Abstract
OBJECTIVE
The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) trial demonstrated that combination therapy with metformin plus rosiglitazone provided superior durability of glycemic control compared with metformin alone, with significantly lower treatment failure rates (38.6 vs. 51.7%), and metformin plus lifestyle was intermediate. Herein we describe the temporal changes in measures of β-cell function and insulin sensitivity over a 4-year period among the three treatments.
RESEARCH DESIGN AND METHODS
TODAY participants (699) were tested periodically with an oral glucose tolerance test to determine insulin sensitivity (1/fasting insulin [1/IF]), insulinogenic index (△I30/△G30) or C-peptide index (△C30/△G30), and β-cell function relative to insulin sensitivity (oral disposition index [oDI]).
RESULTS
During the first 6 months, metformin plus rosiglitazone exhibited a significantly greater improvement in insulin sensitivity and oDI versus metformin alone and versus metformin plus lifestyle; these improvements were sustained over 48 months of TODAY. Irrespective of treatment, those who failed to maintain glycemic control had significantly lower β-cell function (∼50%), higher fasting glucose concentration, and higher HbA1c at randomization compared with those who did not fail.
CONCLUSIONS
The beneficial change in insulin sensitivity and the resultant lower burden on β-cell function achieved in the first 6 months with metformin plus rosiglitazone appear to be responsible for its superior glycemic durability over metformin alone and metformin plus lifestyle. However, initial β-cell reserve and HbA1c at randomization are independent predictors of glycemic durability. Therefore, efforts to preserve β-cell function before significant loss occurs and to reduce HbA1c may be beneficial in the treatment of youth with type 2 diabetes.
Despite the escalating rates of obesity-driven type 2 diabetes in youth, therapeutic options remain limited to metformin, the only FDA-approved oral hypoglycemic agent for children, and insulin when the former fails (1). Even though metformin was effective in the short-term over 16 weeks (2), it remained unknown whether this effect was durable until the results of the TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) trial showed >50% failure rates on metformin over an average follow-up of 3.86 years (3). TODAY was a multicenter, randomized clinical trial that compared metformin monotherapy (M) with metformin plus rosiglitazone (M+R) or metformin plus intensive lifestyle intervention (M+L) on time to treatment failure, i.e., loss of glycemic control defined as either HbA1c ≥8% over a 6-month period or inability to wean from temporary insulin therapy within 3 months of acute metabolic decompensation (3,4). The results revealed that the combination of M+R was superior to M in sustaining durable glycemic control, and M+L was intermediate (3).
Similar to adults, the pathophysiology of type 2 diabetes in youth involves peripheral and hepatic insulin resistance, together with impaired β-cell function, which progressively worsens over time (5–9). The deterioration in β-cell function in youth appears to be accelerated compared with that observed in adults (10–14). Cross-sectional observations, including the TODAY study, show an inverse relationship between HbA1c and β-cell function but not insulin sensitivity, suggesting that residual β-cell function relative to insulin sensitivity is a determinant of glycemic control in youth with type 2 diabetes (5,15). Based on the TODAY outcome of better glycemic durability with M+R, we hypothesized that the combination of M+R was superior in improving β-cell function relative to insulin sensitivity compared with M or M+L. We describe the temporal changes in measures of β-cell function and insulin sensitivity derived from an oral glucose tolerance test (OGTT) over a 4-year period among the three treatments of TODAY.
RESEARCH DESIGN AND METHODS
Study design
Detailed description of the TODAY protocol and the primary outcome results have been published (3,4,16,17). In brief, the TODAY trial consisted of a screening phase and a run-in phase followed by the randomized clinical trial. After initial screening, eligible participants entered a 2–6-month run-in period with goals of weaning from nonstudy diabetes medications, tolerating metformin up to a dose of 1,000 mg twice daily but no less than 1,000 mg/day, attaining HbA1c <8.0% for at least 2 months on metformin alone, and demonstrating adherence to study medications and visit attendance (4,16,17). After the run-in phase, 699 overweight youths, 10–17 years of age, with a mean duration of diagnosed type 2 diabetes of 7.8 months, were randomly assigned to receive M, M+R, or M+L (3,4). HbA1c was obtained at screening, randomization, and every study visit thereafter. OGTTs were performed after a 10–14-h overnight fast, at randomization, and at 6 and 24 months and annually thereafter, and blood samples were analyzed for glucose, insulin, and C-peptide. This report uses temporal data related to measures of insulin sensitivity, secretion, and glycemic control from the randomized participants.
Assays and calculations
All assays were performed at the TODAY central laboratory (Northwest Lipid Research Laboratory, University of Washington, Seattle, WA) (4). HbA1c (high-performance liquid chromatography), C-peptide (two-site immunoenzymatic assay), and insulin (double-antibody radioimmunoassay) were performed as previously described (15).
