Abstract
OBJECTIVE
To determine the relationship between markers of insulin resistance (fasting insulin and homeostasis model assessment of insulin resistance), markers of adiposity (BMI, waist circumference, and body fat), HbA1c, and cognitive performances in a middle-aged population–based sample free of diabetes.
RESEARCH DESIGN AND METHODS
Our study sample consisted of 1,172 people aged 35–64 years (49% women), free of diabetes, and recruited between 2005 and 2007 in the MONA LISA survey. Cognitive functions (memory, attention, and processing speed) were evaluated by neuropsychological tests: word-list learning test, digit symbol substitution test (DSST), word fluency test, and Stroop Test. Multiple logistic regressions were used to estimate the relationship between cognitive performance and metabolic markers. We serially adjusted for age, sex, education, and occupational status (model A), additionally for income, smoking, alcohol consumption, sedentarity, and psychotropic substance use (model B), and finally, included variables linked to the metabolic syndrome (hypertension, dyslipidemia, vascular disease, and C-reactive protein) and depression (model C).
RESULTS
Elevated markers of adiposity were associated with poor cognitive performance in tests evaluating processing speed. The probability of being in the lowest quartile of each test was nearly doubled for participants in the upper quartile of BMI, compared with those in the lowest one [BMI, adjusted odds ratio (OR) 2.18, P = 0.003 (DSST), and OR 2.09, P = 0.005 (Stroop Test)]. High HbA1c was associated with poor cognitive performance in DSST (adjusted OR 1.75, P = 0.037). Waist circumference was linked to poor cognitive performance in men but not in women.
CONCLUSIONS
Poor cognitive performance is associated with adiposity and hyperglycemia in healthy middle-aged people.
Recent results from the Whitehall II prospective cohort study have provided evidence of a cognitive decline related to aging, occurring at any age from 45 to 70 years, even among those 45–49 years of age at baseline (1). In a relatively young population, cognitive decline may be the first expression of poor cognitive reserve, which facilitates the expression of dementia in old age (2). The global prevalence of dementia was estimated in the world at 24.3 million in 2001, with the number of people affected by dementia projected to double every 20 years (3). A World Health Organization report has estimated that dementia contributes to 11.2% of years spent living with a disability in people >60 years of age, more than stroke, cardiovascular disease, and cancer (4). There are currently few effective treatment options to prevent or treat dementia. Therefore, identifying modifiable risk factors and the critical window for an effective intervention is important. To this end, there has been a recent focus on the identification of potential preventive factors for dementia, and epidemiological research has suggested various candidates, including modifiable lifestyle factors, such as social contacts, leisure activities, physical exercise, and diet, as well as some pharmacological strategies, such as ginkgo biloba, and treatments of vascular risk factors, such as diabetes and hypertension (5). Randomized trials have assessed the efficacy of intervention on cognitive outcomes, but few interventions seem to be effective in preventing cognitive decline or dementia. For example, diabetes is associated with cognitive decline and late-onset dementia (6), but the Action to Control Cardiovascular Risk in Diabetes–Memory in Diabetes (ACCORD-MIND) trial recently failed to demonstrate a positive impact of intensive glucose control on cognitive function in type 2 diabetic patients (mean age 62.5 ± 5.8 years) (7). We can hypothesize that interventions are more likely to be effective if they are applied over a long period, starting with the beginning of cognitive decline.
Diabetes is a progressive disease, frequently associated with adiposity and an asymptomatic state of metabolic dysregulation, consisting of insulin resistance, hyperinsulinemia, and a slight increase in glycemia. We hypothesize that metabolic dysregulation (insulin resistance, adiposity, and increased glycemia) is associated with a poor cognitive outcome in healthy middle-aged people. We therefore aimed to determine the relationship between clinical markers of adiposity (elevated BMI, body fat percentage, and waist circumference), biological markers of insulin resistance [high fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR)], HbA1c, and cognitive functions in a middle-aged population sample that is free of diabetes.
RESEARCH DESIGN AND METHODS
Study population
The 2005–2007 MONA LISA cross-sectional survey was carried out in three French centers: Lille, Strasbourg, and Toulouse areas, respectively, in northern, northeastern, and southwestern France.
Participants (aged 35–74 years) were randomly selected from electoral rolls after stratification by town size, sex, and age, in order to obtain 200 participants for each sex and each 10-year age-group. Participation rates were 50% for men and 51% for women. Cognitive function was assessed among participants from the Toulouse center exclusively. Consequently, the present analysis was restricted to this sample. A total of 1,627 participants completed the recruitment procedure in the Toulouse center, and our study population consisted of 1,172 participants without diabetes that were between 35 and 64 years of age. The excluded participants were 402 subjects who were 65 years of age or older and 53 subjects who suffered from diabetes. We decided to exclude participants with diabetes because diabetes treatment (i.e., insulin or metformin) and poor glycemic control interfere with the measurement of fasting insulin and HOMA (8,9).
