values as a result of medial arterial calcifications (7–9). Nam et al. (7) showed that the most significant factor affecting the validity of ABI was diabetes with an OR of 4.36 for false-negative results. In addition, although ABI is usually considered simple and reproducible, it may be affected by the experience of practicing physicians (24). However, a recent paper has observed that the predictive value for cardiovascular and all-cause mortality may be similar in subjects with and without type 2 diabetes (25).
TcPO2 is a noninvasive and highly reproducible measure of skin oxygenation and reflects very well the metabolic state of lower limbs (10–12). Given that TcPO2 measurement is not affected by arterial calcification, it is particularly useful in evaluating and managing vascular disease in diabetic patients (13). Several studies have analyzed the use of TcPO2 measurement in determining amputation level, the need of revascularization (11,12,26), and wound healing evaluation (11,12,27), but no studies are available in the literature about the use of TcPO2 as a predictor of cardiovascular events. The main original findings of our investigation are that there is a strong independent association between TcPO2 and future occurrence of MACE and that this association is stronger than that between ABI and MACE. This greater predictive value of TcPO2 may be due to the fact that TcPO2 directly reflects tissue perfusion, while ABI is affected by artery calcifications often present in diabetes (11). These new findings could have interesting clinical implications. Indeed, although all diabetic patients have a high cardiovascular risk, those with a TcPO2 ≤46 mmHg might have a particularly high cardiovascular risk. The assessment of TcPO2 could be useful to better stratify the cardiovascular risk of diabetic patients and could imply that specific treatments should be performed. This may be particularly useful in diabetic subjects at relatively lower cardiovascular risk, as were those enrolled in the current study. Indeed, only persons with uncomplicated diabetes were evaluated. Certainly, ABI remains a routine tool for screening PAD among diabetic patients. It is interesting to note that although ABI is significantly associated with MACE in univariate analysis and in the Kaplan-Meier survival curve, it does not enter the model in the multivariate analysis. However, we can exclude that ABI may be independently associated with MACE in a larger study population. But specific studies should clarify when TcPO2 should be used to more effectively identify subjects at higher cardiovascular risk. The recent study by Hanssen et al. (25) has shown that ABI has similar predictive value for mortality both in diabetic and nondiabetic subjects. We cannot exclude that TcPO2 may have a higher predictive value also in nondiabetic patients, but specific work is needed to test this hypothesis. However, our study population may be rather small, since it is difficult to accurately estimate a sample size. Indeed, there are no previous studies on the prevalence of abnormal TcPO2 in patients without foot ulcers and with PAD; in addition, an established cutoff of TcPO2 does not exist. This implies that our data should be interpreted with caution and larger studies are needed to confirm our findings.
Another finding of the current study relates to the cutoff of TcPO2. We have identified a cutoff that seems to identify very well patients at risk for MACE, but there is still not an established TcPO2 value to predict the need for revascularization or for the diagnosis of PAD. It is important to remember that no study has evaluated the potential use of TcPO2 in asymptomatic PAD patients; therefore, it is not possible to directly compare our data with those of previous studies that have evaluated subjects with critical limb ischemia or an amputation threshold. Indeed, some studies considered 30 mmHg as the best threshold for TcPO2 to identify patients who need revascularization (28). The TransAtlantic Inter-Society Consensus stated that TcPO2 <30 mmHg is related to critical limb ischemia (4). Faglia et al. (28) identified a cutoff of 34 mmHg for revascularization needs, stating that for values between 34 and 40 mmHg there exists an elevated amputation probability. Other work suggested a TcPO2 value between 30 and 50 mmHg for the diagnosis of PAD, even if this range appears to be too wide for clinical purposes (11). In our investigation, a threshold of 46 mmHg has been identified as the most efficient cut point for the identification of the patients at high risk for MACE. However, we have also evaluated a TcPO2 cutoff equal to ≤40 mmHg, which represents the threshold beyond which the amputation risk is drastically reduced (28) and which was used in our previous study (15). The multivariate analysis showed that a cutoff of 40 mmHg is also able to effectively identify subjects at risk for MACE. In any case, specific large studies are needed to establish both the best cutoff for the diagnosis of PAD and the most adequate threshold to predict MACE, even if it is likely that the two cutoffs may coincide.
