Abstract
OBJECTIVE
Wnt/β-catenin signaling is related to the pathogenesis of several diseases. Sclerostin is an inhibitor of Wnt/β-catenin signaling. However, there are few data regarding the sclerostin levels and vascular disease. Our aim was to examine the relationship between serum sclerostin and atherosclerotic disease (AD) in type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
We performed a cross-sectional study including 78 T2DM patients (45.3% females, mean age 59 ± 5.7 years; 54.7% males, 57.4 ± 6.7 years).
RESULTS
Serum sclerostin concentrations of T2DM patients in the AD group were significantly higher than in the non-AD group (P = 0.006). For each increase of 1 pmol/L in sclerostin level, there was a 4% increase of the risk of AD in T2DM patients. A concentration of ≥42.3 pmol/L showed a sensitivity of 69% and a specificity of 54.8% to detect an increased risk of AD. In males, sclerostin levels were higher in those with AD (P = 0.04), abnormal intima-media thickness (IMT) (P = 0.004), carotid plaques (P < 0.001), and aortic calcification (P < 0.001). In females, higher levels of sclerostin were related to abnormal IMT (P = 0.03) and aortic calcifications (P = 0.004). Homocysteine (β = 0.319 [95% CI 0.561–2.586], P = 0.003) and IMT (β = 0.330 [14.237–67.693], P = 0.003) were positively correlated with sclerostin.
CONCLUSIONS
Circulating sclerostin is increased in T2DM patients with atherosclerotic lesions. Although the sample size of our study was small, these data suggest that sclerostin levels could be a major modulator of Wnt signaling in AD with implications in T2DM patients.
Type 2 diabetes mellitus (T2DM) enhances the risk of macrovascular complications (coronary artery disease, peripheral artery disease, and cerebrovascular disease) and disorders of bone metabolism with serious consequences on morbidity and mortality. Atherosclerosis is the main pathological mechanism in macrovascular disease, inducing an inappropriate proliferation of vascular smooth muscle cells (VSMCs), which is linked to thickening of the arterial wall, atheroma plaque formation, and vascular calcification (1).
The canonical Wnt or Wnt/β-catenin pathway is increasingly related to the regulation of proliferation, migration, and survival of VSMCs (2–4). Furthermore, a gene mutation implicated in this pathway has been associated with hyperlipidemia, hypertension, and early coronary artery disease in metabolic syndrome patients (5). In these patients, abnormal canonical Wnt signaling has been also implicated in disturbances of the lipids, glucose, and bone homeostasis (6–9).
The Wnt/β-catenin pathway results from Wnt proteins binding to its receptors Frizzled and its coreceptors LRP-5 and -6 on the cell surface. The formation of the complex increases the stability of β-catenin, which leads to its translocation in the nucleus and induces transcription of Wnt target genes (10). The canonical Wnt pathway is modulated by several Wnt antagonists, including a family of proteins such as soluble Frizzled-related receptors (sFRPs) and dickkopfs (DKKs), which have been shown in physiological and pathological processes to be related to vascular injury in experimental mice (9,11–13) and humans (9,14).
On the other hand, sclerostin is an endogenous antagonist secreted almost always exclusively by osteocytes, and it has been extensively studied as a major regulator of canonical Wnt pathway in bone metabolism (15,16). We have previously reported that circulating sclerostin is increased in T2DM and its relationship with bone turnover and bone mass. Moreover, in T2DM sclerostin levels are related to duration of T2DM and HbA1c (17). Notably, sclerostin was highly expressed in calcified aorta tissues from a diabetic murine model (18) and in human aortic samples from three patients with atherosclerosis (19). Recently, besides sclerostin production by osteocytes, in vitro assays under a calcifying environment showed sclerostin expression in VSMCs (20) that were able to undergo phenotypic transition to mineralizing osteoblast-like cells, expressing several osteogenic genes—among them, the protein product of the SOST gene (sclerostin). These findings suggest an additional role for sclerostin on vascular pathology, but at present this fact remains to be evaluated. In this context, our aim was to study the relationship between serum sclerostin and atherosclerotic disease (AD) and vascular calcification in T2DM.
RESEARCH DESIGN AND METHODS
Our cross-sectional study included 78 T2DM patients with diagnosis of diabetes according to American Diabetes Association criteria (2005). From January 2006 to December 2007, we consecutively recruited patients who had been referred to our outpatient clinic from primary care centers for treatment of diabetes. Patients were classified into two groups according to the presence of AD: AD group (n = 44) and non-AD group (n = 31). Inclusion criteria for patients with AD were cerebrovascular disease (ischemic stroke or transient ischemic attack), coronary heart disease (previous myocardial infarction, diagnosed stable or unstable angina, or coronary revascularization surgery), or ischemic peripheral arterial disease. There are some local administrative constraints for referring patients to Endocrinology in our area, and patients with longer diabetes duration and with comorbidities are more likely to be referred than those without.