This study received ethical approval from the appropriate ethics committee for the protection of people participating in biomedical research, and all participants provided written informed consent.
Data collection
After participants had provided written informed consent, a fasting blood sample was drawn. No fasting blood analysis was missing. Then, participants filled out a standard questionnaire on socioeconomic characteristics, previous medical history, cardiovascular risk factors, and drug treatments, and clinical measurements were taken by a specially trained nurse.
Assessment of adiposity
Anthropometric measurements included height, body weight, and waist circumference (at a midlevel between the lower rib margin and the iliac crest, to the nearest 0.5 cm). The participant’s body fat percentage was evaluated by the bioelectrical impedance analysis method (Tanita TBF-300 GS; Tanita France, Neuilly sur Seine, France). There were no missing data.
Participants gave their weight at 20 years of age. Weight change was the difference between the weight measured and weight at 20 years of age.
Assessment of insulin resistance
Biological measurements included fasting insulin and glucose measurement. All measurements were performed in a core laboratory (Pasteur Institute of Lille, Lille, France). Glucose was measured by the enzymatic colorimetric method (Olympus). Fasting insulin was measured by the enzyme immuno-assay technique (Beckman Coulter). Insulin resistance was estimated using the HOMA-IR, a standardized measure of insulin sensitivity. The HOMA-IR formula is defined as fasting insulin (mUI/L) multiplied by fasting glucose (mmol/L) divided by 22.5.
Clinically relevant cutoffs defining hyperinsulinemia were used: fasting insulin >12.2 mUI/L and HOMA >2.6 mUI/L ⋅ mmol/L (10). Only 49 participants (4.2%) had fasting insulin >12.2 mUI/L, and 68 participants (5.8%) presented with HOMA >2.6 mUI/L ⋅ mmol/L. No data on the clinical or biological assessment of insulin resistance were missing.
Assessment of HbA1c and diabetes
Diabetes was determined by the use of insulin or other antidiabetes medications or fasting glucose concentration ≥126 mg/dL or HbA1c ≥6.5% (11). Fifty-three participants had diabetes and were not included in our analyses.
HbA1c is an integrated measure of circulating glucose levels of the preceding 2–3 months. HbA1c was measured using high-performance liquid chromatography assays standardized to Diabetes Control and Complications Trial (DCCT) values (Chromsystems HPLC). No data were missing.
Measures of cognitive function
Four neuropsychological tests were used to assess cognitive function in the following order: 1) a word-list learning test (WLT) in three trials, each followed by immediate free recall adapted from the Rey auditory verbal learning test (12), 2) the digit symbol substitution test (DSST) from the revised version of the Wechsler Adult Intelligence Scale (WAIS-R III) (13), 3) a semantic word fluency test (WFT), and 4) a short version of the Stroop Test (14). All tests were run by a trained examiner.
The WLT consisted of three successive trials where the participant had to learn 16 words orally and loudly presented at a speed of one word per second. Each trial was followed by an immediate free recall. Results were given as the number of words correctly recalled. The WLT typically assesses episodic memory.
The DSST consisted of nine digit-symbol pairs, which served as a model, followed by a list of digits. The participant had to write down the corresponding symbol under as many digits as possible. The score was the total number of symbols correctly translated within the allotted time of 90 s. The DSST is considered as widely reflecting processing speed (15).
In the WFT, the participant was asked to name as many fruits as possible. The score was the number of correct items named within 1 min. The WFT is a category fluency test that reflects the efficiency of brain structures involved in semantic memory and executive functions.
The adapted version of the Stroop Test consisted of two successive tasks. In the first task, participants were given 40 patches colored either in blue, yellow, green, or red and were asked to write in corresponding boxes the initial of the color name (B, Y, G, and R). In the second task, participants were given 40 names of one of the four colors, with the names always printed in a different ink color than the color name (e.g., the name green was printed in yellow). They were asked to write in corresponding boxes the initial of the color name. Participants were instructed to perform the two tasks as quickly and accurately as possible. The duration of the first task evaluates processing speed. The task 1–task 2 duration difference evaluates the interference between the ink color seen and the color name read. It reflects the participant’s ability to inhibit their automatic and powerful tendency to name the color of the ink instead of the written name.
For each cognitive test, we defined that a participant had a poor performance if he/she was in the lowest quartile of the distribution of the test.
Assessment of covariates
Participants provided information on demographic variables, socioeconomic status, and medical history, including medication use, smoking, and alcohol consumption, using a questionnaire that was completed with the help of medical staff.
Educational level was assessed by the subject’s stated number of completed years of education (from starting primary school until graduation or school dropout). The education data from six individuals were missing. Workers were categorized according to their current occupation, and retired subjects were categorized according to their last occupation. We distinguished blue-collar and white-collar workers.