In our study, no difference in aspirin use was observed between patients with and without MACE. There are several possible reasons for this surprising finding. First, some patients may have taken aspirin in a noncontinuous manner or may have stopped taking it during the follow-up. On the other hand, this is not a trial to evaluate the efficacy of a drug. So, we do not have complete information on treatments during the study period. In addition, there is evidence that aspirin may be less effective in preventing MACE in diabetic patients than in nondiabetic people (29).
It is evident that in our study, only people “at relatively low cardiovascular risk” were enrolled; indeed, all patients with conditions or risk factors, including age >75 years, associated with an increased incidence of MACE were excluded. But if one considers the incidence of MACE during the follow-up period, our study population seems to be “at high cardiovascular risk.” Indeed, we have observed an incidence of events greater than that reported in other investigations (30,31). Nevertheless, this seems mainly due to the fact that we have used a broad composite clinical outcome that includes all vascular complications, including those transient, such as transient ischemic attack, as also done in previous studies (14,16). In addition, we cannot exclude that a slight increased evidence of cardiac events may be due to the systematic screening for silent CAD in all men with erectile dysfunction in our clinic (19,32).
A study limitation may be represented by the fact that intra- and interobserver errors for TcPO2 were not tested. Most studies found inter/intraobserver errors for TcPO2 of ~20%. This may imply that a single reading in a single foot could make a large difference to the cutoff value. This is true even if one considers that in our study, TcPO2 was measured in both legs but only the lowest value was reported in the results and used for the analyses. Therefore, other larger studies are needed to test the predictive power of TcPO2 and the exact cut point value.
In conclusion, the present longitudinal study showed that TcPO2 could possibly be used as a predictor of MACE among patients with uncomplicated type 2 diabetes and that its predictive value may be greater than that of ABI.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
C.G. and A.C. were the principal investigators, designed the study, wrote the article, and participated in all analyses and interpretation of data. C.F. and C.L. participated in the study design, collected and checked data, and contributed to writing the paper. T.M. and E.B. performed statistical analysis and contributed to writing the paper. P.G. participated in the study design, collected and checked data, and contributed to writing the paper. A.P. performed statistical analysis and contributed to writing the paper. S.B.S. participated in the study design, collected and checked data, and contributed to writing the paper. G.P. and A.G. were responsible for overall supervision of the research and interpretation of data and contributed to writing the paper. C.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S14–S21 [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146 [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33(Suppl. 1):S1–S75 [DOI] [PubMed] [Google Scholar]
- 5.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation 2006;113:388–393 [DOI] [PubMed] [Google Scholar]
- 6.Fowkes FG, Murray GD, Butcher I, et al. Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam SC, Han SH, Lim SH, et al. Factors affecting the validity of ankle-brachial index in the diagnosis of peripheral arterial obstructive disease. Angiology 2010;61:392–396 [DOI] [PubMed] [Google Scholar]
- 8.Cao P, Eckstein HH, De Rango P, et al. Chapter II: Diagnostic methods. Eur J Vasc Endovasc Surg 2011;42(Suppl. 2):S13–S32 [DOI] [PubMed] [Google Scholar]
- 9.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg 2011;41:110–116 [DOI] [PubMed] [Google Scholar]
- 10.Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother 2004;58:427–431 [DOI] [PubMed] [Google Scholar]
