Abstract
OBJECTIVE
The risk of cardiovascular death before the age of 40 is 20-fold higher in patients with type 1 diabetes mellitus (T1DM). Endothelial progenitor cells (EPCs) predict cardiovascular morbidity and mortality in patients without diabetes. We hypothesized that EPCs are modified in children with T1DM and are related to characteristics of T1DM such as glycemic control.
RESEARCH DESIGN AND METHODS
Children (n = 190; 156 T1DM subjects and 34 control subjects) were included in an observational cohort study and matched for age and sex. EPCs were enumerated by flow cytometry at the beginning (cross-sectional) and 1 year later (longitudinal). To analyze changes of variables during the observation, Δ values were calculated.
RESULTS
EPCs were significantly reduced in T1DM children versus control subjects (609 ± 359 vs. 1,165 ± 484, P < 0.001). Multivariate regression modeling revealed that glycated hemoglobin A1c (HbA1c) was the strongest independent predictor of EPCs (β = −0.355, P < 0.001). Overall glycemic control at the beginning and end of study did not differ (7.8 ± 1.2 vs. 7.8 ± 1.2 relative %, P = NS), but we observed individual HbA1c changes of −4.30/+3.10 relative %. The strongest EPC increase was observed in the patients with the most favorable HbA1c lowering during the 1-year follow-up. Accordingly, the strongest EPC decrease was demonstrated in the patients with the strongest HbA1c worsening during the time period.
CONCLUSIONS
This is the first prospective study demonstrating diminished EPCs in children with T1DM. The association of better glycemic control with an increase in EPC numbers within 1 year suggests that a reduction of the high cardiovascular disease burden might be mediated likewise.
Over the last 30 years, a marked improvement in diabetic nephropathy, retinopathy, and neuropathy was observed in patients with type 1 diabetes mellitus (T1DM) (1–3). However, no difference for the incidence of cardiovascular disease (CVD) was observed in those patients (1–3). Thus, one can assume that late improvements in diabetes care do not reduce cardiovascular risk (1–3).
Even more, despite dramatic improvement in CVD therapy, such as interventional therapy, statins, and clopidogrel, CVD mortality did not improve in T1DM over the last 10 years (1–3). In fact, the mortality of CVD before 40 years of age is 20-fold higher in patients with T1DM compared with age- and sex-adjusted healthy subjects. Between 30–40 years of age, CVD is already the first cause of death in those patients (4,5).
The main contributor to the increased cardiovascular risk might be unsatisfactory glycemic control, which emerges from the very beginning of T1DM (childhood) (6–8). Other mechanisms speculated to be associated or involved independently might be endothelial dysfunction (9) and/or systemic vascular inflammation (10).
Recent analysis of the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) trial strengthened the suspicion that endothelial dysfunction and vascular inflammation might be involved in the increased susceptibility to vascular disease (11,12) in T1DM. However, the exact mechanisms linking those pathophysiological findings to manifest clinical disease are not understood.
A possible link might be vascular progenitor cells, which are involved in vascular hemostasis and counteract the aberrances of vascular inflammation. Werner and Nickenig (13) argued that endothelial progenitor cells (EPCs) are the common feature of atherosclerosis, from its beginning as endothelial dysfunction to its result as end-stage ischemic heart disease. EPCs measured by colony-forming assays were demonstrated to correlate with the Framingham Risk Score and endothelial function (14), and furthermore, when measured by flow cytometry, EPCs were found to be associated with cardiovascular outcome (15).
In accordance with the theory of endothelial continuum of EPCs (13), EPCs were demonstrated to be reduced in adults with T1DM in a pilot study (16). We have added that EPCs are directly related to stages of retinopathy in adults with T1DM (17) and also T2DM (18). Recently, three very interesting cross-sectional pilot-like studies have been reported. First, Sibal et al. (19) showed an association of EPCs and premature atherosclerosis in young adults with T1DM by investigating flow-mediated dilatation in those patients. However, they did not obtain a significant difference for CD34+/CD309+ cells between T1DM and control subjects. Second, DiMeglio et al. (20) demonstrated a reduction of EPCs already in young adults (mean age 20.3 ± 1.4 years) with T1DM compared with those without. Third, Palombo et al. (21) confirmed a reduction of EPCs in 16 young adults with T1DM and added an association of EPCs and intima media thickness. All three studies failed to identify any association of EPCs with characteristics of T1DM, such as glycemic control or total insulin dosage.
It is tempting to speculate that diminished EPCs could be one of the pathophysiological mechanisms linking the elevated CVD risk to young patients with T1DM. We assumed that elevated inflammation and/or impaired glucose control might be associated cross-sectionally and longitudinally with diminished levels of EPCs, thereby leading to the increased risk for CVD at such a young age.