Insulin sensitivity was calculated as 1/fasting insulin (1/IF), which correlates strongly with hyperinsulinemic-euglycemic clamp–derived in vivo insulin sensitivity in obese youth with or without type 2 diabetes (18). During the OGTT, the insulinogenic index (△I30/△G30) and C-peptide index (△C30/△G30) were calculated as the ratio of the incremental insulin, C-peptide, and glucose responses over the first 30 min of the test, as reported for the TODAY baseline (15). These indices reflect similar trends in first-phase insulin from the hyperglycemic clamp in obese youth across the glucose tolerance spectrum (19). The oral disposition index (oDI), a measure of β-cell function relative to insulin sensitivity, was calculated as the product of insulin sensitivity multiplied by the insulinogenic index (1/IF × △I30/△G30 and 1/IF × △C30/△G30). In obese youth, the oDI correlates strongly with clamp-derived DI, identifies comparable decrements in β-cell function across the glucose tolerance groups (as does the clamp DI), and has analogous predictive power to that of clamp DI for the 2-h glucose concentration of the OGTT (20). We used the C-peptide index of insulin secretion (△C30/△G30) in addition to the insulinogenic index (△I30/△G30), since some participants had received insulin prior to screening/enrollment in TODAY, which could have potentially resulted in circulating insulin antibodies interfering with the insulin assay. In addition, differences in insulin clearance in different racial groups (21,22) could confound the insulinogenic index data. Metabolic assessments performed after participants reached the primary end point of treatment failure are not reported because accurate assessment of β-cell function is hindered by the impact of exogenous insulin therapy on the parameters of insulin secretion. Thus, treatment group differences in the above measures over time may be influenced by the successive removal of subjects reaching treatment failure. Sensitivity analyses were used to assess the potential impact of bias.
Statistical methods
Of the 2,043 insulinogenic index values obtained through the 4-year visits, 75 (3.67%) were ≤0, and for the C-peptide–based index, 74 (3.61%) were ≤0. Although mathematically possible, such values were judged biologically implausible and were treated as missing values, similar to approaches used in adult type 2 diabetes trials (12). These improbable responses were observed in 64 subjects (average of 1.13 per such subject), of whom 18 had a response ≤0 at baseline, necessitating their exclusion from the longitudinal analyses. There were a total of 74 missing oDI values due to the combination of 72 missing insulin sensitivity values and insulinogenic index ≤0.
Kruskal-Wallis or F tests were used to compare baseline variables among the treatment groups for continuous variables, and the χ2 test was used for categorical variables. Longitudinal linear models were used to estimate mean levels of the parameters over time within groups over the follow-up period using all available data. Analyses of the reciprocal of fasting insulin, insulinogenic index, and oDI used the natural log transformation to better approximate a normal distribution. Mean change from baseline to 6 months, describing the acute effects of therapy on the outcomes, and the average rate of change from 6 months to 48 months were estimated from linear contrast of the model-estimated means over time (12). Data in the figures are presented as baseline adjusted geometric means ± SE asymmetric limits (obtained as exp[mean ± SE of the log values]). Baseline predictors for glycemic failure and odds ratios (ORs) were examined by multiple logistic regression.
RESULTS
Demographic and metabolic characteristics
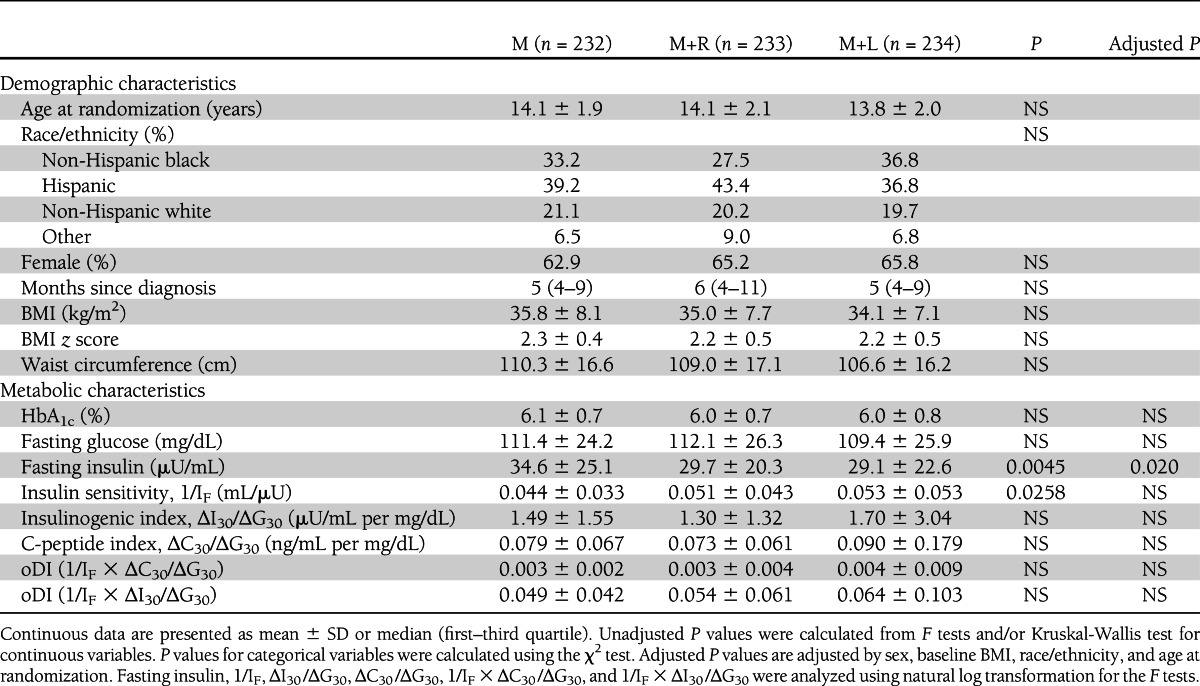
Screening, entry, and run-in information were described previously (4,15–17). Mean screening HbA1c levels were not different among the three groups (M, 7.6 ± 2.0%; M+R, 7.4 ± 2.0%; M+L, 7.4 ± 2.0%). At randomization (Table 1), age, sex, race/ethnicity, BMI, waist circumference, and duration of diabetes were similar among the three treatment groups. Fasting insulin was highest and insulin sensitivity lowest in the M group before adjusting for age, sex, BMI, and race. After these adjustments, the between-group difference in insulin sensitivity disappeared. There were no differences in the remaining metabolic parameters, including randomization HbA1c, fasting glucose, insulinogenic index, and oDI, among the three groups.