The household income tax level was reported (the data from 45 individuals were missing). Total alcohol intake was expressed as grams of alcohol per day. In terms of smoking exposure, subjects were categorized as never smokers, former smokers, and current smokers. A participant was considered sedentary if she/he performed no leisure-time physical activity.
A participant was considered to have cerebrovascular disease (CVD) if she/he reported a history of stroke or transient ischemic attack and to have coronary heart disease (CHD) if she/he reported angina pectoris or myocardial infarction.
Blood pressure measurements were performed with a standard sphygmomanometer (OMRON 705IT). The data from four individuals were missing. The average of the two blood pressure measurements was used for statistical analysis. High blood pressure was defined as systolic blood pressure ≥160 mmHg or mean diastolic blood pressure ≥95 mmHg. Participants were considered to have hypertension if they had high blood pressure on examination or if they were taking antihypertensive drugs. Dyslipidemia was considered present if the subject was taking a lipid-lowering drug or had total cholesterol ≥250 mg/dL and/or triglycerides ≥300 mg/dL. Cholesterol and triglyceride concentrations were measured using enzyme assays (Olympus).
Statistical analyses
Participants were categorized according to quartiles of markers of adiposity (BMI, body fat percentage, and waist circumference), markers of insulin resistance (fasting insulin and HOMA), and HbA1c. Participants were compared using the χ2 test or Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables.
Multiple logistic regressions were used to estimate the independent relationship between poor cognitive performance and metabolic markers and hyperglycemia after controlling for potential confounders. The potential confounders were variables associated with either cognitive performance or metabolic measures in univariate analyses, using P < 0.10 as the cutoff for significance, and variables previously found in the literature to influence cognitive performance.
We performed sequential adjustments. Model A was adjusted for age, sex, education, and occupational status. Model B included the same terms as model A plus environmental variables (poor level of income, smoking, alcohol consumption, sedentarity, and psychotropic substance use). Model C included the same terms as model B plus variables linked to the metabolic syndrome (hypertension, dyslipidemia, CHD, CVD, and C-reactive protein level) and depression. In additional analyses, we examined the possible interactions between metabolic markers and others covariates, using model B. We solely found a significant interaction between sex, waist circumference, and cognitive performance (P = 0.019). Consequently, the link between waist circumference and all cognitive tests was presented separately in men and women. No age interaction was found.
The accuracy of each model was assessed by estimating the area under the receiver operator characteristic curve (AUC). Bootstrap validation was also performed to correct the estimation. This is a technique, described in detail by Efron and Gong (16), in which the model is developed with all participants and then reanalyzed on repeated random samples of the data set. Resampling occurred 100 times for each bootstrap validation. Only corrected AUCs are presented.
The sensitivity of conclusions to classification of the cognitive measures by categories of quartiles or continuous linear outcomes was investigated. We repeated each analysis using multiple regression models.
To have an indirect assessment of the temporal link between BMI and cognitive performance, we performed sensitivity analyses using multiple logistic regressions to estimate the independent relationship between cognitive performance and BMI at 20 years of age and weight change. Analyses were performed using the Stata software package, version 11 (StataCorp, College Station, TX).
RESULTS
The study population consisted of 1,172 participants without diabetes, 35–64 years of age. Sixty-five participants (5.5%) did not complete the whole cognitive evaluation [17 performed no cognitive test, 1 performed the WLT only, 1 responded to all tests except the WLT, 1 to all tests except the DSST, and 45 did not fill in the two tasks of the Stroop Test (20 of them were color-blind)]. There were no significant differences in metabolic markers or HbA1c between patients with or without missing data on cognitive tests.