All were Caucasians and ambulatory, had normal values of serum calcium and phosphorus, and did not have renal, hepatic, gastrointestinal, or thyroid diseases. All patients were on medications for diabetes, including metformin, sulfonylureas, insulin, and a combination of these drugs. None of them had been treated with calcium supplements, vitamin D preparations, hormone therapy, antiresorptive therapy, thiazides, steroids, or other medications that might affect bone metabolism. Patients treated with thiazolidinediones were also excluded.
The study was conducted with the approval of the ethics committee of the San Cecilio University Hospital and conformed to the relevant ethics guidelines for human and animal research. Written informed consent was obtained from all subjects.
Clinical evaluation
Height, weight, and waist circumference were measured at baseline according to standard procedures. Weight was measured to the nearest 100 g using digital electronic scales. Height and waist circumference were measured to the nearest 1 mm using a stadiometer and a metal anthropometric tape, respectively. BMI was calculated as weight divided by the square of height in meters.
Blood pressure was measured in a standardized manner. After subjects remained at rest for at least 5 min, blood pressure was measured twice using a standard mercury sphygmomanometer (12 cm long and 35 cm wide). The mean of the two values was used for analysis. We defined hypertension as values ≥140/90 mmHg and/or antihypertension treatment. Participants reported alcohol use, smoking status, and level of physical activity with a specific health questionnaire. Patients were classified as having a significant alcohol intake if it was >40 g/day in males and 24 g/day in women. Smoking status was categorized as no tobacco use or current tobacco use. Physical activity was collected through a specific questionnaire in which study subjects considered activity levels on a scale from 0 (none) to 10 (sport >1 h four times per week). Based on the results, the study sample was divided into two groups: sedentary (<5 on the scale) and not sedentary (≥5 on the scale).
Biochemical measurements
Biochemical parameters, including fasting plasma glucose, HDL cholesterol, LDL cholesterol, triglycerides, and creatinine, were measured by standard biochemical methods. Dyslipidemia was defined according to Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) criteria or current treatment with statins. HbA1c was determined by high-performance liquid chromatography (ADAMS A1c, HA-8160; Menarini), and homocysteine levels were determined by immunoassay (Abbott, Wiesbaden, Germany).
Serum sclerostin was measured using a commercially available ELISA (Biomedica, Vienna, Austria) according to the manufacturer’s instructions. In our laboratory, we assay duplicates for all values. Precision was tested by determination of intra-assay and interassay variation. Two samples of known concentrations were tested six times for assessment of intra-assay variability, which resulted in 4%. Moreover, two samples of known concentrations were tested in three assays from two different operators to assess interassay variability, which was 3%. Sclerostin measurements are reported in picomoles per liter, and the lower limit of detection was <10 pmol/L.
Carotid intima-media thickness and aortic calcification measurements
Ultrasonographic examination of the carotid arteries was performed with patients in the supine position using Doppler ultrasonography (Toshiba PowerVision 6000). The maximum intima-media thickness (IMT) at the carotid bifurcation (BIF) was determined between the near and far walls of the BIF on the right and left sides. Each part was measured from views of both longitudinal and vertical sections at the BIF. If a discrepancy was observed in the measured values between longitudinal and vertical sections, the smaller value was selected to avoid overestimation. BIF-IMT was defined as the mean of the measurements from the right and left sides. The value for each side was obtained from the mean of 10 wall measurements. The BIF-IMT, measured in millimeters, was considered pathological if it was ≥0.9 mm and was considered carotid atherosclerosis if the BIF-IMT was ≥1.2 mm or 50% greater than the BIF-IMT in the adjacent area (21). Plaques were identified as calcified by findings of bright white echoes on sonography. A single trained sonographer performed the ultrasonographic study in all subjects.
The presence of aortic calcification was evaluated on lateral-view conventional X-rays of the thoracic and lumbar spine (T4–L5). Severity of anterior and posterior aortic calcifications was graded individually on a scale of 0–3 at each lumbar segment, and the results were summarized (22). Patients with calcification in one or more lumbar segments were considered to have aortic calcification. The radiographs were analyzed by two independent investigators who were blinded to each other's readings. In instances of disagreement, M.M.-T. analyzed the radiograph to confirm the diagnosis of aortic calcification.