- 11.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine 2012;41:384–397 [DOI] [PubMed] [Google Scholar]
- 12.de Meijer VE, Van’t Sant HP, Spronk S, Kusters FJ, den Hoed PT. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J Vasc Surg 2008;48:382–388 [DOI] [PubMed] [Google Scholar]
- 13.Abou-Zamzam AM, Jr, Gomez NR, Molkara A, et al. A prospective analysis of critical limb ischemia: factors leading to major primary amputation versus revascularization. Ann Vasc Surg 2007;21:458–463 [DOI] [PubMed] [Google Scholar]
- 14.Gazzaruso C, Coppola A, Montalcini T, et al. Screening for asymptomatic coronary artery disease can reduce cardiovascular mortality and morbidity in type 2 diabetic patients. Intern Emerg Med 2012;7:257–266 [DOI] [PubMed] [Google Scholar]
- 15.Gazzaruso C, Coppola A, Montalcini T, et al. Lipoprotein(a) and homocysteine as genetic risk factors for vascular and neuropathic diabetic foot in type 2 diabetes mellitus. Endocrine 2012;41:89–95 [DOI] [PubMed] [Google Scholar]
- 16.Gazzaruso C, Solerte SB, Pujia A, et al. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with asymptomatic coronary artery disease angiographically proven. A potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol 2008;51:2040–2044 [DOI] [PubMed] [Google Scholar]
- 17.Gazzaruso C, Garzaniti A, Giordanetti S, Falcone C, Fratino P. Silent coronary artery disease in type 2 diabetes mellitus: the role of Lipoprotein(a), homocysteine and apo(a) polymorphism. Cardiovasc Diabetol 2002;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 19.Gazzaruso C, Giordanetti S, De Amici E, et al. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation 2004;110:22–26 [DOI] [PubMed] [Google Scholar]
- 20.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–386 [DOI] [PubMed] [Google Scholar]
- 21.Kallio M, Forsblom C, Groop PH, Groop L, Lepäntalo M. Development of new peripheral arterial occlusive disease in patients with type 2 diabetes during a mean follow-up of 11 years. Diabetes Care 2003;26:1241–1245 [DOI] [PubMed] [Google Scholar]
- 22.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004;109:733–739 [DOI] [PubMed] [Google Scholar]
- 23.Fine JJ, Hopkins CB, Hall PAX. Abnormal ankle brachial indices may predict cardiovascular disease among diabetic patients without known heart disease. Circ J 2005;69:798–801 [DOI] [PubMed] [Google Scholar]
- 24.Andersen CA. Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg 2010;52(Suppl.):76S–80S [DOI] [PubMed] [Google Scholar]
- 25.Hanssen NMJ, Huijberts MS, Schalkwijk CG, Nijpels G, Dekker JM, Stehouwer CDA. Associations between the ankle-brachial index and cardiovascular and all-cause mortality are similar in individuals without and with type 2 diabetes: nineteen-year follow-up of a population-based cohort study. Diabetes Care 2012;35:1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redlich U, Xiong YY, Pech M, et al. Superiority of transcutaneous oxygen tension measurements in predicting limb salvage after below-the-knee angioplasty: a prospective trial in diabetic patients with critical limb ischemia. Cardiovasc Intervent Radiol 2011;34:271–279 [DOI] [PubMed] [Google Scholar]
- 27.Ladurner R, Küper M, Königsrainer I, et al. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med Sci Monit 2010;16:CR273–CR277 [PubMed] [Google Scholar]
- 28.Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Morabito A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg 2007;33:731–736 [DOI] [PubMed] [Google Scholar]
- 29.Angiolillo DJ. Antiplatelet therapy in diabetes: efficacy and limitations of current treatment strategies and future directions. Diabetes Care 2009;32:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young LH, Wackers FJ, Chyun DA, et al. DIAD Investigators Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc 2012;1:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazzaruso C, Coppola A, Montalcini T, et al. Erectile dysfunction can improve the effectiveness of the current guidelines for the screening for asymptomatic coronary artery disease in diabetes. Endocrine 2011;40:273–279 [DOI] [PubMed] [Google Scholar]