Participant characteristics
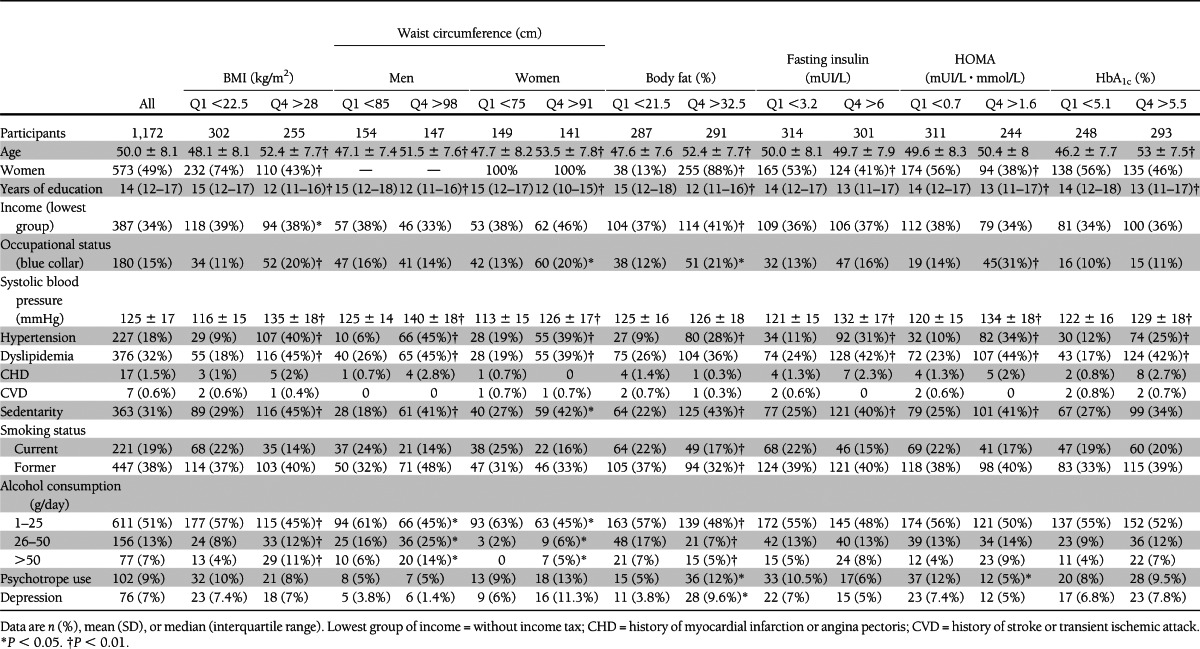
The general characteristics of the study population sample are presented in Table 1, for all people and for those in the first and fourth quartile of markers of adiposity, markers of insulin resistance, and HbA1c. The mean age of the entire cohort was 50.0 ± 8.1 years; half were women (49%). The level of education was high with a median of 14 (12–17) years. Approximately half of the sample had never smoked and one-third was sedentary. Participants in the fourth quartile of BMI, HOMA, and body fat percentage were more likely to be men and to present hypertension (P < 0.01).
Table 1.
General characteristics of the study population by markers of insulin resistance and HbA1c status
Metabolic characteristics and performance in cognitive tests are presented in Table 2. Few participants had hyperinsulinemia. Only 49 participants (4.2%) had fasting insulin >12.2 mUI/L, and 68 participants (5.8%) had HOMA >2.6.
Table 2.
Metabolic characteristics and performance in cognitive tests
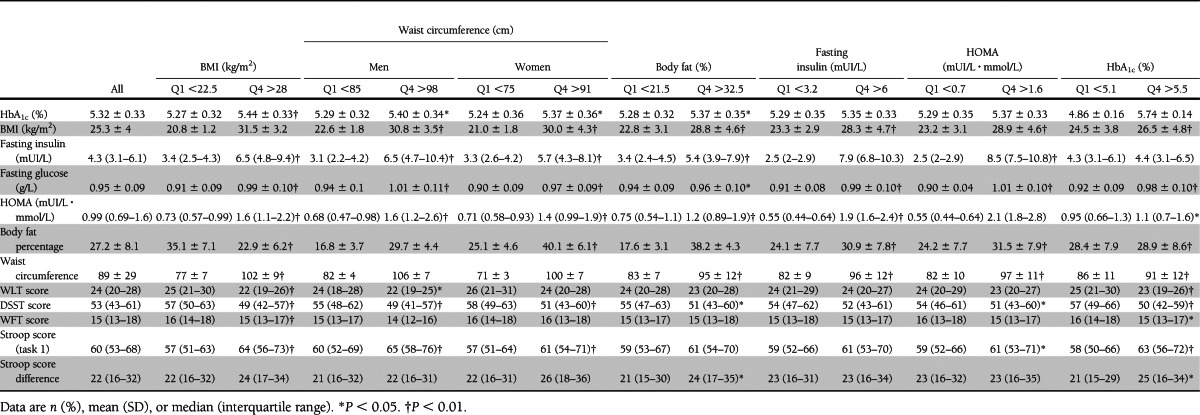
A poor cognitive performance was defined by a score of <21 words for the WLT, 45 symbols for the DSST, or 14 fruits for the WFT. For the Stroop Test, poor performance corresponded to a >69-s duration for the Stroop task 1 and to a >33-s duration for the Stroop task 1–task 2 difference.
Relationship between markers of insulin resistance, HbA1c, and cognitive function
Table 3 presents associations between markers of insulin resistance, HbA1c, and cognitive function. Only the first and fourth quartiles are presented in the table, but the second and third quartiles were also included in each multiple logistic regression model. Figure 1 presents the association between markers of adiposity, markers of insulin resistance, HbA1c, and tests evaluating processing speed.
Table 3.