Statistical analysis
Data were expressed as means ± SD. Data for categorical variables are presented as percentages. Kolmogorov-Smirnov test was used to test the normality of distribution of continuous variables. For continuous variables, mean values between two groups were compared by unpaired Student t test for normally distributed variables and Mann-Whitney U test for skewed variables. The χ2 test was used to compare categorical variables between groups. Pearson (normal distribution) and Spearman correlation analyses (nonnormal distribution) were used to assess the correlations between serum sclerostin and other continuous parameters, and we used partial correlations to correct the possible influence of age on sclerostin values.
Multiple backward model of logistic regression analysis was performed to identify sclerostin as an independent predictor of AD (dependent variable) in T2DM patients. The model included established atherosclerotic risk factors (age, sex, BMI, hypertension, dislipidemia, smoking, sedentarism, HbA1c, creatinin, homeocysteine, and IMT). AD-defining parameters (cerebrovascular disease, coronary heart disease, or ischemic peripheral arterial disease) were not included in the multiple logistic regression model. To determine the independent variables correlated with sclerostin (dependent variable), the parameters that correlate significantly in univariate analysis and others that are biologically linked to sclerostin were tested in multiple backward model linear regression analysis. The usefulness of serum sclerostin as a marker of high risk of AD in T2DM was analyzed using a receiver operating characteristic (ROC) curve. A P value <0.05 was considered significant (two-tailed). Data were recorded and analyzed using SPSS, version 18.0, software (SPSS, Chicago, IL).
RESULTS
Baseline characteristics of the study population
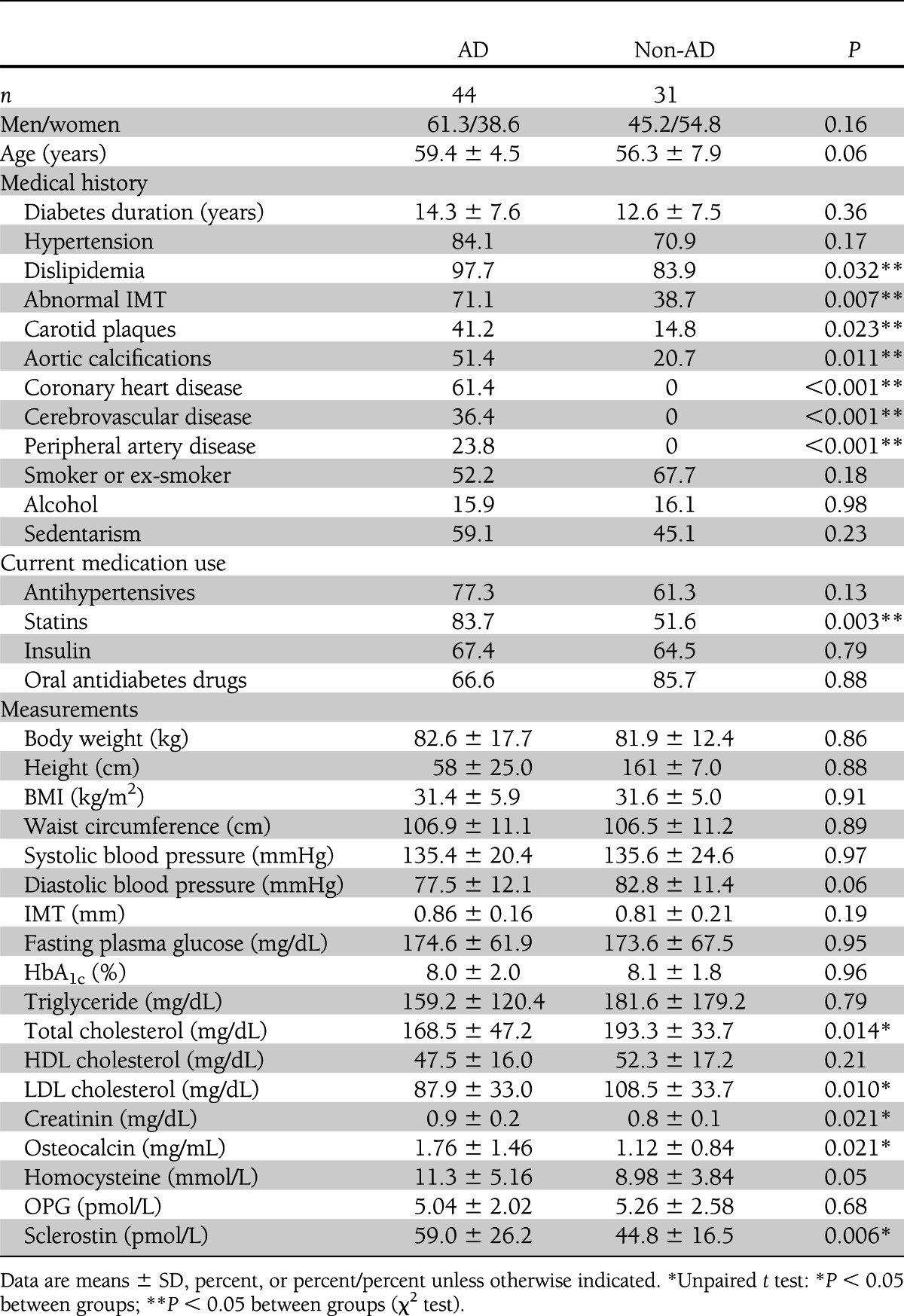
The clinical characteristics of the T2DM patients are summarized in Table 1. Both groups were comparable in clinical, anthropometric, and biochemical parameters except AD-defining parameters and AD surrogate markers.