RESEARCH DESIGN AND METHODS
This study was approved by the institutional ethics committee and complies with the Declaration of Helsinki (22), including current revisions and the Good Clinical Practice Guidelines (23,24). The procedures followed were in accordance with institutional guidelines, and all subjects, respectively their parents, gave written informed consent before the study. Patients and control subjects were enlisted at the outpatients’ Department of Pediatrics and Adolescent Medicine at the Medical University of Vienna and Vienna General Hospital. Children and adolescents between 8 and 16 years of age who were seen for their yearly check-up of T1DM at the outpatients’ care department of the Department of Pediatrics and Adolescent Medicine were eligible for the study. By protocol, T1DM patients would have to be excluded from the study if they had proliferative diabetic retinopathy or serum creatinine >2.0 mg/dL at the time of inclusion. However, we did not identify any patients with the latter two complications of T1DM. Age- and sex-matched control subjects were recruited at the same institution. Controls were not eligible if they had endocrine disorders requiring hormone therapy or cancer.
Comorbidities
Comorbidities among the 156 patients with T1DM included seven cases of autoimmune thyroiditis, one case of Hashimoto thyroiditis, one case of myasthenia gravis and Hashimoto thyroiditis, two cases of allergies, one case of allergic asthma, four cases of celiac disease, one case of celiac disease and Hashimoto thyroiditis, and three cases of atopic dermatitis.
Familial history of diabetes
Of the control subjects, neither the father nor the mother had a history of T1DM. One mother of the control subjects had gestational diabetes and two fathers of the control subjects suffered from T2DM. Of the patients with T1DM, three mothers had T1DM, six fathers had T1DM, six mothers had gestational diabetes, three mothers had T2DM, and two fathers had T2DM.
Cross-sectional study
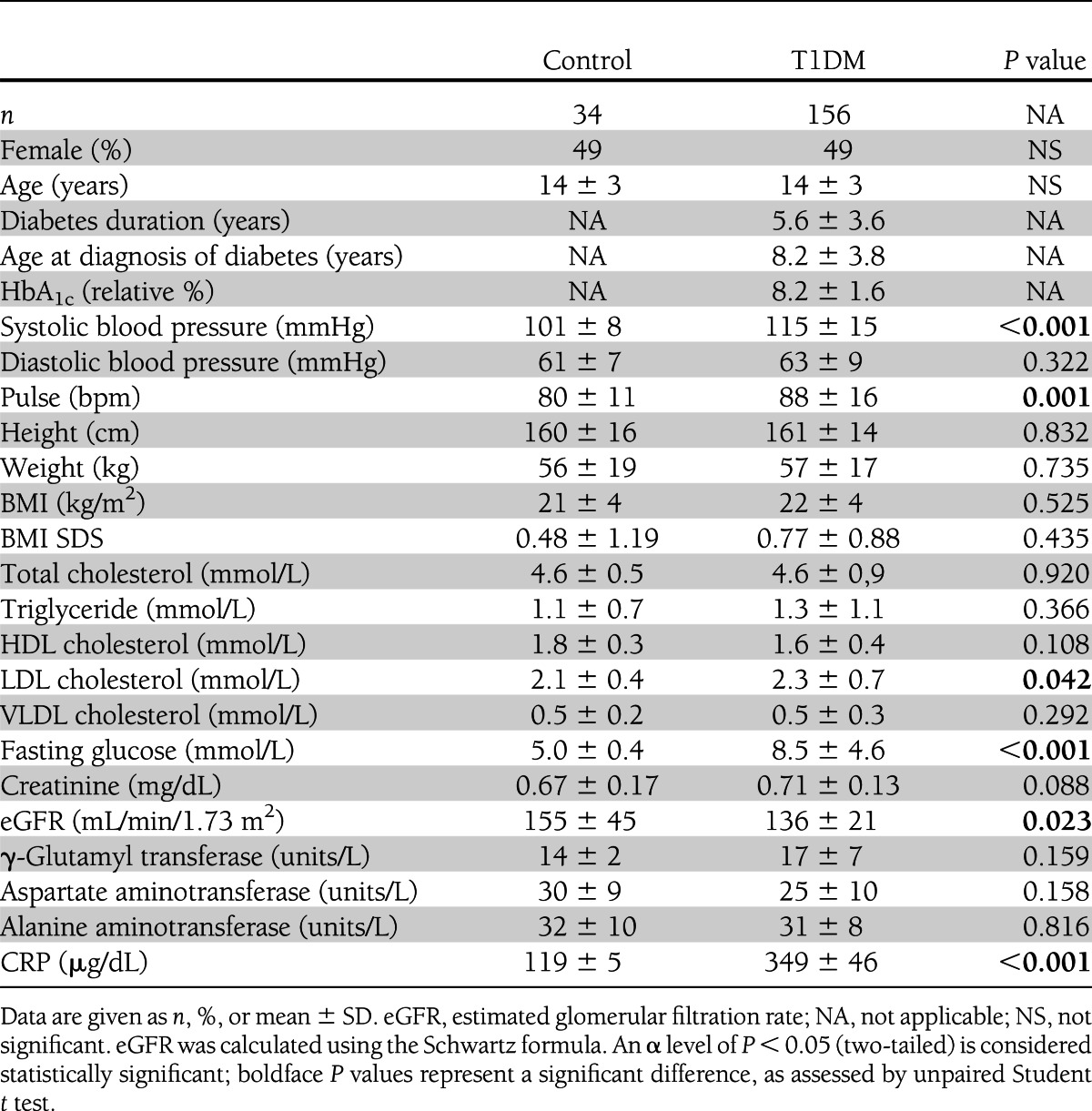
Patients with T1DM (n = 156) and control subjects (n = 34) were studied in a cross-sectional approach. Clinical characteristics of the 190 study participants are shown in Table 1. Patients and control subjects were well matched and did not differ in age (14 ± 3 years), sex (49% female), and BMI (21 ± 4 kg/m2). The mean diabetes duration was 5.6 ± 3.6 years, and the age at diagnosis of diabetes was 8.2 ± 3.8 years.