Associations between markers of adiposity, markers of insulin resistance, HbA1c, and cognitive function
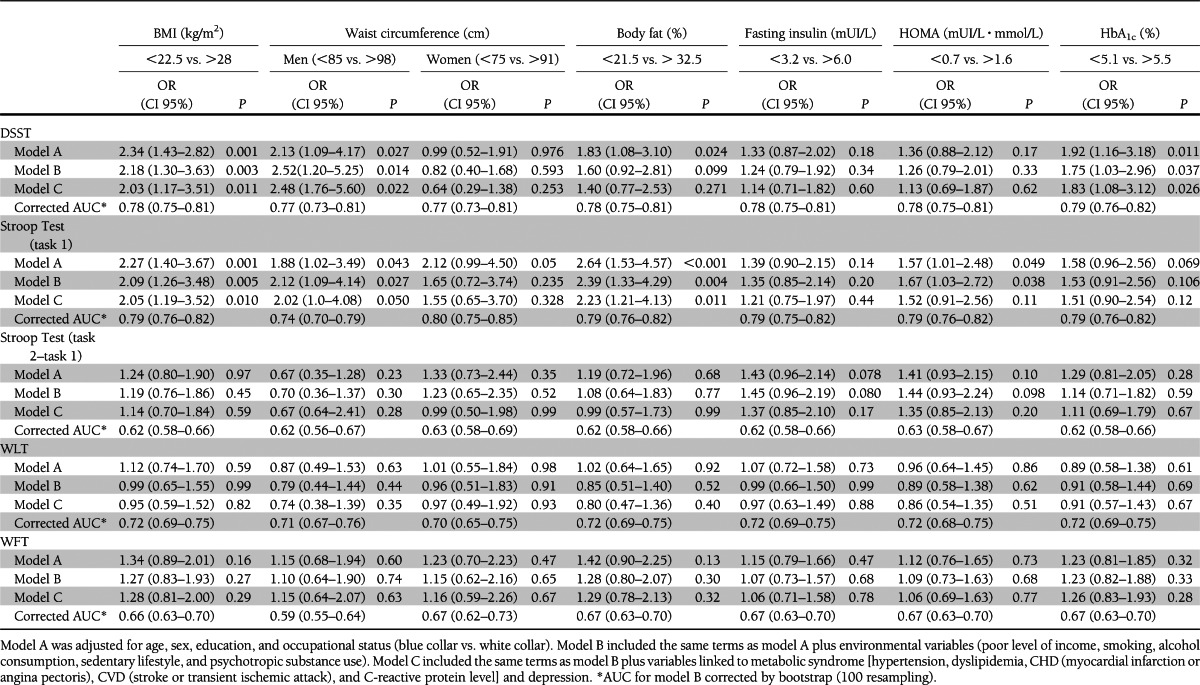
Figure 1.
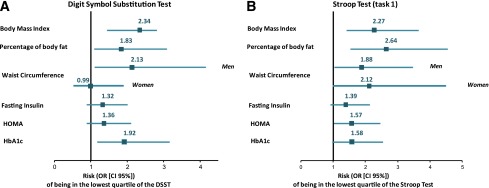
Associations between tests evaluating processing speed and markers of insulin resistance, markers of adiposity, and HbA1c. This figure presents the risk of being in the lowest quartile of the cognitive tests [DSST (A) and Stroop Test (task 1) (B)] for participants in the fourth quartile compared with participants in the first quartile of BMI, body fat percentage, waist circumference, fasting insulin, HOMA, and HbA1c. Results are adjusted for age, sex, educational level, and occupational status. (A high-quality color representation of this figure is available in the online issue.)
High BMI was significantly associated with poor cognitive performance in the DSST and the Stroop Test (task 1), two tests evaluating processing speed. The risk of being in the lowest quartile of each test was nearly doubled for participants in the fourth quartile of BMI distribution compared with participants in the first quartile after adjustment for age, sex, years of education, and occupational status [DSST, OR 2.34 (95% CI 1.43–2.82); Stroop Test, OR 2.27 (1.40–3.67)]. The association between BMI and cognitive performance persisted in models B and C.
In men, high waist circumference was significantly associated with poor cognitive performance in tests evaluating processing speed [model B: DSST, OR 2.52 (95% CI 1.20–5.25); Stroop Test, OR 2.12 (1.09–4.14)]. However, in men, the association between high waist circumference and poor cognitive performance was not significant after adjustment for BMI or percentage of body fat.
In women, the waist circumference was not associated with poor cognitive performance. However, after adjustment for BMI or percentage of body fat, women with a high waist circumference had better performance in the DSST than women with a low waist circumference [model B + BMI, OR 0.20 (95% CI 0.10–0.58); model B + percentage of body fat, OR 0.38 (0.15–0.98)].
High HOMA was associated with poor cognitive performance in the Stroop Test (task 1). The risk of being in the lowest quartile of this test was increased by 50% for participants in the upper quartile of HOMA distribution compared with participants in the first quartile [model B: Stroop Test, OR 1.67 (1.03–2.72)]. However, the association between a high HOMA and poor cognitive performance was not significant after adjustment for markers of adiposity. Table 4 presents these additional adjustments.