Table 1.
Anthropometric and biochemical parameters of study subjects according to AD status
Association of serum sclerostin levels in T2DM patients with AD
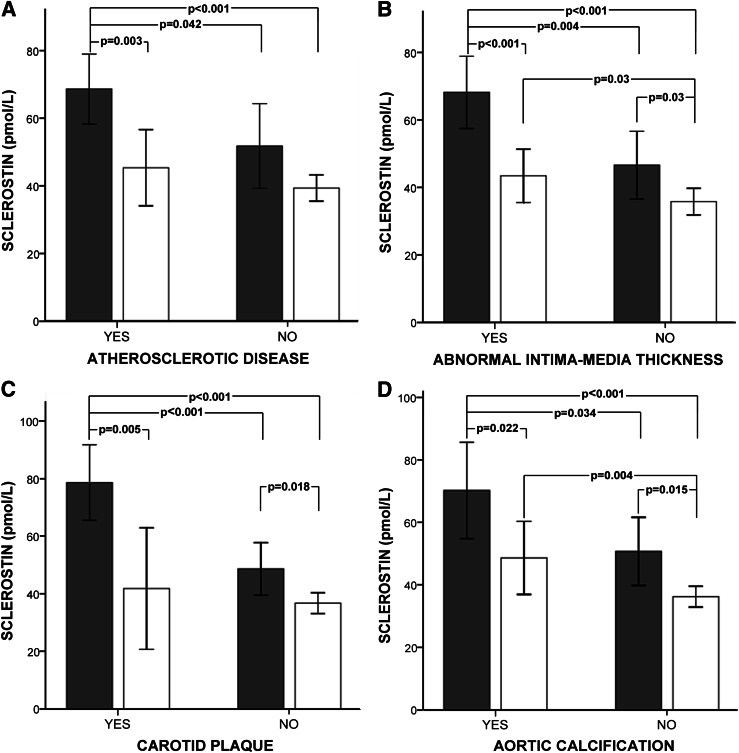
Serum sclerostin concentrations of T2DM patients in the AD group were significantly higher than in the non-AD group (59.0 ± 26.2 vs. 44.8 ± 16.5 pmol/L, P = 0.006) (Table 1). When subjects were further divided according to sex (Fig. 1A), we found that serum sclerostin differences were maintained in AD males compared with non-AD males and females. Furthermore, serum sclerostin levels were higher in males than in females in both groups but only reached significance in the AD group.
Figure 1.
Sclerostin serum levels in female (white bars) and male (gray bars) T2DM patients with and without AD (A), abnormal IMT (B), carotid plaque (C), and aortic calcifications (D). Data are means ± 95% CI. Significant differences between group regions are indicated by a bar with the P value given above.
A model of logistic regression analysis was performed using the presence of AD as a dependent variable. Independent variables were serum sclerostin levels and atherosclerotic risk factors (age, sex, BMI, hypertension, dislipidemia, smoking, sedentarism, HbA1c, creatinin, homocysteine, and IMT). Only serum sclerostin levels were independent predictors of the presence of AD in T2DM (odds ratio 1.040 [95% CI 1.009–1.072]; P = 0.012). Therefore, for each picomole per liter of serum sclerostin level increase, there is a 4% increase risk in AD in T2DM patients.
