Abstract
OBJECTIVE
To evaluate the effect of removal of the duodenum on the complex interplay between incretins, insulin, and glucagon in nondiabetic subjects.
RESEARCH DESIGN AND METHODS
For evaluation of hormonal secretion and insulin sensitivity, 10 overweight patients without type 2 diabetes (age 61 ± 19.3 years and BMI 27.9 ± 5.3 kg/m2) underwent a mixed-meal test and a hyperinsulinemic-euglycemic clamp before and after pylorus-preserving pancreatoduodenectomy for ampulloma.
RESULTS
All patients experienced a reduction in insulin (P = 0.002), C-peptide (P = 0.0002), and gastric inhibitory peptide (GIP) secretion (P = 0.0004), while both fasting and postprandial glucose levels increased (P = 0.0001); GLP-1 and glucagon responses to the mixed meal increased significantly after surgery (P = 0.02 and 0.031). While changes in GIP levels did not correlate with insulin, glucagon, and glucose levels, the increase in GLP-1 secretion was inversely related to the postsurgery decrease in insulin secretion (R2 = 0.56; P = 0.012) but not to the increased glucagon secretion, which correlated inversely with the reduction of insulin (R2 = 0.46; P = 0.03) and C-peptide (R2 = 0.37; P = 0.04). Given that the remaining pancreas presumably has preserved intraislet anatomy, insulin secretory capacity, and α- and β-cell interplay, our data suggest that the increased glucagon secretion is related to decreased systemic insulin.
CONCLUSIONS
Pylorus-preserving pancreatoduodenectomy was associated with a decrease in GIP and a remarkable increase in GLP-1 levels, which was not translated into increased insulin secretion. Rather, the hypoinsulinemia may have caused an increase in glucagon secretion.
Bariatric surgery has recently been suggested as a new treatment for type 2 diabetes. After gastric bypass, glycemic improvements in type 2 diabetes occur after few days—long before weight loss. This suggests that mechanisms related to the reconstruction of the gastrointestinal tract and the secretion of gastrointestinal hormones may be responsible for the antidiabetes effect (1). According to Rubino and Marescaux (2), exclusion of the duodenum provides the key explanation: because of the exclusion, an “anti-incretin factor” is no longer secreted (“the upper gut hypothesis”). Other studies have supported the concept that early exposure of the distal ileum to undigested nutrients (“lower gut hypothesis”) with a subsequent release of antidiabetes hormones gives rise to this phenomenon (1,3–7). Nevertheless, these studies do not rule out that exclusion of the duodenum may contribute to the antidiabetes effects of gastric bypass surgery. In the current study, we examined the effect of duodenum removal in patients undergoing the duodenum pancreatectomy for reasons different from obesity and diabetes. This surgical procedure is associated with a reduction in β-cell mass, which leads to decreasing systemic insulin levels. Since hemipancreatectomy, while lowering insulin levels, is not expected to modify the intraislet relationship between α- and β-cells, we also looked at the relationship between postsurgical insulin and glucagon levels.
RESEARCH DESIGN AND METHODS
A total of 10 patients (6 male and 4 female) undergoing pylorus-preserving pancreatoduodenectomy with curative intent at the Hepato-biliary Surgery Unit, Department of Surgery, Agostino Gemelli University Hospital, Rome, Italy, were consecutively enrolled. Indication for surgery was tumor of ampulla of Vater. Pancreatoduodenectomy was carried out according to the pylorus-preserving technique (8). Briefly, the pancreatic head, duodenum, common bile duct, and gallbladder were removed en bloc, leaving a functioning pylorus at the gastric outlet intact. All adjacent lymph nodes were carefully removed. The continuity of the gastrointestinal tract was restored by an end-to-side invaginated pancreaticojejunostomy. Further downstream, an end-to-side hepaticojejunostomy and side-to side gastroenterostomy or an end-to-side pylorus jejunostomy was made. The pancreas volume removed during the surgery is ~50%, as previously reported by Schrader et al. (9). Figure 1 gives a schematic presentation of the surgical procedure. Only patients with normal cardiopulmonary and kidney functions, as determined by medical history, physical examination, electrocardiography, and urinalysis; without known diabetes; and free of any antidiabetes medication were enrolled for the studies. Patients were studied 1 week before and after a variable period of recovery from the surgical procedure (a sufficient recovery period was judged on normalization of inflammatory parameters such as C-reactive protein and erythrosedimentation rate, stability of weight, and normal diet without any clear symptoms of abnormal intestinal motility or exocrine pancreatic deficiency). The study protocol was approved by the local ethics committee.