Table 4.
Associations between markers of adiposity, markers of insulin resistance, HbA1c, and tests evaluating processing speed (additional adjustments)
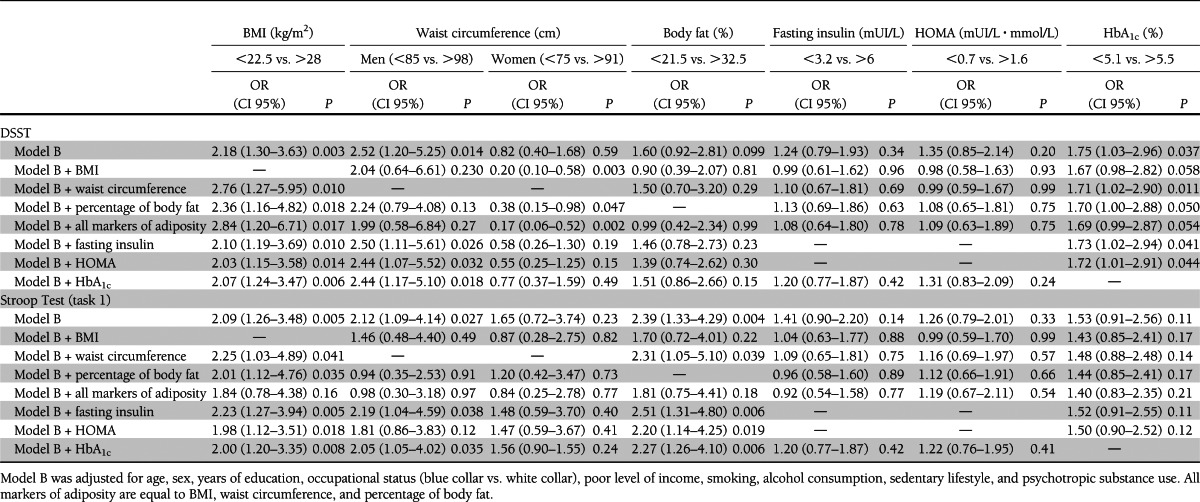
High HbA1c was associated with poor cognitive performance in the DSST but not in the Stroop Test [model B: DSST, OR 1.75 (95% CI 1.03–2.96); Stroop Test, OR 1.53 (0.91–2.56)]. The association between HbA1c and DSST score persisted in models B and C. None of the metabolic markers were associated with poor cognitive performance in the WLT or in the WFT.
Sensitivity analyses were performed to assess whether using linear regression analyses instead of logistic regression models would change overall conclusions. Each variable was coded in the same way as in the logistic regression models. These analyses revealed no substantive differences in the conclusions from using logistic models with the lowest quartile of cognitive performance as the outcome.
In additional analyses, the BMI at 20 years of age (BMI <19.5 vs. BMI >23.5) was associated with poor cognitive performance in the DSST [model A: OR 1.89 (95% CI 1.16–3.08), P = 0.010] but not in other cognitive tests. Weight gain (no change or weight loss vs. weight gain >11 kg) was associated with poor cognitive performance in the Stroop Test [model A: OR 1.69 (1.02–2.80), P = 0.043] but not in other cognitive tests.
CONCLUSIONS
In a population of middle-aged adults without diabetes, we found that adiposity and a high level of HbA1c were both associated with poor cognitive performance in tests assessing processing speed. Waist circumference was linked to poor cognitive performance in men but not in women. After adjustment for BMI or percentage of body fat, waist circumference was not yet linked to cognitive performance in men, and high waist circumference was linked to good cognitive performance in women.
The main strengths of this study include its large sample size and population-based design and the recruitment of younger participants with lower levels of insulin resistance than in previous studies. Additional strengths are the use of different clinical and biological measures of insulin resistance and adiposity and a low rate of missing data. The main limitation is its cross-sectional design. The lack of temporal depth of cross-sectional studies raises concerns with issues of causal directionality. Participants with poor cognitive performance could have unhealthy behaviors (eating more and exercising less), which would lead to adiposity and insulin resistance. Moreover, associations could be secondary to confounding by education status, for example. To address this issue, we adjusted for years of education, occupational status, and income tax level, and we estimated the relationship between cognitive performance and BMI at 20 years of age to have an indirect assessment of the temporal link between BMI and cognitive performance. In our sample, BMI at 20 years of age and weight gain were associated with poor cognitive performance in tests assessing processing speed 15–45 years later. Nevertheless, the causal relationship between adiposity and cognitive function still needs to be evaluated in longitudinal studies.