Table 1.
Demographic and metabolic characteristics of TODAY participants by treatment groups at randomization
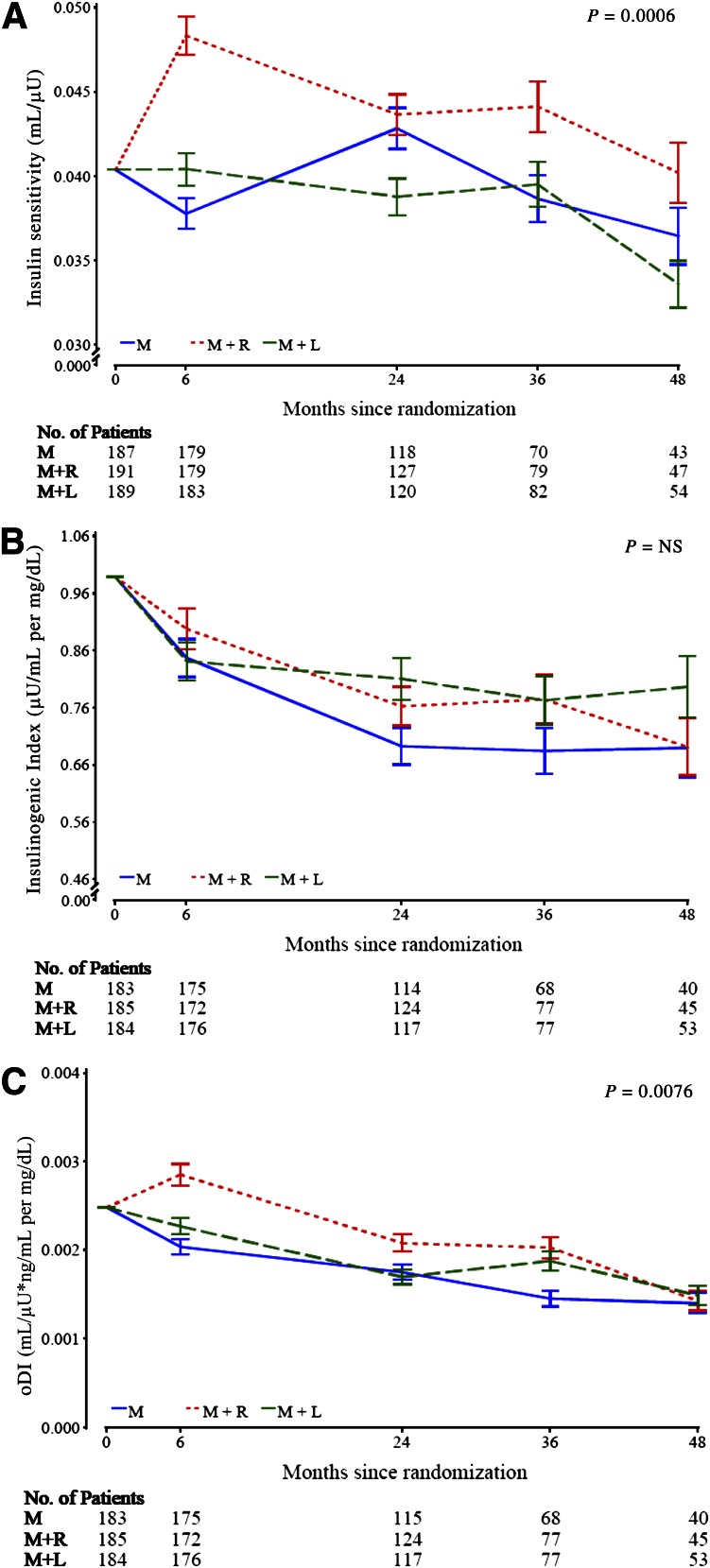
Temporal patterns of insulin sensitivity, insulinogenic index, and oDI
Only participants with a baseline and follow-up evaluation for each outcome measure contributed data to the longitudinal analyses of the measures depicted in Fig. 1. The longitudinal models present data over 48 months of follow-up by treatment group for insulin sensitivity (Fig. 1A), insulinogenic index (Fig. 1B), and oDI (Fig. 1C). During the first 6 months, insulin sensitivity and oDI increased in the M+R group relative to the other two groups but fell thereafter in all groups similarly.