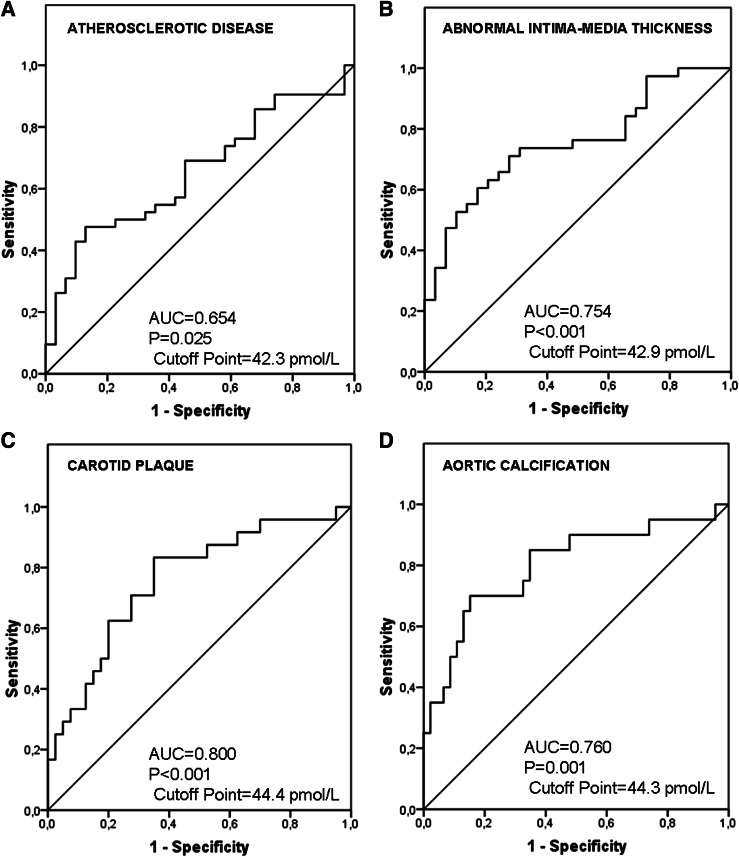
In the ROC curve analysis to evaluate the usefulness of sclerostin as a marker for high risk of AD (Fig. 2A), the area under the curve was 0.654 (P = 0.025). A concentration of ≥42.3 pmol/L showed a sensitivity of 69% and a specificity of 54.8% for identifying an increased risk of AD.
Figure 2.
Sclerostin ROC curve for AD (A), abnormal IMT (B), carotid plaque (C), and aortic calcifications (D). AUC, area under the curve.
Association of serum sclerostin levels in T2DM patients with surrogate markers of AD
In addition, in the entire cohort we analyzed the associations between serum sclerostin levels and surrogate markers of AD, such as abnormal IMT (Fig. 1B), carotid plaque (Fig. 1C), and aortic calcifications (Fig. 1D). Male patients had higher concentrations of serum sclerostin compared with females.
In males, serum sclerostin levels were higher patients with versus without abnormal IMT, carotid plaques, and aortic calcifications. In females, similar results were found, except for a lack of significance in serum sclerostin concentrations in patients with versus without carotid plaques.
The ROC curve analysis to evaluate the usefulness of sclerostin as a marker for high risk showed an area under the curve of 0.754 ± 0.059 (P < 0.001) for abnormal IMT thickness (Fig. 2B), 0.800 ± 0.064 (P < 0.001) for carotid plaque (Fig. 2C), and 0.760 ± 0.062 (P = 0.001) for aortic calcification (Fig. 2D). A concentration of ≥42.9 pmol/L showed a sensitivity of 73.7% and a specificity of 69% to identify an increased risk for abnormal IMT. A concentration of ≥44.4 pmol/L showed a sensitivity of 85% and a specificity of 65.2% to identify an increased risk for carotid plaque, and a concentration of ≥44.3 pmol/L showed a sensitivity of 75% and a specificity of 65% to identify an increased risk for aortic calcification.