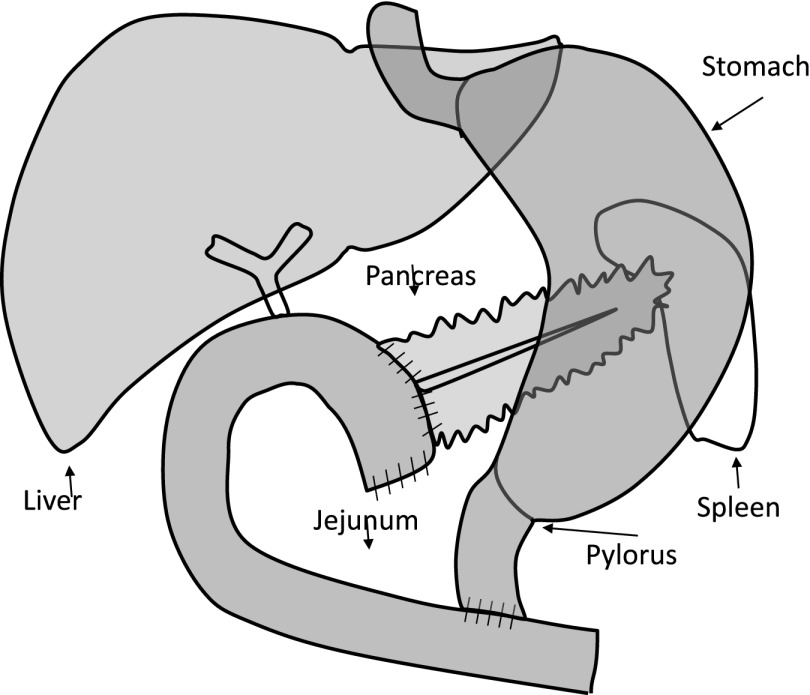
Figure 1.
Surgical and reconstruction procedure: the pancreatic head, the duodenum, the common bile duct, and the gallbladder were removed en bloc, leaving the functioning pylorus at the gastric outlet intact. The continuity of the gastrointestinal apparatus was restored by an end-to-side invaginated pancreaticojejunostomy. Further downstream, an end-to-side hepaticojejunostomy and side-to side gastroenterostomy or an end-to-side pylorus jejunostomy was made. This figure is designed to simplify the understanding of the anatomical changes on which our model is based.
Anthropometric parameters were determined according to standard procedures (10). BMI was calculated as weight in kilograms divided by the square of height in meters. All patients had blood samples taken for serum lipid assays (total, HDL, and LDL cholesterol). Blood samples were taken in the morning after an 8-h overnight fast. All the procedures were performed with subjects in a supine position throughout the experiments. The hyperinsulinemic-euglycemic clamp test was performed after a 12-h overnight fast using insulin (40 mU/min/m2 body surface) according to the methodology of DeFronzo et al. (11). On the day after, all patients underwent a mixed-meal challenge test using a liquid meal of 830 kcal (107 kcal from protein, 353 kcal from fat, and 360 kcal from carbohydrates) consumed within 15 min. Blood samples were drawn twice in the fasting state and at 30-min intervals over 240 min (time 0′, 30′, 60′, 90′, 120′, 150′, 180′, 210′, and 240′) afterward for the measurement of plasma glucose, insulin, C-peptide, glucagon, glucagon-like peptide-1 (GLP-1), and gastric inhibitory peptide (GIP) concentrations. Blood for glucagon, total GLP-1, and intact GIP was sampled in tubes containing EDTA and a dipeptidyl peptidase-4 inhibitor (Millipore); after centrifugation (1,000 rpm for 10 min at 4°C), they were stored at −80°C until analyzed. Insulin levels were determined using a commercial RIA kit (Medical System; Immulite DPC, Los Angeles, CA). Plasma glucose concentrations were determined by the glucose oxidase technique, using a glucose analyzer (Beckman Instruments, Palo Alto, CA). Plasma C-peptide was measured by autoDELPHIA automatic fluoroimmunoassay (Wallac, Turku, Finland), with a detection limit of 17 pmol/L. Immunoreactive glucagon was measured in ethanol-extracted plasma by RIA using antibody code no. 4305 directed against the COOH terminus of glucagon and reacting specifically with pancreatic glucagon (12,13). Total GLP-1 concentrations were measured using antiserum no. 89390, reacting equally with intact GLP-1(7-36)amide and its primary NH2-terminally truncated metabolite GLP-1(9-36) amide (14). Intact GIP was measured using antiserum no. 98171, reacting with the NH2-terminal of GIP but not with the metabolite, GIP 3-42 (15).