Figure 1.
Baseline adjusted geometric mean ± SE asymmetric limits (obtained as exp[mean ± SE of log values]) of OGTT-derived measures of insulin sensitivity (1/IF) (A), insulinogenic index (△I30/△G30) (B), and oDI ([1/IF] × [△C30/△G30]) (C) in the three treatment groups over 48 months of follow-up in TODAY, analyzed using log-transformed values. The P value refers to the overall effect of treatment group assignment in the longitudinal models for the various parameters under question within the groups.
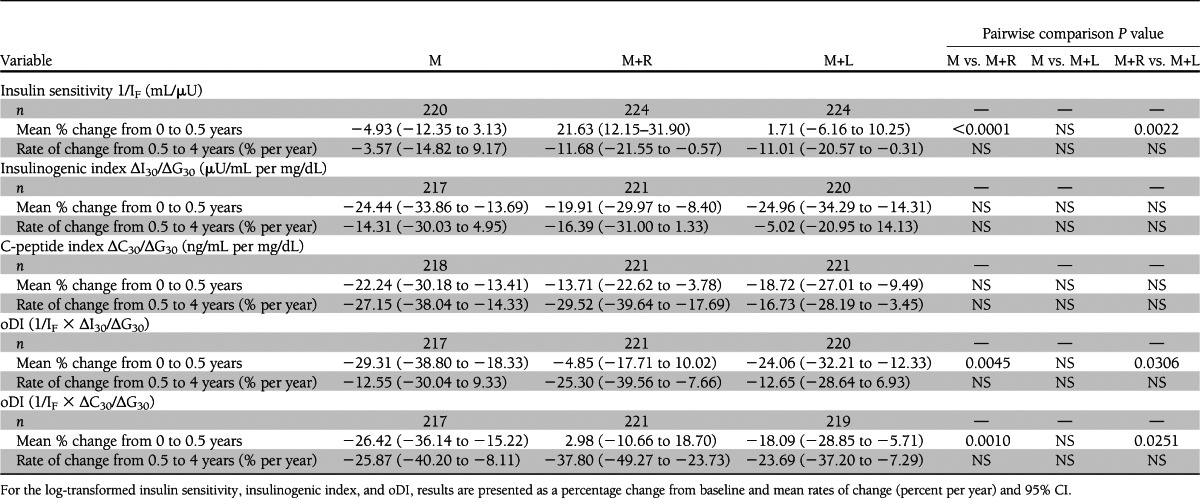
Table 2 shows the short-term (first 6 months) effect of therapy as the mean percent change from baseline to 6 months, and the longer-term (chronic) effect as the rate of change, percent per year, from 6 to 48 months. M+R produced a significantly greater short-term increase in insulin sensitivity and oDI over the first 6 months than M or M+L, whereas the decline in the insulinogenic index among the three groups was similar. Thereafter (6–48 months), the decline in insulin sensitivity and β-cell function relative to insulin sensitivity was parallel among the three groups.
Table 2.
Changes in OGTT-derived measures of insulin sensitivity, insulinogenic index, and β-cell function relative to insulin sensitivity (oDI) for the full TODAY cohort from randomization to 0.5 years and rates of change among means from 0.5 to 4 years based on a longitudinal model adjusted for baseline factors
Baseline characteristics in those who failed versus did not fail treatment
Those reaching the primary outcome of treatment failure in TODAY, regardless of treatment group assignment, had significantly higher HbA1c levels and fasting glucose concentrations at randomization (Table 3) and lower insulinogenic index, C-peptide index, and oDI compared with those who did not fail. Insulin sensitivity was not different between those who failed versus those who did not fail in each treatment group. In the M treatment group, more non-Hispanic blacks failed; in the M+R group, fewer females failed. Duration of diagnosed diabetes was significantly different between those who failed and did not fail in the M+L treatment group, but not in the other two treatment groups.
Table 3.