Previous studies reported an association between BMI, HbA1c, insulin resistance, and cognitive performance in middle-aged adults. In a cohort of 2,223 workers 32–62 years of age at baseline, a higher BMI was associated with lower cognitive scores and a higher cognitive decline over a 5-year follow-up (17). Using a cohort of 2,439 individuals from the Framingham Offspring study, Tan and al. (18) found that HbA1c, HOMA, and fasting insulin were related to poorer executive function scores and a smaller total cerebral brain volume. In this cohort, mean age was 61 years and more than half of the sample had hyperinsulinemia. In the Atherosclerosis Risk in Communities (ARIC) cohort, fasting insulin and HOMA were associated with a lower baseline score in DSST, delayed word recall, and phonemic word fluency (19). In this cohort, mean age was 53 years and nearly one-third of the sample had hyperinsulinemia. In the Framingham Offspring study and the ARIC cohort, data on adiposity were not presented. In our sample, markers of insulin resistance were not associated significantly with cognitive performance after adjustment of markers of adiposity.
Our population was younger, had a lower prevalence of insulin resistance (<8% of participants, even after inclusion of diabetic subjects), and was representative of a Western European middle-aged population free of diabetes. Our results show that even in healthy middle-aged adults without diabetes, adiposity and a high (but still normal) HbA1c level are negatively associated with cognitive performance. When we included diabetic patients, the relationship was not significantly modified. Our results offer evidence for an early link between slower processing speed and metabolic dysregulation.
As individuals age, many aspects of information processing become less efficient and processing speed seems to decline earlier than other cognitive domains (20). Young adults with type 1 diabetes and altered metabolic control had a poorer performance on tests assessing processing speed than those with better control, whereas performance on others tests (memory, attention, learning, etc.) was not significantly different between the two groups (21). Furthermore, in a French cohort of 3,777 elderly subjects, a decline in cognitive performance was observed about 12 years before the fulfillment of dementia criteria. The first measurable decline in cognitive performance was in the Isaacs Set Test, a multidetermined test involving semantic memory. Response rapidity is one important component of this test (22).
Mechanisms explaining the relationship between adiposity, glycemia, and cognitive function remain unclear and might involve various effects of Alzheimer disease neuropathology and/or cerebral microvascular disease (6,23).
Adiposity is a continuum without a clear or ideal threshold identifying pathological status. An increase in adiposity is associated with an increase in insulin resistance, inflammation, and risk of cardiovascular disease (24). However, in our study, insulin resistance seems not to be the most important component explaining the link between adiposity and cognitive performance.
Adipocytes produce free fatty acids and induce overexpression of tumor necrosis factor-α, a cytokine involved in inflammation. Free fatty acids inhibit extracellular degradation of amyloid β peptide by competition for insulin-degrading enzyme (25) and stimulate in vitro assembly of amyloid and τ filaments (26). Tumor necrosis factor-α is elevated in the brain and the cerebrospinal fluid of patients with Alzheimer disease and in adults with mild cognitive impairment, and it inhibits amyloid β peptide transport from the brain to the periphery (23).
Waist circumference was associated with poor cognitive performance in tests assessing processing speed in men but not in women. After adjustment for BMI or percentage of body fat, high waist circumference was linked to better cognitive performance in women. Participants in the fourth quartile of waist circumference distribution may mainly fulfill one or more criteria of the metabolic syndrome (27). In the Health ABC study, women with the metabolic syndrome had more subcutaneous abdominal fat than men (370.9 ± 125.9 vs. 271.0 ± 89.5 cm2), whereas the proportion of visceral fat was quite similar in the two groups (162.3 ± 61.5 cm2 in women vs. 195.4 ± 73.2 cm2 in men) (28). Such a difference in fat distribution has a hormonal impact. A part of oestrone, the second major human circulating estrogen in premenopausal women and the predominant one in postmenopausal women, is derived from aromatization of androstenedione in adipose tissue and subcutaneous adipose tissue (28). Consequently, women in the fourth quartile of waist circumference distribution may have a high level of oestrone, and estrogens seem to have a protective effect on neurocognition in middle-aged women (5,29).
Our results show that a high (but still normal) HbA1c level is negatively associated with cognitive performance even in healthy middle-aged adults without diabetes. An increase in glycemia is linked to an increase in retinopathy prevalence (30). Retinal microvascular abnormalities seem to reflect small vessel damage in the brain and are independently associated with poor cognitive function, especially in processing speed tests, and MRI images of cerebral atrophy (31,32). Chronic exposure to hyperglycemia is believed to initiate a cascade of biochemical and physiological changes that ultimately lead to microvascular damages. Interestingly, young adults with type 1 diabetes and poor metabolic control (HbA1c >8.8%) performed more slowly on measures of psychomotor efficiency than those with better control (HbA1c <7.4%) (21).