Statistical analysis
Statistical analysis was carried out using two-way ANOVA or Student t test, as appropriate, and regression analyses using SPSS version 9 (SPSS, Chicago, IL). The changes in variables during the mixed-meal test were assessed by two-way ANOVA for repeated measures. A post hoc analysis with a two-tailed paired t test was used to assess differences at individual time periods in the study, with Bonferroni correction used for multiple comparisons. A P value of <0.05 was taken to indicate significant differences.
RESULTS
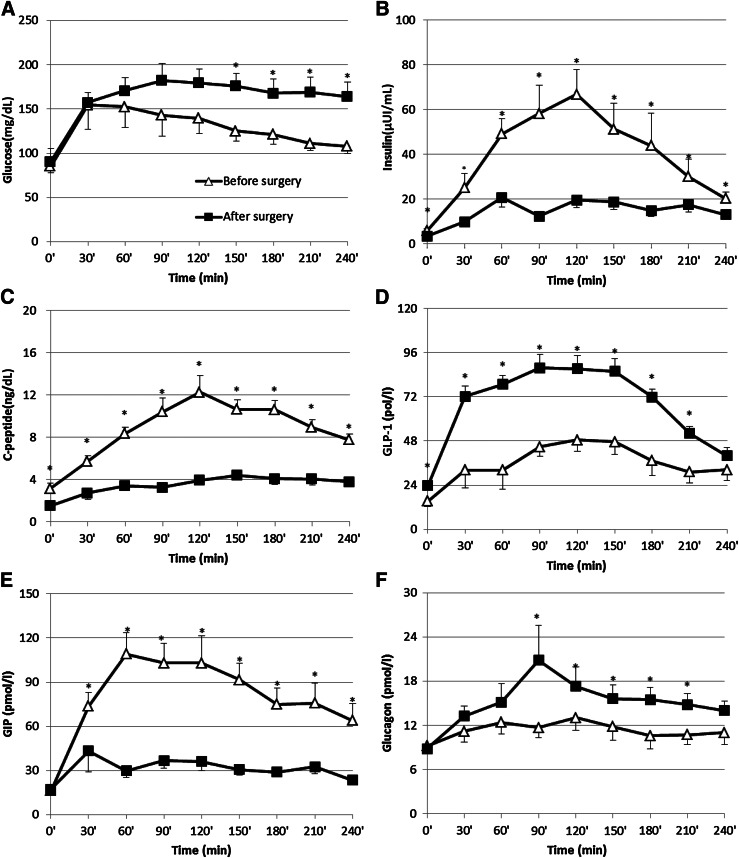
The mean ± SD age of patients, six male and four female, was 61 ± 19.3 years (median interval 66; range 22–82), and BMI was 27.9 ± 5.3 kg/m2 before and 26.8 ± 5.0 kg/m2 after surgery. Table 1 reports metabolic characteristics and mean values of all the measured parameters during the mixed-meal test. Fasting and postchallenge glucose excursion increased after surgery (P = 0.0001) (Fig. 2A), and there was a significant reduction of postprandial insulin levels (P = 0.002) (Fig. 2B) accompanied by a parallel reduction of C-peptide (P < 0.001) (Fig. 2C). In comparison with the preoperative study, the meal ingestion elicited a markedly greater increase in GLP-1 concentrations (P = 0.02) (Fig. 2D) along with a decrease in GIP responses (P < 0.01) (Fig. 2E). We also explored whether the changes in GLP-1, insulin, and glucagon secretion were related after surgery. Interestingly, the greater the increase in the GLP-1 secretion after surgery, the smaller the reduction in insulin secretion (R2 = 0.56; P = 0.012) and C-peptide (R2 = 0.30; P = 0.04), calculated as percentage of reduction of area under the curve (AUC). While no difference was found in fasting glucagon levels, after surgery glucagon concentrations were higher, particularly 90 min after meal ingestion (P = 0.031) (Fig. 2). The increase correlated with the reduction in insulin as well as C-peptide responses to the meal (individual differences between the preoperative and postoperative AUCs) (R2 = 0.46, P = 0.03, and R2 = 0.37, P = 0.04, respectively); GLP-1 and glucose AUCs did not correlate with insulin or C-peptide AUCs. GIP AUCs were not related to glucose, insulin, C-peptide, or glucose levels. No change in insulin sensitivity was found after surgery (glucose uptake changing from 4.27 ± 1.32 to 3.97 ± 1.04 mg · kg−1 · min−1; P = 0.44).