Table 1.
Basic characteristics of the cross-sectional study
Longitudinal study
Of the 156 patients with T1DM, 97 patients attended a predefined follow-up visit ∼1 year later. Thus, the mean observation time was 11.5 ± 1.1 months. Of those 97 patients, the mean diabetes duration at the time of inclusion was 5.3 ± 3.6 years, and the age at diagnosis of diabetes was 8.0 ± 3.8 years (Supplementary Table 1).
Insulin treatment
All patients with T1DM were treated with insulin. Three patients with T1DM were additionally treated with metformin; other antidiabetic drugs were not used. Glycated hemoglobin A1c (HbA1c) did not change during the 1-year follow-up in the 97 patients available (start: 7.8 ± 1.2 vs. end: 7.8 ± 1.2, P = 0.660) (Supplementary Table 1).
At the beginning of the longitudinal study, 19 patients were on conventional insulin therapy (CT), 57 were on intensified insulin therapy (IT), and 21 were on continuous subcutaneous insulin (CSII) therapy. At the end of the study, 7 patients were on CT, 62 were on IT, and 28 were on CSII therapy. The HbA1c in the individual treatment groups at the start and end of the study were not significantly different (as assessed by ANOVA): 7.6 ± 1.1 vs. 8.3 ± 1.6 vs. 7.6 ± 1.0 (CT vs. IT vs. CSII), P = 0.075 at the start; 7.7 ± 1.2 vs. 7.9 ± 1.2 vs. 7.8 ± 1.1 (CT vs. IT vs. CSII), P = 0.883 at the end. To compare insulin dosage in T1DM, mean daily insulin dosage per kilogram body weight and day was calculated.
Calculations
The Schwartz formula was applied to compare renal function in children and adolescents (25). For comparison of weight changes over time, in addition to the classical BMI calculations, SD scores (SDS) based on a German investigation (26) were calculated. An obtained SDS of 1.282 resembles the 75th percentile, 0.674 the 50th percentile, and 0 the 25th percentile.
Measurement of standard laboratory parameters
Venous blood (35 mL) was taken after an overnight fast for the measurement of biochemical and inflammatory factors. Blood glucose, cholesterol, HDL cholesterol, and triglycerides were measured by enzymatic in vitro tests (Roche Diagnostics GmbH, Graz, Austria). The intra-assay and interassay coefficients of variation were 1.1 and 2.9%, 0.8 and 1.7%, 1.3 and 2.6%, and 1.5 and 1.8%, respectively. HbA1c was measured by DCA 2000 (Bayer Corporation, Elkhart, IN). C-reactive protein (CRP) was measured with a nephelometric assay in serum (Dade Behring, Marburg, Germany). The intra-assay and interassay variability coefficients for CRP were 4.7 and 8.3%.
EPC enumeration
As Fadini et al. (27) stated in their review “any definitions of the antigenic phenotype of EPC overlaps with cells of other lineages,” several different antigen combinations have been applied to facilitate EPC enumerations. Nevertheless, several antigen combinations have been acknowledged to identify vascular progenitor cells (28–30). To discriminate from true EPCs, bone marrow–derived angiopoietic progenitor cells are circulating endothelial cells, which are shed from damaged vessel walls (31). For the investigation of different subsets of vascular progenitor cells, several cell types were defined according to their antigen expression. Cells in peripheral blood with hemangioblastic potential were named circulating progenitor cells (CPCs) and were defined as double positive for CD34 and CD133 (17,18,28,30), and classic EPCs were defined as triple positive for CD34, CD133, and CD309 (17,18,32).