Relationship of sclerostin serum levels in T2DM patients with anthropometric and biochemical parameters of AD risk
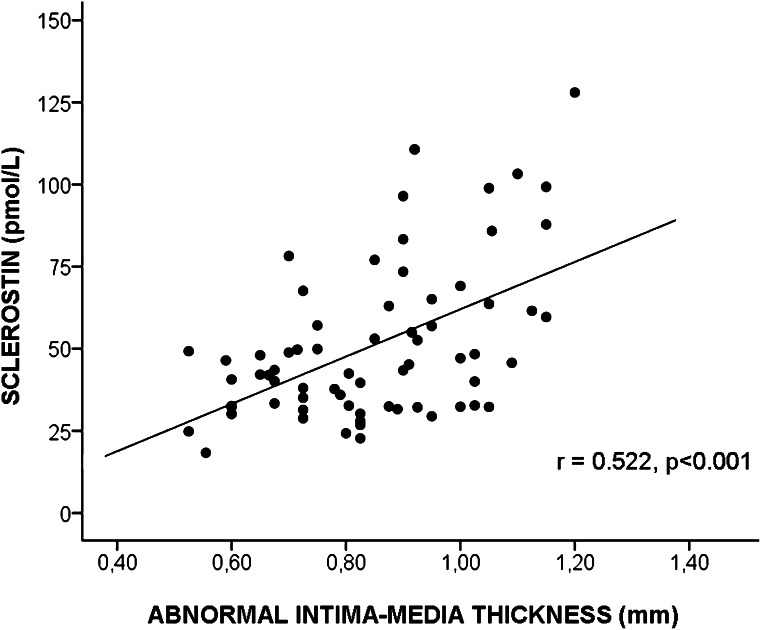
In male T2DM patients, significant positive correlations were observed between serum sclerostin levels and age (r = 0.34, P = 0.03), IMT (r = 0.48, P = 0.002), serum creatinin (r = 0.43, P = 0.006), and homeocysteine (r = 0.33, P = 0.047). After further adjustment for age, only creatinin (r = 0.43, P = 0.02) and homocysteine (r = 0.42, P = 0.02) remained significant. In contrast, we found no relation between sclerostin levels and age in female T2DM patients. However, in this group, sclerostin concentrations were positive related to HbA1c (r = 0.46, P = 0.008) and homocysteine (r = 0.56, P = 0.0.002) and remained significant after adjustment for age. Moreover, in the entire cohort we found a positive correlation between levels of sclerostin and IMT (r = 0.52, P < 0.001) (Fig. 3).
Figure 3.
Statistically significant positive correlation between sclerostin concentration and IMT in the whole T2DM population.
Linear regression analysis was performed to determine the influence of independent factors identified in univariate correlation analysis, including sex, age, HbA1c, creatinin, homocysteine, and IMT as independent variables that explain serum sclerostin levels. The analysis demonstrated that homocysteine (β = 0.319 [95% CI 0.561–2.586], P = 0.003) and IMT (β = 0.330 [14.237–67.693], P = 0.003) were positively associated with serum sclerostin, independently of sex.
CONCLUSIONS
Our cross-sectional study shows for the first time that higher sclerostin levels are associated independently with AD in T2DM patients. Secondly, high concentrations of sclerostin were associated with abnormal IMT, carotid plaques, and aortic calcifications in T2DM males. We found similar differences in T2DM females with abnormal IMT and aortic calcifications. Thirdly, we found a significant positive correlation among sclerostin levels, homocysteine, and IMT in T2DM patients, independently of sex.
The higher levels of sclerostin observed in T2DM patients with AD suggest a specific role of sclerostin in this process, confirming data from preclinical studies. Previous reports on the Wnt signaling antagonist sFRP (11,13) illustrated that its upregulation after injury was involved in healing and homeostasis of vascular tissue. In support of this hypothesis, data with the Wnt signaling agonist Dishevelled (Dvl) (23), which acts as a positive regulator of the Wnt pathway, showed that activation of Wnt signaling was sufficient and critical for the induction of vascular injury. Therefore, sclerostin might also be indicative of a defensive mechanism activated in order to block or to attenuate the canonical Wnt pathway. The overexpression of sclerostin may influence disease progress, leading to restoration of quiescent Wnt signaling observed under health conditions.
We found an increase of serum sclerostin in T2DM patients with abnormal IMT and advanced aortic calcification. Moreover, T2DM males with carotid plaques had increased levels of sclerostin compared with those without carotid plaques. Our finding of a relationship between sclerostin and vascular status might be explained, at least in part, by sclerostin production not only by osteocyte cells from skeleton but also, mainly, by sclerostin upregulation in vascular cells previously transformed to osteocytic phenotype after osteogenic regulation, such as has been shown recently in VSMCs under calcifying conditions (20). Several studies on Wnt inhibitors have shown an increase of its expression in advanced carotid plaques (DKK1 antagonist) and in calcified aortas (SFRP1, -2, and -4), supporting the role of inhibitors in the establishment of a defensive response to reduce the activation of the Wnt pathway and, accordingly, to reduce ossification and avoid further atherosclerotic progression (9,13). By contrast, the positive association between IMT and serum sclerostin seen in our study with T2DM patients was different from the findings of another study in chronic kidney disease (24), which showed a lack of correlation between sclerostin and a surrogate marker of arterial stiffness.