Randomization demographic and metabolic characteristics of TODAY participants who failed versus those who did not fail treatment by treatment group
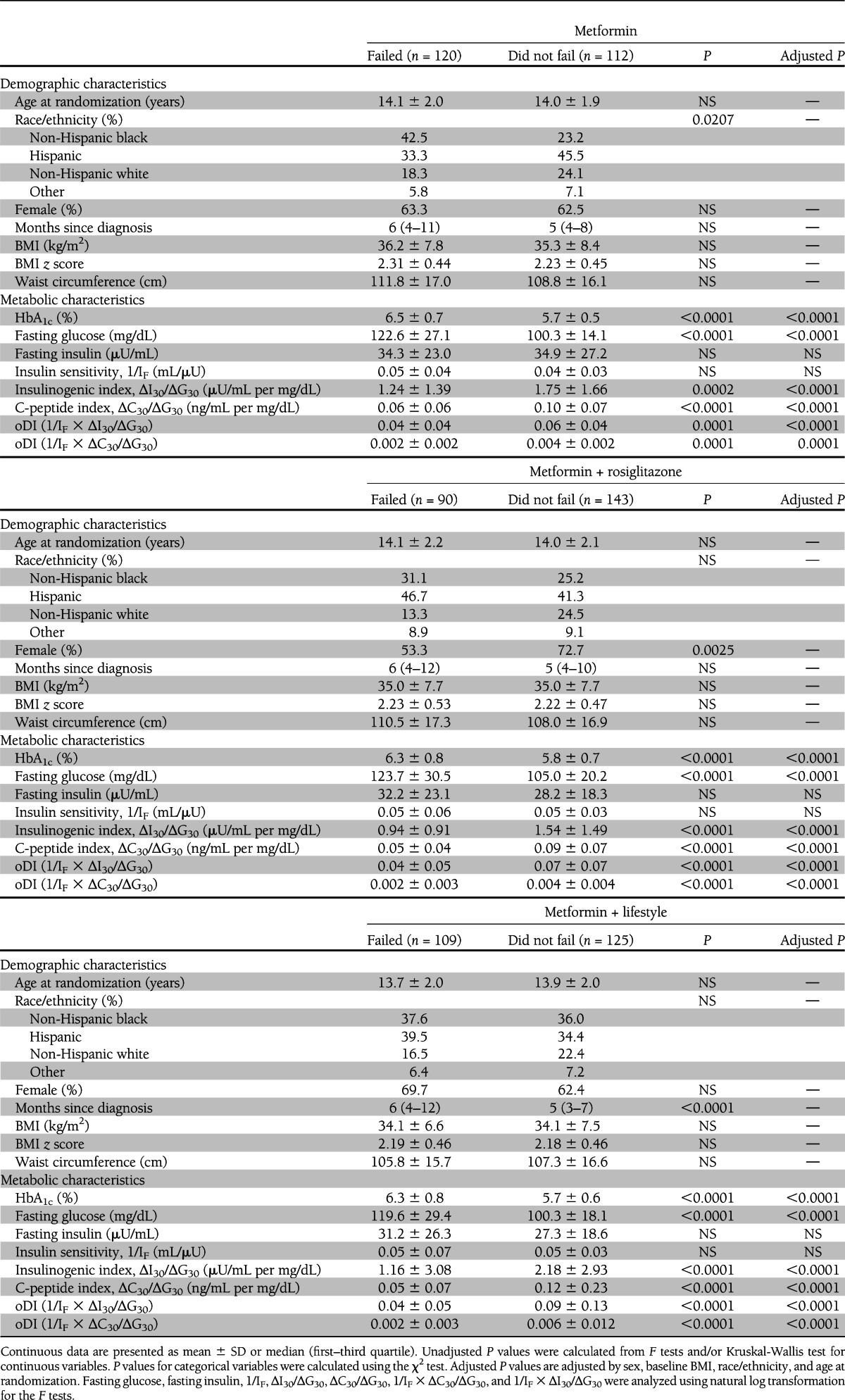
To identify baseline predictors of treatment failure, logistic regression analysis was performed, with the outcome being treatment failure and the independent variables at randomization being diabetes duration, HbA1c, and oDI in addition to age, sex, race/ethnicity, and BMI. The best prediction model for failure included randomization oDI (P = 0.0071) and HbA1c (P < 0.0001). Replacing oDI in the model with insulinogenic index (P = 0.0521) or insulin sensitivity (P = ns) showed no predictive power for either. OR analyses revealed that for every 0.5% increase in HbA1c at randomization, the OR for future glycemic failure was 1.83 (95% CI 1.59–2.12), and for every 0.002 unit increase in oDI at randomization, the OR for glycemic failure was 0.84 (0.74–0.95).
Similar to randomization HbA1c, screening HbA1c (4,15) was higher in those who failed to maintain glycemic control versus those who did not fail (8.0 ± 2.1 vs. 7.1 ± 1.8%, P < 0.001). Using logistic regression analysis to identify screening predictors of treatment failure (independent variables in the model included age, sex, race/ethnicity, duration of diabetes, and HbA1c), screening HbA1c was the only significant (P < 0.001) predictor of glycemic failure, with OR of 1.12 (95% CI 1.08–1.17) for every 0.5% increase in screening HbA1c.