Enumeration was performed according to our previous EPC studies (17,18). A detailed protocol can be found in the Supplementary Data.
Statistics
The data are presented as mean ± SD or percentages. The differences between patient groups were analyzed by unpaired Student t test, paired Student t test, ANOVA, χ2 test, or correlation as appropriate. The data were tested for association within each other by univariate and multivariate regression analyses. To investigate associations of changing variables over time, Δ values were calculated: valueat start – valueat end. An α level of P < 0.05 (two-tailed) was considered significant. All statistical analyses were performed with the statistical software package SPSS 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Basic characteristics
Baseline characteristics are depicted in Table 1 (cross-sectional study) and Supplementary Table 1 (longitudinal study). Patients and control subjects differed significantly: systolic blood pressure (115 ± 15 vs. 101 ± 8 mmHg, P < 0.001), pulse (88 ± 16 vs. 80 ± 11 bpm, P = 0.001), fasting glucose (8.5 ± 4.6 vs. 5 ± 0.4 mmol/L, P < 0.001), and CRP (349 ± 46 vs. 120 ± 5 µg/dL, P < 0.001). During the follow-up period, patients with T1DM showed a significant increase in height (163 ± 13 vs. 159 ± 14 cm, P < 0.001), weight (59 ± 15 vs. 55 ± 15 kg, P < 0.001), BMI (22 ± 3 vs. 21 ± 3 kg/m2, P < 0.001), VLDL cholesterol (0.5 ± 0.3 vs. 0.6 ± 0.4 mmol/L, P = 0.001), and creatinine (0.70 ± 0.13 vs. 0.74 ± 0.17 mg/dL, P = 0.004). In contrast, total cholesterol showed a significant reduction (4.5 ± 0.8 vs. 4.3 ± 0.8 mmol/L, P = 0.014), as did LDL cholesterol (2.3 ± 0.6 vs. 1.9 ± 0.7 mmol/L, P < 0.001) and γ-glutamyl transferase (16 ± 4 vs. 14 ± 4 units/L, P < 0.001).
Levels of CPCs and EPCs in patients with T1DM and control subjects
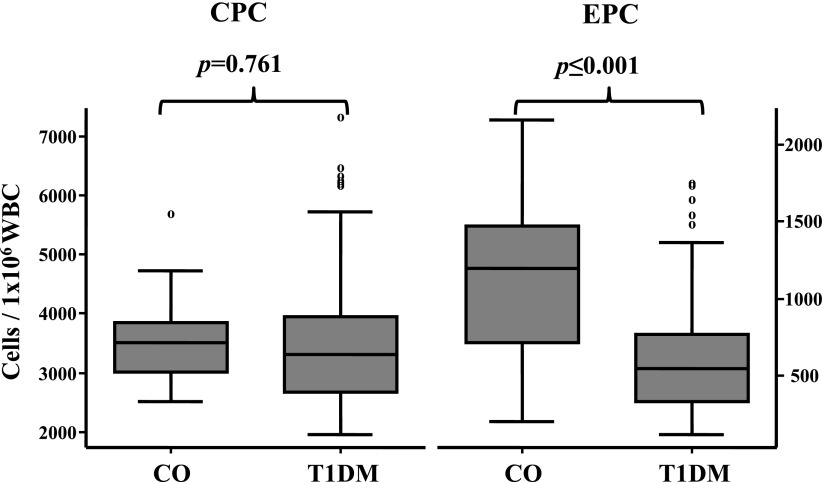
Total CPCs were not different in patients with T1DM and control subjects: 3,473 ± 1,073 vs. 3,532 ± 673/1 × 106 white blood cells (WBCs), P = 0.761 (Fig. 1). In contrast, progenitor cells restricted to the endothelial lineage (EPCs) were significantly different in T1DM versus control subjects: 609 ± 359 vs. 1,165 ± 484/1 × 106 WBCs, P < 0.001 (Fig. 1). Since we assumed that glucose control and/or inflammation might be associated with levels of CPCs and EPCs, we first tested HbA1c as an estimation of glucose control in patients with T1DM at the time of inclusion. In the 156 patients, the glycemic control estimated by HbA1c correlated with the levels of EPCs (R = −0.355, P < 0.001) but not with CPCs (R = −0.068, P = 0.412) (Supplementary Fig. 1).
Figure 1.
CPCs and EPCs in patients with T1DM. CO, control subjects. An α level of P < 0.05 (two-tailed) is considered statistically significant.