Table 1.
Patients’ anthropometric and metabolic characteristics
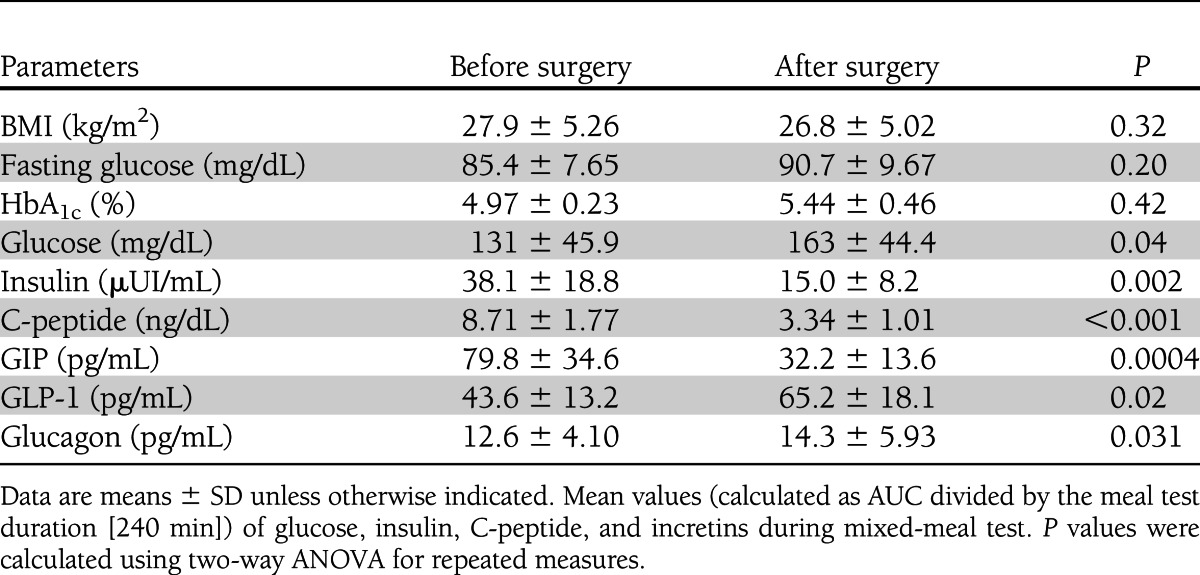
Figure 2.
Plasma concentrations of glucose (A), insulin (B), C-peptide (C), GLP-1 (D), glucagon (E), and GIP (F) in patients examined before and after surgery. At t = 0 min, an oral mixed meal was ingested. Data are presented as means ± SEM. P values were calculated using repeated measures by ANOVA. Significant difference (P < 0.05) at individual time points (Bonferroni post hoc test). *Significant (P ≤ 0.05) differences at individual time points. Increased glucose levels (A) are consequent to decreased insulin and C-peptide levels after surgery (B and C). Incretin adaptation (D and F) to removal of duodenum and consequent reduction of GIP (E) are shown. The incretins showed an opposite trend after the surgery, i.e., a major reduction of GIP secretion and increase of GLP-1 release. The rise in glucagon concentration after the removal of duodenum reached the statistical significance after the first hour of the mixed-meal test.