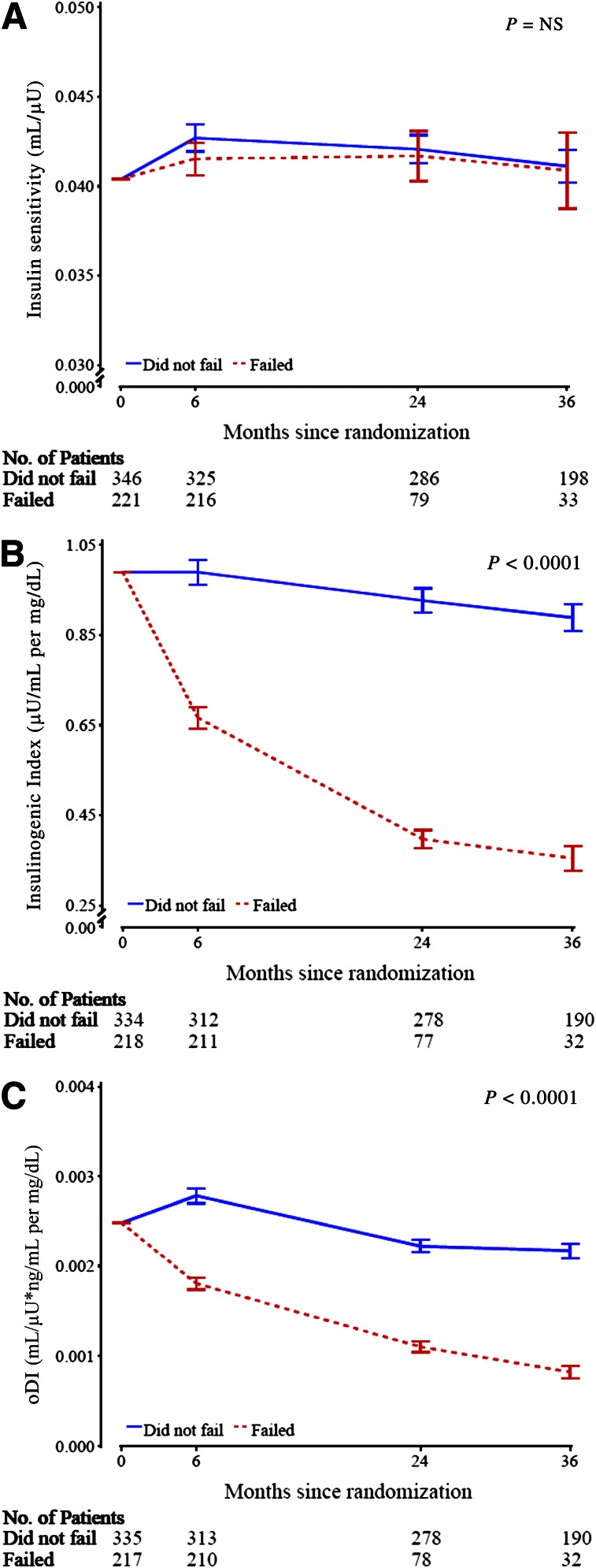
Temporal patterns of insulin sensitivity, insulinogenic index, and oDI in those who failed versus did not fail treatment
Figure 2 shows the change in insulin sensitivity (A), insulinogenic index (B), and oDI (C), with the three therapeutic groups combined, in those who failed treatment and those who did not. Data are shown for up to 36 months because in the group who failed therapy, once the subjects were put on insulin, they were not included in the analysis, as stated above. Due to successive failure, the remaining numbers of subjects dwindled over time, with only 15 subjects available before primary outcome at 48 months, which did not allow for adequate statistical analysis. Insulin sensitivity was not different over time between those who failed versus those who did not fail, but insulinogenic index and oDI deteriorated rapidly and progressively over time in those who failed in contrast to those who did not fail.
Figure 2.
Baseline adjusted geometric mean ± SE asymmetric limits (obtained as exp[mean ± SE of log values]) of OGTT-derived measures by treatment failure with the three treatment groups combined, analyzed using log-transformed values. A: Insulin sensitivity (1/IF). B: Insulinogenic index (△I30/△G30). C: oDI ([1/IF] × [△C30/△G30]). The P value refers to the overall effect of failed vs. not failed group assignment in the longitudinal models for the various parameters under question within the two groups.
CONCLUSIONS
The present investigation demonstrates that 1) over the first 6 months, the M+R group exhibited a statistically significant greater acute improvement in insulin sensitivity and oDI versus the other two groups, 2) after the first 6 months and up to 4 years, the changes in glucose homeostasis parameters were not different among the three treatment groups, 3) HbA1c and oDI were significant baseline predictors of glycemic failure, 4) insulinogenic index and oDI were ∼40–50% lower at baseline in those who failed to maintain glycemic control versus those who did not fail, and 5) insulinogenic index and oDI deteriorated rapidly and progressively in those who failed versus those who did not fail therapy, but insulin sensitivity over time was not different between the two groups.
The pathogenesis of youth-onset type 2 diabetes involves peripheral and hepatic insulin resistance combined with β-cell failure, which progressively worsens (5–9). Thus, therapeutic approaches that directly improve or preserve β-cell function or those that improve insulin resistance and consequently lessen the burden on the β-cell are desirable. Limited longitudinal data in youth using clamp methodology show that there is a relatively rapid deterioration of β-cell function over time with no significant change in peripheral or hepatic insulin sensitivity in the absence of weight or BMI change (10,11,23). After a median duration of 20 months of diagnosed diabetes, youth with type 2 diabetes lost on average 20% per year of their first-phase insulin or C-peptide secretion measured with the hyperglycemic clamp (11). These data are consistent with the TODAY data (Table 2), which show 20–35% decline in β-cell function per year, depending on treatment group. These data in youth and adolescents contrast with adult data showing a lower rate of decline in β-cell function. The ADOPT (A Diabetes Outcome Progression Trial) study of drug-naive adults with type 2 diabetes showed that the insulinogenic index declined at a rate of ∼7–11% per year (12). In the UK Prospective Diabetes Study, the estimated rate of decline of β-cell function, using the homeostasis model assessment %B index, was ∼7% per year (13,14).