Our study shows that cognitive impairment associated with adiposity and glycemia is already evident in middle-aged people. Provided these results could be confirmed by longitudinal data, the next step would be to examine whether early interventions aimed at reducing glycemia, BMI, and adiposity in middle-aged people would be able to delay cognitive decline and dementia in nondiabetic subjects. Pharmacological and lifestyle intervention in middle-aged adults have already demonstrated their ability to prevent type 2 diabetes (33–36). These interventions were feasible and led to weight loss, waist circumference reduction, and a decrease in the HbA1c level and thus could possibly be helpful in preventing cognitive decline in adults.
Acknowledgments
The MONA LISA study was supported by a grant from the Agence Nationale de la Recherche (ANR-05-PNRA- 018), the Institut National de Veille Sanitaire, INSERM, and a grant from Pfizer. This study was also supported by a grant for young researchers from the Toulouse University Hospital. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. No other potential conflicts of interest relevant to this article were reported.
C.M.S. performed statistical analyses, interpreted the data, and wrote the manuscript. J.-B.R. contributed to the conception and design of the study, advised on all statistical aspects, interpreted the data, and reviewed the manuscript and approved the final version to be published. V.B. contributed to the conception of the study, collected and standardized the database, and reviewed the manuscript and approved the final version to be published. J.-C.M. was a cognitive functioning expert, chose the neuropsychological tests used in the study, and reviewed the manuscript and approved the final version to be published. H.H. reviewed the manuscript and approved the final version to be published. J.F. contributed to the conception of the study and reviewed the manuscript and approved the final version to be published. S.A. interpreted the data and reviewed the manuscript and approved the final version to be published. J.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the nurses, physicians, dieticians, computer scientists, and secretaries of Toulouse, the Genomic Analysis Laboratory and the Department of Biological Sciences of the Pasteur Institute of Lille, the Department of Cardiology of the Toulouse University Hospital, and the city halls that have participated.
References
- 1.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460 [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, et al. Alzheimer’s Disease International Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet 2011;377:1019–1031 [DOI] [PubMed] [Google Scholar]
- 5.Coley N, Andrieu S, Gardette V, et al. Dementia prevention: methodological explanations for inconsistent results. Epidemiol Rev 2008;30:35–66 [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 7.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 2001;86:5457–5464 [DOI] [PubMed] [Google Scholar]
- 9.Katsuki A, Sumida Y, Urakawa H, et al. Neither homeostasis model assessment nor quantitative insulin sensitivity check index can predict insulin resistance in elderly patients with poorly controlled type 2 diabetes mellitus. J Clin Endocrinol Metab 2002;87:5332–5335 [DOI] [PubMed] [Google Scholar]
- 10.McAuley KA, Williams SM, Mann JI, et al. Diagnosing insulin resistance in the general population. Diabetes Care 2001;24:460–464 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rey A. Clinical Tests in Psychology Paris, Presses Universitaires de France, 1964 [in French] [Google Scholar]
- 13.Wechsler D. Manual for the Wechsler Adult Intelligence Scale New York, Psychological Corporation, 1955 [Google Scholar]
- 14.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662 [Google Scholar]
- 15.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996;103:403–428 [DOI] [PubMed] [Google Scholar]
- 16.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat 1983;37:36–48 [Google Scholar]
- 17.Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006;67:1208–1214 [DOI] [PubMed] [Google Scholar]
- 18.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 2011;34:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young SE, Mainous AG, 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care 2006;29:2688–2693 [DOI] [PubMed] [Google Scholar]
- 20.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging 2002;17:299–320 [PubMed] [Google Scholar]
- 21.Jacobson AM, Musen G, Ryan CM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol 2008;64:492–498 [DOI] [PubMed] [Google Scholar]
- 23.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol 2009;66:300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirier P, Giles TD, Bray GA, et al. American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918 [DOI] [PubMed] [Google Scholar]
- 25.Hamel FG, Upward JL, Bennett RG. In vitro inhibition of insulin-degrading enzyme by long-chain fatty acids and their coenzyme A thioesters. Endocrinology 2003;144:2404–2408 [DOI] [PubMed] [Google Scholar]
- 26.Gamblin TC, King ME, Kuret J, Berry RW, Binder LI. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry 2000;39:14203–14210 [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–783 [DOI] [PubMed] [Google Scholar]
- 29.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol 2011;69:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng YJ, Gregg EW, Geiss LS, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: implications for diabetes diagnostic thresholds. Diabetes Care 2009;32:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki R, Cheung N, Mosley T, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:1826–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492 [DOI] [PubMed] [Google Scholar]
- 33.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 34.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 35.Sanz C, Gautier JF, Hanaire H. Physical exercise for the prevention and treatment of type 2 diabetes. Diabetes Metab 2010;36:346–351 [DOI] [PubMed] [Google Scholar]
- 36.Knowler WC, Narayan KM, Hanson RL, et al. Preventing non-insulin-dependent diabetes. Diabetes 1995;44:483–488 [DOI] [PubMed] [Google Scholar]