CONCLUSIONS
As expected because of the pancreas resection, insulin secretion in our patients decreased postoperatively, and this was associated with prolonged postprandial hyperglycemia. Another predictable consequence was a marked decrease in GIP secretion—presumably a direct consequence of the duodenectomy and the shunting of chyme to a site more distally in the jejunum. The GIP-producing K cells are the so-called open type, being able to react to luminal nutrients via their cytoplasmic processes, which are equipped with microvilli protruding into the lumen of the proximal gut (16); therefore, duodenectomy and bypass of the most proximal small intestine probably explain the markedly decreased GIP responses. It is more difficult to explain the increased secretion of GLP-1, which reached levels comparable with those observed after gastric bypass surgery (17). After bypass surgery, the GLP-1 increase is usually explained by delivery of nutrients to regions of the gut where the density of the GLP-1–producing L cells is higher than in the proximal small intestine (18). Since the L cells seem to respond to digested nutrients (monosaccharides, free fatty acids, and amino acids) rather than undigested nutrients (19), the region of origin of the exaggerated secretion is probably the so-called common limb, i.e., the small intestinal segment distal to the entry of the secretory limb carrying the digestive juices. In addition, since there is no retardation of ingested food in the gastric pouch, the passage of nutrients into the distal intestine is very rapid, and this is thought to contribute to the exaggerated secretion of GLP-1 (18,20).
In our patients, the reconstruction of continuity in the gastrointestinal tract after duodenectomy also involves a Roux-en-Y maneuver, but the site of the gastroduodeno-jejunal anastomosis is much more proximal than the entry of the secretory limb after gastric bypass; furthermore, because of the preservation of the stomach plus the pylorus in our patients there is little or no accelerated gastric emptying. In fact, decelerated emptying has been reported to result from this operation (21,22). In support of this notion, our postprandial glucose levels showed no signs of accelerated gastric emptying, with peak levels actually being reached later after compared with before the operation. Our results are more reminiscent of those obtained after insertion of an endoluminal sleeve (the “endobarrier”), which has a length of only 60 cm and causes increases in GLP-1 secretion and decreases in GIP secretion similar to these observed here (23,24). Interestingly, insertion of an endobarrier is associated with both antidiabetes effects and weight loss. This raises the possibility that bypassing the duodenum/proximal jejunum has beneficial metabolic effects; these may be related to the increasing secretion of GLP-1 but could also lack secretion of a duodenal antidiabetes factor as suggested by Rubino and coworkers (25). The nature of this duodenal factor is unknown, and although GIP has been reported to be able to elicit a neuroendocrine loop resulting in stimulation of GLP-1 secretion in rats (26), a similar mechanism seems unlikely in our patients because of the markedly lowered GIP responses. Recent research has indicated that part of the early GLP-1 response to meal ingestion may derive not from classical ileal or colonic L cells but, rather, from proximal duodenal GLP-1–producing cells, some of which may also express GIP and cholecystokinin (27,28). This is in contrast to the duodenal GIP-expressing cells, which, as a rule, do not express GLP-1. A transfer of nutrients from the duodenum and 60 cm down the small intestine might possibly explain the exaggerated GLP-1 response. At any rate, this response was not associated with concomitant GIP secretion. It may also be noted that the exaggerated GLP-1 response occurred in the face of a decreased GIP response, indicating that under these conditions a mechanism for GLP-1 secretion involving duodenal GIP (or cholecystokinin) is unlikely to be of importance.