The TODAY study allowed the prospective evaluation of changes in glucose metabolism over 4 years in patients randomized to M, M+R, or M+L. In the first 6 months, M+R improved insulin sensitivity by ∼20%. Even though this change was statistically significant, it is uncertain if it was clinically meaningful, especially bearing in mind the severity of insulin resistance in youth with type 2 diabetes (50% lower in vivo insulin sensitivity compared with age-, BMI-, and adiposity-matched nondiabetic controls) (5). This acute improvement in insulin sensitivity in the M+R group may have translated to lowering the short-term secretory burden on the β-cells. Whereas the insulinogenic index did not differ among the three treatment groups, either in the short- or long-term, the β-cell function relative to insulin sensitivity remained relatively stable in the M+R group but deteriorated (∼25%) in the M and M+L groups in the short-term (Table 2). After the first 6 months, there were no significant group differences in the decline in oDI; all showed continued deterioration. Similar to our data, results from the adult ADOPT study showed that, during the first 6 months, insulin sensitivity increased more in the rosiglitazone group than the metformin group (12,24). However, contrary to our findings, the ADOPT study showed a continued increase in insulin sensitivity in both groups after the first 6 months, whereas in TODAY, there was continued and comparable deterioration in insulin sensitivity in all three groups. The mean percent change in insulin sensitivity with metformin monotherapy in TODAY was remarkably lower (−4.93% [95% CI −12.3 to 3.1]) than that in ADOPT (∼13%) (12). This could be indicative of a more severe impairment in peripheral insulin sensitivity in youth with type 2 diabetes that may not be adequately managed with the hepatic insulin sensitizer metformin, but may require more potent peripheral insulin sensitizers.
In the TODAY cohort, M+R provided superior durability of glycemic control compared with M, with significantly lower treatment failure rates (38.6 vs. 51.7%), whereas M+L was intermediate (46.6%) and not significantly different from either of the other two groups. These treatment failure rates are in stark contrast to adult studies showing lower failure rates; 21% with metformin monotherapy and 15% with rosiglitazone monotherapy at 5 years (25). These higher failure rates in youth despite combination therapy (M+R) in TODAY are suggestive of a more severe disease process in youth.
Irrespective of treatment group assignment, those who ultimately failed to maintain glycemic control were metabolically in a more advanced disease state at baseline, characterized by higher HbA1c and fasting glucose levels, lower insulinogenic index, and ∼50% lower β-cell function relative to insulin sensitivity, with no difference in insulin sensitivity (Table 3). Moreover, logistic regression analyses revealed that both HbA1c and oDI were significant independent baseline predictors of glycemic failure. For every 0.5% increase in HbA1c at the time of randomization, the OR for failure increased to 1.83, and for every doubling of oDI, the OR decreased to 0.84. Analyses of longitudinal data between those who failed to maintain glycemic control and those who did not fail demonstrated a lack of beneficial effects of treatment among those who failed. Whereas the pattern of insulin sensitivity over time was similar between those who failed and those who did not fail, insulinogenic index deteriorated rapidly and relentlessly in those who failed (Fig. 2), with a similar pattern of progressive deterioration in oDI over time in those who failed (∼27% by 6 months, ∼56% by 24 months, and ∼67% by 36 months). Thus, when β-cell impairment is far advanced, as was the case in those who failed to maintain glycemic control, none of the three treatments proved effective in maintaining glycemic durability.
Studies of youth with type 2 diabetes have demonstrated a strong inverse relationship between HbA1c and β-cell function relative to insulin sensitivity (5). In the TODAY study, at screening and randomization, insulin secretion indices declined with increasing HbA1c quartiles (15). This inverse relationship between HbA1c and β-cell function may either reflect the impact of deficient insulin secretion on the outcome of glycemic control or could be viewed as a glucotoxic phenomenon impairing β-cell function. In the current study, the group that failed to maintain glycemic control already had significantly impaired oDI at randomization compared with the group that did not fail, and did not show beneficial effects of treatment. This observation, combined with our findings that both HbA1c and oDI are predictors of glycemic failure, would suggest that treatment before significant β-cell impairment and deterioration in glycemic control occur may be prudent in achieving better therapeutic success in youth with type 2 diabetes.
Since it was not feasible to institute clamp experiments across the many participating clinics in TODAY, we used surrogate estimates of insulin sensitivity and β-cell function derived from the OGTT. These surrogate estimates correlate strongly with clamp-measured parameters in youth (18–20). A potential limitation of TODAY is that in 3.6% of the tests, the value for the insulinogenic index was ≤0. This is in complete agreement with previous adult trials of type 2 diabetes (12) and is reported in individuals with normal and impaired glucose tolerance (24). Because of the high failure rates, statistical analysis was not possible past the 36-month visit in the cohort that failed since the number of subjects became very limited (15 subjects at 48 months). Lastly, despite the favorable therapeutic outcome of M+R in TODAY, a limitation is the use of rosiglitazone. However, TODAY was designed and initiated prior to the discovery of the adverse effects of rosiglitazone (26).