Several groups have previously reported opposed findings concerning the correlation between serum sclerostin concentrations and age (17,25,26). We found positive correlations in serum sclerostin levels with aging in male but not in female T2DM patients. This finding may reflect imbalances in vascular remodeling seen with aging in males in addition to skeletal remodeling. Also, higher sclerostin levels were observed in males compared with females as previously documented (17,25). This sex difference could result from an influence of sex hormones on sclerostin production (27). In particular, large differences were found when sclerostin levels were compared in male versus female T2DM patients with AD or presence of abnormal IMT, carotid plaque, or aortic calcification. Differences can be explained by the scarce association between traditional risk factors for AD in females compared with males (28–31).
The significant positive correlation between sclerostin and creatinin values in male T2DM patients can be explained by a lack or reduction of clearance of the protein sclerostin, which, owing to its molecular weight of 22 KD, would be cleared by the kidney. Thus, we noticed that sclerostin concentrations were positively associated with HbA1c levels in female T2DM patients. Hyperglycemia has effects on vascular complications (32) and also on the formation of advanced oxidation protein products that induce vascular calcification by promoting osteoblastic trans-differentiation of VSMCs (33), which could explain, at least in part, overexpression of sclerostin in T2DM patients. To determine the factors independently associated with sclerostin, we included sex and all significant variables correlating with sclerostin (age, HbA1c, creatinin, homocysteine, and IMT) in a multiple linear regression analysis. We found that homocysteine and IMT were factors independently associated with sclerostin levels. High plasma homocysteine level is an independent risk factor for the development of atherosclerosis, cardiovascular events, and stroke (34). Thus far, there is only one report showing the relationship between sclerostin metabolism and homocysteine in postmenopausal women (35). On the other hand, carotid IMT is a strong predictor of vascular events (36). To our knowledge, this is the first report that reveals a relationship between serum sclerostin levels and IMT. Future studies are required to uncover the relationship among sclerostin, homocysteine metabolism, and AD.
Our cross-sectional study has some limitations. First, the cross-sectional design does not allow establishment of a cause-effect relationship. Second, the sample size is relatively small and might affect the statistical power. However, we believe that our findings are consistent. Moreover, pharmacologic treatment of patients with vascular disorders may have influenced the results. Strengths of our study are the evaluation of circulating serum sclerostin in patients with T2DM and AD for the first time and the exhaustive evaluation of biochemical and clinical parameters of atherosclerotic risk.
In summary, our observation that sclerostin circulates in a significant amount in T2DM patients with atherosclerotic lesions may support the hypothesis that sclerostin action is not only on the regulation of bone formation. Although the sample size of our study is small, we suggest that sclerostin circulating levels are a major modulator of Wnt signaling in AD, and they are implicated in the vascular integrity in T2DM. Nevertheless, it remains to be established whether higher levels of sclerostin have a protective role in the survival of patients with AD. The usefulness of sclerostin as a serum marker of atherosclerotic risk and vascular lesions in T2DM patients merits further prospective studies.
Acknowledgments
This work was supported by grants from Fondo de Investigación Sanitaria (Instituto de Salud Carlos III [PI081302]) and Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RD06/0013/1014).
No potential conflicts of interest relevant to this article were reported.