Univariate and multivariate determinants of CRP at time of inclusion
Since only EPCs, but not CPCs, were correlated with HbA1c, we next asked whether CRP might explain both variables. Interestingly, neither CPCs (β = 0.025, P = 0.735) nor EPCs (β = −0.061, P = 0.408) were associated with CRP in a univariate fashion. Since CRP was significantly different between patients and control subjects (P < 0.001), we investigated which parameters were associated with the levels of CRP in patients and control subjects. Among all variables tested for an association with CRP levels, we found that age (β = 0.231, P = 0.002), systolic blood pressure (β = 0.260, P < 0.001), diastolic blood pressure (β = 0.159, P = 0.036), BMI (β = 0.303, P < 0.001), total cholesterol (β = 0.167, P = 0.023), triglycerides (β = 0.242, P = 0.001), HDL cholesterol (β = −0.158, P = 0.033), alanine aminotransferase (β = 0.188, P = 0.018), and HbA1c (β = 0.192, P = 0.022) were associated with CRP in a univariate fashion. Multivariate modeling revealed BMI (β = 0.264, P < 0.001) and triglycerides (β = 0.188, P = 0.009) as final explaining variables.
Univariate and multivariate determinants of EPCs at time of inclusion
Since CRPs and EPCs were not associated with each other, we investigated which variables were correlated with EPCs in T1DM patients. We used all quantitative variables that were different between patients and control subjects and associated with EPCs in a univariate fashion (systolic blood pressure, fasting glucose, and HbA1c). After multiple backward regression analysis, only HbA1c remained significantly associated with EPCs (β = −0.355, P < 0.001) (Supplementary Table 2). Thus, of our initial hypothesis of an association of glucose control and/or inflammation with CPCs/EPCs, there remained only the association of HbA1c with EPC levels from the cross-sectional study.
EPCs during 1 year of follow-up
Overall, EPCs were not different (P = 0.513) at time of inclusion (607 ± 368 per 1 × 106 WBCs) and 1 year later (637 ± 282 per 1 × 106 WBCs) (Supplementary Fig. 2). The baseline characteristics of patients with T1DM at inclusion and 1 year later are depicted in Supplementary Table 1. In association with the 1-year ageing, weight and BMI increased as expected. Apart from these ageing-related parameters, the patients also differed in total cholesterol, LDL cholesterol, VLDL cholesterol, creatinine, and γ-glutamyl transferase. To investigate the associations of changing variables over time, Δ values were calculated: valueat start − valueat end. However, neither Δ of the latter variables was significantly associated with ΔEPCs in a univariate fashion. During the study period of 1 year, the patients did not change in HbA1c overall (7.8 ± 1.2 vs. 7.8 ± 1.2 relative %, P = 0.660). Total insulin dosage significantly increased from 0.78 ± 0.27 to 0.85 ± 0.24 IE/kg body weight/day, P = 0.006. However, changes of CPCs (R = −0.81, P = 0.430) and EPCs (R = 0.022, P = 0.830) were not associated with changes of total insulin dosage. In addition, neither at the time of inclusion nor 1 year later were CPCs and EPCs associated with total insulin dosage (at inclusion: CPCs, R = −0.064, P = 0.434, and EPCs, R = −0.079, P = 0.338; 1 year later: CPCs, R = 0.138, P = 0.179, and EPCs, R = 0.111, P = 0.279).
Association of EPCs with HbA1c during the observation
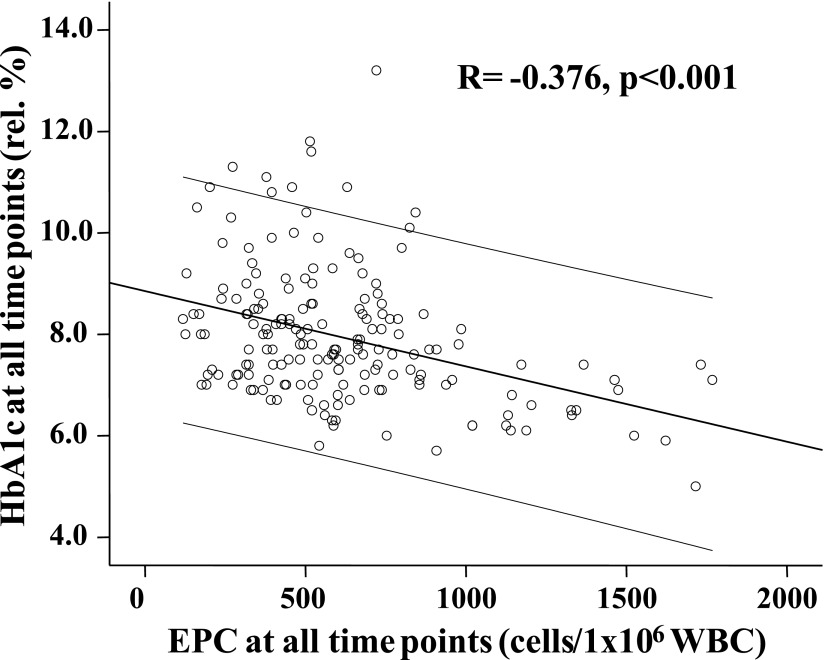
In the next step, we investigated the association of EPCs and HbA1c over time. First, we studied the correlation of all EPC values we had available to all HbA1c values. Since mean HbA1c values were identical at baseline and 1 year of follow-up investigation, all EPC and HbA1c values were analyzed together for analysis for a potential relationship. Figure 2 shows the significant association of EPCs to HbA1c, including HbA1c and EPC values at the beginning and follow-up (R = −0.363, P < 0.001).