Contrasted with gastric bypass operations or insertion of an endobarrier, the partial pancreatectomy in the present operation was associated with impairment of glucose tolerance (although fasting glucose concentrations were not affected). Insulin resistance was also unaltered, so the glucose intolerance is probably best explained by the reduction in insulin secretory capacity, brought about by resection of the head of the pancreas. The secretion of the other pancreatic glucoregulatory hormone, glucagon, did not show a similar reduction, however, but showed a significant elevation after the operation. The so-called intraislet hypothesis for glucagon secretion assumes that glucagon secretion is regulated by intraislet levels of insulin in a paracrine manner (29,30). Thus, if insulin secretion falls, glucagon secretion is thought to increase, as it is observed in diabetes with absolute or relative β-cell insufficiency (31). However, in our case the intraislet hypothesis does not seem to be able to explain the hyperglucagonemia, since the intraislet relationship between insulin and glucagon would not be expected to change after hemipancreatectomy. However, systemic insulin levels may also influence glucagon secretion, and it remains possible that the subnormal insulin responses observed here may have contributed to the exaggerated glucagon responses observed. Similar arguments were recently presented by Schrader et al. (9) based on studies of glucagon secretion after hemipancreatectomy. In the present studies involving duodenectomy as well, we also have increased GLP-1 levels. Exogenous GLP-1 is a powerful inhibitor of glucagon secretion (32), and blocking of the actions of endogenous GLP-1 is associated with increasing glucagon levels (32,33). It is therefore surprising in our study that glucagon levels increase markedly in the face of elevated GLP-1 levels. However, elevations may be seen after gastric bypass in spite of similarly elevated GLP-1 levels (17), suggesting that similar mechanisms are being activated. One possibility might be secretion of glucagon from the proximal gut. The pro–glucagon-producing L cells of the gut are capable of secreting fully processed glucagon, as demonstrated in patients after total pancreatectomy (34), and given that the stimulus intensity is increased in our patients as well as after gastric bypass, the possibility that the gut is actually the source of the increased glucagon levels cannot be excluded.
In morbidly obese patients with type 2 diabetes, gastric bypass may often cause complete resolution of diabetes, presumably secondary to greatly exaggerated GLP-1 secretion, which is capable of enhancing β-cell function sufficiently to normalize glucose levels (35,36). In support of this notion, GLP-1 secretion was tightly related to insulin secretion (R2 = 0.56; P = 0.012). In our patients, there was postoperative glucose intolerance and abnormal, low insulin responses, but nevertheless insulin and GLP-1 responses were correlated, suggesting that without GLP-1 secretion, insulin secretion and therefore diabetes might have been even more impaired.
Our study has several limitations that should be noted. First, this study was performed in patients undergoing both hemipancreatectomy and removal of duodenum; this prevented a “pure” evaluation of the role of duodenum. Second, the patients were affected by cancer. However, we selected patients affected by ampulloma, which is a low-grade tumor malignancy, with good prognosis (37,38), and usually does not affect glucose metabolism. Third, the postsurgery evaluation was carried out at varying intervals after surgery (30–50 days to allow for different rates of full recovery after surgery). This variability could have some influence on the results. Although well characterized by mixed meal and clamp, the limited number of patients may represent a limitation.
In conclusion, the current study has shown that pylorus-preserving pancreatoduodenectomy was associated with impaired glucose tolerance, presumably related to decreasing insulin secretory capacity. Decreased GIP levels may also be involved, whereas greatly enhanced GLP-1 responses did not translate into increased insulin secretion. In spite of the resection, glucagon levels were increased in spite of elevated GLP-1 levels. The glucagon increases were related to decreases in insulin secretion, perhaps suggesting that systemic hypoinsulinemia might be involved. An endocrine suppressive factor, normally released from the duodenum, might also be involved.
Acknowledgments
This study was supported by grants to A.G. from Università Cattolica del Sacro Cuore (Fondi Ateneo Linea D.3.2 Sindrome Metabolica) and from Fondazione Don Gnocchi. G.M. is the recipient of the Umberto Di Mario Prize by the Società Italiana di Diabetologia 2011.
No potential conflicts of interest relevant to this article were reported.