S.M.-S. wrote the manuscript and researched data. B.G.-F. researched data and contributed to discussion. A.G.-M. reviewed and edited the manuscript. P.R.-M. and J.A.G.-S. researched data. R.R.-G. reviewed and edited the manuscript. J.A.G.-S. and M.M.-T. contributed to discussion and reviewed and edited the manuscript. M.M.-T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes—part I: pathways of vascular disease in diabetes. Vascul Pharmacol 2011;54:68–74 [DOI] [PubMed] [Google Scholar]
- 2.Couffinhal T, Dufourcq P, Duplàa C. Beta-catenin nuclear activation: common pathway between Wnt and growth factor signaling in vascular smooth muscle cell proliferation? Circ Res 2006;99:1287–1289 [DOI] [PubMed] [Google Scholar]
- 3.Tsaousi A, Williams H, Lyon CA, et al. Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res 2011;108:427–436 [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the β-catenin/T-cell factor signaling cascade in vascular remodeling. Circ Res 2002;90:340–347 [DOI] [PubMed] [Google Scholar]
- 5.Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007;315:1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chafey P, Finzi L, Boisgard R, et al. Proteomic analysis of β-catenin activation in mouse liver by DIGE analysis identifies glucose metabolism as a new target of the Wnt pathway. Proteomics 2009;9:3889–3900 [DOI] [PubMed] [Google Scholar]
- 7.Jin T. The WNT signalling pathway and diabetes mellitus. Diabetologia 2008;51:1771–1780 [DOI] [PubMed] [Google Scholar]
- 8.Manolagas SC, Almeida M. Gone with the Wnts: β-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol 2007;21:2605–2614 [DOI] [PubMed] [Google Scholar]
- 9.Ueland T, Otterdal K, Lekva T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1228–1234 [DOI] [PubMed] [Google Scholar]
- 10.Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 2010;106:1798–1806 [DOI] [PubMed] [Google Scholar]
- 11.Barandon L, Couffinhal T, Ezan J, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 2003;108:2282–2289 [DOI] [PubMed] [Google Scholar]
- 12.Mastroiacovo F, Busceti CL, Biagioni F, et al. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab 2009;29:264–276 [DOI] [PubMed] [Google Scholar]
- 13.Román-García P, Carrillo-López N, Fernández-Martín JL, Naves-Díaz M, Ruiz-Torres MP, Cannata-Andía JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 2010;46:121–128 [DOI] [PubMed] [Google Scholar]
- 14.Goliasch G, Wiesbauer F, Kastl SP, et al. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012;222:251–256 [DOI] [PubMed] [Google Scholar]
- 15.Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 2005;19:1842–1844 [DOI] [PubMed] [Google Scholar]
- 16.van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 2005;16:319–327 [DOI] [PubMed] [Google Scholar]
- 17.García-Martín A, Rozas-Moreno P, Reyes-García R, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:234–241 [DOI] [PubMed] [Google Scholar]
- 18.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 2005;115:1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics 2010;9:2048–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu D, Mackenzie NC, Millán JL, Farquharson C, MacRae VE. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 2011;6:e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junyent M, Gilabert R, Núñez I, et al. [Carotid ultrasound in the assessment of preclinical atherosclerosis. Distribution of intima-media thickness values and plaque frequency in a Spanish community cohort] Med Clin (Barc) 2005;125:770–774 [DOI] [PubMed] [Google Scholar]
- 22.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997;132:245–250 [DOI] [PubMed] [Google Scholar]
- 23.Malekar P, Hagenmueller M, Anyanwu A, et al. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension 2010;55:939–945 [DOI] [PubMed] [Google Scholar]
- 24.Thambiah S, Roplekar R, Manghat P, et al. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 2012;90:473–480 [DOI] [PubMed] [Google Scholar]
- 25.Mödder UI, Hoey KA, Amin S, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 2011;26:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cejka D, Jäger-Lansky A, Kieweg H, et al. Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 2012;27:226–230 [DOI] [PubMed] [Google Scholar]
- 27.Mödder UI, Clowes JA, Hoey K, et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 2011;26:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997;337:1360–1369 [DOI] [PubMed] [Google Scholar]
- 29.Manolio TA, Pearson TA, Wenger NK, Barrett-Connor E, Payne GH, Harlan WR. Cholesterol and heart disease in older persons and women. Review of an NHLBI workshop. Ann Epidemiol 1992;2:161–176 [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328:1450–1456 [DOI] [PubMed] [Google Scholar]
- 31.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993;328:1444–1449 [DOI] [PubMed] [Google Scholar]
- 32.Cefalu WT. Glycemic control and cardiovascular disease—should we reassess clinical goals? N Engl J Med 2005;353:2707–2709 [DOI] [PubMed] [Google Scholar]
- 33.You H, Yang H, Zhu Q, et al. Advanced oxidation protein products induce vascular calcification by promoting osteoblastic trans-differentiation of smooth muscle cells via oxidative stress and ERK pathway. Ren Fail 2009;31:313–319 [DOI] [PubMed] [Google Scholar]
- 34.Petramala L, Acca M, Francucci CM, D'Erasmo E. Hyperhomocysteinemia: a biochemical link between bone and cardiovascular system diseases? J Endocrinol Invest 2009;32(Suppl. 4):10–14 [PubMed] [Google Scholar]
- 35.Urano T, Shiraki M, Ouchi Y, Inoue S. Association of circulating sclerostin levels with fat mass and metabolic disease–related markers in Japanese postmenopausal women. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1218. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–467 [DOI] [PubMed] [Google Scholar]