In summary, changes in insulin sensitivity and β-cell function relative to insulin sensitivity in the first 6 months of treatment appear to be responsible for the different degrees of glycemic durability observed with M+R, M, and M+L. The former provided favorable, albeit short-term, changes in both parameters, translating to the lowest treatment failure rates in TODAY. However, initial β-cell reserve and HbA1c were important determinants of glycemic durability. Regardless of treatment assignment, those youth who failed to maintain glycemic control had severe impairment of β-cell function at the beginning of the trial and experienced progressive and faster loss of β-cell function compared with those with durable glycemic control.
Acknowledgments
This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and U01-DK-61254), the National Center for Research Resources General Clinical Research Centers Program (M01-RR00036, Washington University School of Medicine; M01-RR00043-45, Children’s Hospital Los Angeles; M01-RR00069, University of Colorado Denver; M01-RR00084, Children’s Hospital of Pittsburgh; M01-RR01066, Massachusetts General Hospital; M01-RR00125, Yale University; and M01-RR14467, University of Oklahoma Health Sciences Center), and the National Center for Research Resources Clinical and Translational Science Awards (UL1-RR024134, Children’s Hospital of Philadelphia; UL1-RR024139, Yale University; UL1-RR024153, Children’s Hospital of Pittsburgh; UL1-RR024989, Case Western Reserve University; UL1-RR024992, Washington University in St. Louis; UL1-RR025758, Massachusetts General Hospital; and UL1-RR025780, University of Colorado Denver).
S.A. is on an advisory committee for and receives a consulting fee from Bristol-Myers Squibb, is on an advisory board for Novo Nordisk, reports serving on DSMB for Boehringer Ingelheim, is a consultant for Gilead, and is on an advisory board for Sanofi. M.W.H. is on advisory panels for Novo Nordisk and Daiichi Sankyo, on a data safety monitoring committee for Bristol-Myers Squibb, and on a scientific advisory committee for Xeris Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
S.A., S.C., M.W.H., N.H.W., and S.M.W. researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. L.P., F.B., L.L.L., and R.G. researched data, contributed to the discussion, and reviewed and edited the manuscript. M.P. researched data. L.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan; Pfizer; and Sanofi. The TODAY Study Group gratefully acknowledges the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
APPENDIX
The members of the writing group are as follows: Silva Arslanian, MD (chair), Children's Hospital of Pittsburgh; Laura Pyle, PhD, George Washington University Biostatistics Center; Marisa Payan, MS, George Washington University Biostatistics Center; Fida Bacha, MD, Baylor College of Medicine; Sonia Caprio, MD, Yale University School of Medicine; Morey W. Haymond, MD, Baylor College of Medicine; Lynne L. Levitsky, MD, Massachusetts General Hospital for Children; Robin Goland, MD, Columbia University; Neil H. White, MD, Washington University in St. Louis; and Steven M. Willi, MD, Children's Hospital of Philadelphia.
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2393/-/DC1.
A complete list of the members of the TODAY Study Group can be found in the Supplementary Data online. The members of the writing group are listed in the appendix.
A slide set summarizing this article is available online.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
References
- 1.Flint A, Arslanian S. Treatment of type 2 diabetes in youth. Diabetes Care 2011;34(Suppl. 2):S177–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002;25:89–94 [DOI] [PubMed] [Google Scholar]
- 3.Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitler P, Epstein L, Grey M, et al. TODAY Study Group Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacha F, Gungor N, Lee S, Arslanian S. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder DA, Woo JG, D’Alessio DA. Impaired β-cell sensitivity to glucose and maximal insulin secretory capacity in adolescents with type 2 diabetes. Pediatr Diabetes 2010;11:314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 9.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009;58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr 2004;144:656–659 [DOI] [PubMed] [Google Scholar]
- 11.Bacha F, Gungor N, Lee SJ, Arslanian SA. Progressive deterioration of β-cell function in obese youth with type 2 diabetes. Pediatr Diabetes 2013;14:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn SE, Lachin JM, Zinman B, et al. ADOPT Study Group Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC, UK Prospective Diabetes Study (UKPDS) Group UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet Med 1998;15:297–303 [DOI] [PubMed] [Google Scholar]
- 14.Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 15.Bacha F, Pyle L, Nadeau K, et al. TODAY Study Group Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes 2012;13:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffel L, Chang N, Grey M, et al. TODAY Study Group Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copeland KC, Zeitler P, Geffner M, et al. TODAY Study Group Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian S. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 22.Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism 1997;46:53–58 [DOI] [PubMed] [Google Scholar]
- 23.Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes 2005;6:95–99 [DOI] [PubMed] [Google Scholar]
- 24.Faulenbach MV, Wright LA, Lorenzo C, et al. Impact of differences in glucose tolerance on the prevalence of a negative insulinogenic index. J Diabetes Complications 2013;27:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 26.Rosen CJ. The rosiglitazone story-lessons from an FDA Advisory Committee meeting. N Engl J Med 2007;357:844–846 [DOI] [PubMed] [Google Scholar]