Figure 2.
Association of all EPC data with all HbA1c data. An α level of P < 0.05 (two-tailed) is considered statistically significant. Middle line is line of fit. The two additional lines are 95% CIs.
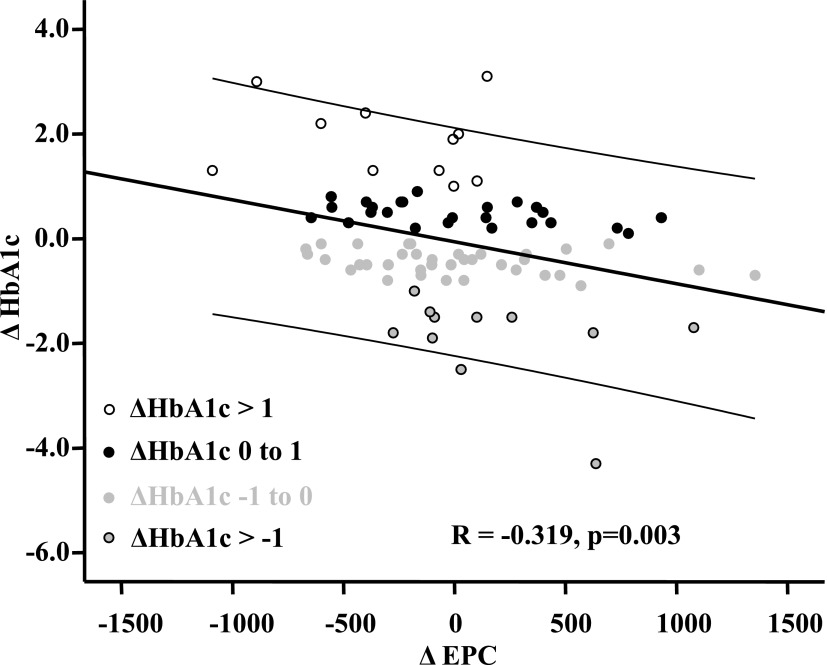
In order to test our results of an association of EPCs and HbA1c in patients and control subjects (n = 190), as well as at the beginning and end of the study in the patients (n = 2 × 97), we investigated the association of ΔEPCs with all Δ variables. Only ΔHbA1c was significantly associated with ΔEPCs in a univariate fashion (β = −0.319, P = 0.003).
Since the EPC and HbA1c values were again tightly associated, we wondered whether changes in HbA1c were not only overall but also individually associated with EPCs. In the patients with T1DM, we observed dramatic changes in HbA1c during the study period (1 year) although the overall HbA1c did not change (Supplementary Table 1). The changes in HbA1c ranged from an increase of 4.3 (maximum) to a decrease of 3.1 (maximum).
In Figure 3, the associations of ΔEPCs with ΔHbA1c are depicted. The changes in patient HbA1c were divided into four groups: 1) an increase in HbA1c >1; 2) an increase in HbA1c between 0 and 1; 3) a decrease in HbA1c between 0 and −1; 4) a decrease in HbA1c greater than −1. In Figure 3, the high increases in HbA1c were associated with strong depressions of EPCs and vice versa. Individual changes of HbA1c and EPC count are presented in Supplementary Fig. 3.
Figure 3.
Association of ΔEPCs with ΔHbA1c. An α level of P < 0.05 (two-tailed) is considered statistically significant. Middle line is line of fit. The two additional lines are 95% CIs.
CONCLUSIONS
This is the first prospective study showing 1) that EPCs are already reduced in children and adolescents with T1DM and 2) an association of changes in glycemic control and EPCs after 1 year of follow-up. EPCs were significantly reduced in children and adolescents with T1DM in comparison with control subjects. EPCs were found to be associated with glucose control but not with systemic inflammation estimated by CRP.
However, CRP was significantly elevated in patients with T1DM versus control subjects. This elevation was found to be associated with BMI and triglycerides. This result was rather surprising because patients and control subjects were virtually identical for weight and BMI (Table 1).
Nevertheless, an association of inflammation (CRP) (34), obesity/overweight (35), and lipids (36) with EPCs has already been demonstrated by others (in adults). We might have missed an association of CRP with EPCs due to our small control. On the other hand, the body at this very young age might be more able to counteract inflammation. Thus, the increase in CRP in our patients might not have a deleterious effect on EPCs, as demonstrated by Verma et al. (34).