G.M. and G.P.S. wrote the manuscript. T.M. generated the data. A.Pr. contributed to discussion and reviewed and edited the manuscript. G.C., G.S., and G.N. researched data. A.Po. reviewed and edited the manuscript. J.J.H. generated data and reviewed and edited the manuscript. A.G. reviewed and edited the manuscript. G.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Umeda LM, Silva EA, Carneiro G, Arasaki CH, Geloneze B, Zanella MT. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg 2011;21:896–901 [DOI] [PubMed] [Google Scholar]
- 2.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004;239:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans S, Pamuklar Z, Rosko J, et al. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc 2012;26:1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Näslund E, Grybäck P, Hellström PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 1997;21:387–392 [DOI] [PubMed] [Google Scholar]
- 5.Van der Schueren BJ, Homel P, Alam M, et al. Magnitude and variability of the glucagon-like peptide-1 response in patients with type 2 diabetes up to 2 years following gastric bypass surgery. Diabetes Care 2012;35:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patriti A, Facchiano E, Annetti C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 2005;15:1258–1264 [DOI] [PubMed] [Google Scholar]
- 7.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005;288:E447–E453 [DOI] [PubMed] [Google Scholar]
- 8.Traverso LW, Longmire WP., Jr Preservation of the pylorus in pancreaticoduodenectomy a follow-up evaluation. Ann Surg 1980;192:306–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrader H, Menge BA, Breuer TG, et al. Impaired glucose-induced glucagon suppression after partial pancreatectomy. J Clin Endocrinol Metab 2009;94:2857–2863 [DOI] [PubMed] [Google Scholar]
- 10.Lohman TG, Roche AF, Martorelli R. Anthropometric Standardization Reference Manual Champaign, IL, Human Kinetics Books, 1988, p. 177 [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003;46:798–801 [DOI] [PubMed] [Google Scholar]
- 13.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 1991;87:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994;43:535–539 [DOI] [PubMed] [Google Scholar]
- 15.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000;85:3575–3581 [DOI] [PubMed] [Google Scholar]
- 16.Holst JJ. On the physiology of GIP and GLP-1. Horm Metab Res 2004;36:747–754 [DOI] [PubMed] [Google Scholar]
- 17.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst JJ. Postprandial insulin secretion after gastric bypass surgery: the role of glucagon-like peptide 1. Diabetes 2011;60:2203–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 2010;12:e1. [DOI] [PubMed] [Google Scholar]
- 20.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 2011;96:2227–2235 [DOI] [PubMed] [Google Scholar]
- 21.Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet 1985;160:1–4 [PubMed] [Google Scholar]
- 22.Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg 1986;204:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdam FJ, Liedorp PR, Geubbels N, et al. EndoBarrier for counteracting obesity and metabolic syndrome. Ned Tijdschr Geneeskd 2012;156:A3844 [in Dutch] [PubMed] [Google Scholar]
- 24.De Jonge C, Verdam FJ, Rensen SS, et al. EndobarrierTM duodenal-jejunal bypass liner rapily improves diabetes parameters paralleled by increased postprandial GLP-1 and PYY levels in obese type 2 diabetic patients. Diabetologia 2011;54(Suppl. 1):S84 [Google Scholar]
- 25.Rubino F, Kaplan LM, Schauer PR, Cummings DE, Diabetes Surgery Summit Delegates The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399–405 [DOI] [PubMed] [Google Scholar]
- 26.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999;140:1687–1694 [DOI] [PubMed] [Google Scholar]
- 27.Mortensen K, Petersen LL, Ørskov C. Colocalization of GLP-1 and GIP in human and porcine intestine. Ann N Y Acad Sci 2000;921:469–472 [DOI] [PubMed] [Google Scholar]
- 28.Habib AM, Richards P, Cairns LS, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012;153:3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 30.Diao J, Asghar Z, Chan CB, Wheeler MB. Glucose-regulated glucagon secretion requires insulin receptor expression in pancreatic alpha-cells. J Biol Chem 2005;280:33487–33496 [DOI] [PubMed] [Google Scholar]
- 31.Kielgast U, Asmar M, Madsbad S, Holst JJ. Effect of glucagon-like peptide-1 on alpha- and beta-cell function in C-peptide-negative type 1 diabetic patients. J Clin Endocrinol Metab 2010;95:2492–2496 [DOI] [PubMed] [Google Scholar]
- 32.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes 2011;60:1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 1999;48:86–93 [DOI] [PubMed] [Google Scholar]
- 34.Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia 1983;25:396–399 [DOI] [PubMed] [Google Scholar]
- 35.Salehi M, Aulinger B, Prigeon RL, D’Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 2010;59:1330–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 2012;303:E122–E131 [DOI] [PubMed] [Google Scholar]
- 37.Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol 2012;105:266–272 [DOI] [PubMed] [Google Scholar]
- 38.Choi SB, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg 2011;100:92–98 [DOI] [PubMed] [Google Scholar]