In addition, CRP and EPC levels were not influenced in patients or control subjects by comedication, since none of our study subjects were treated with statins (37) or angiotensin II receptor blockers (38).
Some of our results could be related to overall glycemic control. However, for our understanding, the patients in our study were adequately treated, although our study was not powered for glucose control. In comparison with the recently published PedPump Study (39), which demonstrated a mean HbA1c of 8.3 ± 1.4% in adolescents (12–18 years of age), by using CSII therapy, our patients showed a mean HbA1c of 7.8 ± 1.2% throughout the study, and only 21.6% (study start)/28.9% (study end) were treated with CSII therapy.
We did not expect a 46% reduction (95% CI −31 to −62%) of EPCs in the T1DM patients in comparison with control subjects. We assumed a 20% difference between patients and control subjects, believing that in the young age, the overall positive effects of youth might minimize the burden of endothelium dysfunction (8,9) and premature atherosclerosis (6), which has been demonstrated in young adults.
The DCCT/EDIC study has demonstrated that previous intensive treatment and lower HbA1c during the DCCT continue to have beneficial effects during the EDIC on retinopathy and nephropathy for ∼7 years (7,40). This benefit persisted, although the large differences in HbA1c between the two originally randomized groups vanished later (7). Although the DCCT/EDIC study could not yet demonstrate a reduction in cardiovascular/macrovascular events later, it is generally assumed that the “metabolic memory” might also benefit atherosclerosis.
There is evidence that improved metabolic control in patients with diabetes improves EPC number and function (41–43) in adults. In one study (42), insulin treatment for 4 weeks increased EPCs by 65.6 ± 62.7% (P = 0.007).
In mature human endothelial cells, numerous studies have shown that high glucose exerts deleterious effects on the function of those cells and that glucose toxicity can be counteracted by insulin cosupplementation, independent of glucose lowering (43). For EPCs, the possible good and bad of glucose and insulin are less investigated. Marchetti et al. (44) have shown that high glucose affects the intracellular signaling of EPCs, resulting in reduced differentiation and tube formation. In their cell culture experiments, glucose lowering by insulin as well as interaction in the phosphatidylinositol 3-kinase signaling pathway by benfotiamine resulted in the restoration of EPC differentiation.
Since our study has only 1 year of follow-up, we cannot answer whether a reduction in EPCs predicts or is related to future cardiovascular events in T1DM patients. However, EPCs have been demonstrated to be tightly associated with CVD (15,45–47). Likewise, in a prospective follow-up study of 519 patients with coronary heart disease (including 147 patients with diabetes), EPCs predicted the occurrence of cardiovascular events and death from cardiovascular causes over 12 months (15).
A limitation of our study is that we did not perform culture experiments for CPCs and EPCs. However, both the colony-forming unit assay for EPCs (14) as well as the culture from peripheral mononuclear cells of EPCs (27) would require an additional substantial amount of blood taken (at least 40 mL), which is not possible for children and adolescents due to ethical concerns. Human clinical cardiovascular outcome data are primarily based on flow cytometry; likewise the largest study, by sample size, demonstrated a clear impact of EPCs on the reoccurrence of cardiovascular events (15).
In addition, we may have found different cell numbers compared with other groups due to the different antibody conjugations we used. We followed the International Society of Hematotherapy and Graft Engineering guidelines (33) and used anti-CD34 in a strong (reddish) color in contrast to others that used a “weaker” anti-CD34 monoclonal antibody in fluorescein isothiocyanate.
We have recently been able to demonstrate that aberrations of microvascular autoregulation already exist in children and adolescents with T1DM (48) after a rather short duration of diabetes. Thus, macro- and microvascular complications might already have their onset in the early “diabetes career.” Therefore, the resolution of diabetes as best as possible might be the most necessary strategy for preventing individual suffering. Likewise, Petrelli et al. (49) have demonstrated an enhancement of EPCs after pancreatic islet transplantations in patients with T1DM.
Since the recently published observation by Juutilainen et al. (50) demonstrated that T1DM patients appear to have a similar risk of total and cardiovascular mortality in insufficiently controlled hyperglycemia as patients with T2DM, intensive glucose control in young patients with T1DM seems mandatory for preventing micro- and macrovascular disease. Maybe additional treatment with statins and angiotensin II blockers should be investigated in the future.
Since low numbers of EPCs are important predictors of future cardiovascular morbidity and mortality in healthy and T2DM high-risk patients, our observation of a reduction of EPCs already in youth could be relevant for understanding the future high cardiovascular risk of T1DM children, adolescents, and young adults.
The association of EPCs with HbA1c that we observed was significant; however, an R2 of 0.10 suggests that HbA1c and EPCs explain each other’s coefficient of variation for ∼10%. Nevertheless, the association of HbA1c with EPCs was consistent from the analyses of patients and control subjects together over patients at both time points to the Δ association of patients and withstood multivariate regression in all analyses. Thus, the observed significant increase of EPCs in our small group of T1DM children with improved diabetic control within 1 year suggests that the optimization of glycemic control could be relevant in reducing the high CVD burden in these patients.
Acknowledgments
This study was partly funded by the Österreichische Diabetes Gesellschaft 2006 Science Grant, awarded to G.H.S. for the project Endothelial Progenitor Cells in Diabetes. This study was also funded in part by the institutional baseline research budget.
No potential conflicts of interest relevant to this article were reported.
T.H., B.R.-M., M.S., K.N., C.H., F.H., R.K., G.S., E.S., and G.-H.S. all contributed substantially to conception and design, acquisition of data or analysis, and interpretation of data. In addition, all authors were involved in the drafting of the article as well as its critical revision for important intellectual content. All authors provided final approval of the version to be published. G.-H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1206/-/DC1.
References
- 1.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 2.Soedamah-Muthu SS, Fuller JH, Mulnier HE, et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006;29:798–804 [DOI] [PubMed] [Google Scholar]
- 3.Waernbaum I, Blohmé G, Ostman J, et al. Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 2006;49:653–659 [DOI] [PubMed] [Google Scholar]
- 4.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 5.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dogra G, Rich L, Stanton K, Watts GF. Endothelium-dependent and independent vasodilation studies at normoglycaemia in type I diabetes mellitus with and without microalbuminuria. Diabetologia 2001;44:593–601 [DOI] [PubMed] [Google Scholar]
- 10.Devaraj S, Cheung AT, Jialal I, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 2007;56:2790–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes-Virella MF, Carter RE, Gilbert GE, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort Study Group Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 2008;31:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Glynn RJ, Rifai N, et al. Inflammation and progressive nephropathy in type 1 diabetes in the diabetes control and complications trial. Diabetes Care 2008;31:2338–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med 2006;10:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600 [DOI] [PubMed] [Google Scholar]
- 15.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005;353:999–1007 [DOI] [PubMed] [Google Scholar]
- 16.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 17.Brunner S, Schernthaner GH, Satler M, et al. Correlation of different circulating endothelial progenitor cells to stages of diabetic retinopathy: first in vivo data. Invest Ophthalmol Vis Sci 2009;50:392–398 [DOI] [PubMed] [Google Scholar]
- 18.Brunner S, Hoellerl F, Schmid-Kubista KE, et al. Circulating angiopoietic cells and diabetic retinopathy in type 2 diabetes mellitus, with or without macrovascular disease. Invest Ophthalmol Vis Sci 2011;52:4655–4662 [DOI] [PubMed] [Google Scholar]
- 19.Sibal L, Aldibbiat A, Agarwal SC, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009;52:1464–1473 [DOI] [PubMed] [Google Scholar]
- 20.DiMeglio LA, Tosh A, Saha C, et al. Endothelial abnormalities in adolescents with type 1 diabetes: a biomarker for vascular sequelae? J Pediatr 2010;157:540–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palombo C, Kozakova M, Morizzo C, et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc Diabetol 2011;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects [Internet]. Helsinki, Finland, 1964; amended in Tokyo, Japan, 1975; Venice, Italy, 1983; Hong Kong 1989; Somerset West, South Africa, 1996; Edinburgh, Scotland, 2000; Washington, USA, 2002; Tokyo, Japan 2004; Seoul, Korea, 2008. Available from http://www.wma.net/en/30publications/10policies/b3/
- 23.ICH Harmonised Tripartite Guideline for Good Clinical Practice [Internet]. Available from http://www.fda.gov/oc/gcp/guidance.html Accessed 12 December 2012
- 24.Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use [Internet]. Official Journal of the European Communities L/21/34 1.5.2001 and the EudraCT Supporting Documentation updated 04/08/05, both available from http://eur-lex.europa.eu/en/index.htm Accessed 11 December 2012
- 25.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259–263 [PubMed] [Google Scholar]
- 26.Kromeyer-Hauschild K, Wabitsch M, Kunze D, et al. Percentiles of body mass index in children and adolescents evaluated from different regional German studies. Monatsschr Kinderheilkd 2001;149:807–818 [in German] [Google Scholar]
- 27.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis 2008;197:496–503 [DOI] [PubMed] [Google Scholar]
- 28.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 29.Gehling UM, Ergün S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000;95:3106–3112 [PubMed] [Google Scholar]
- 30.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000;95:952–958 [PubMed] [Google Scholar]
- 31.Blann AD, Pretorius A. Circulating endothelial cells and endothelial progenitor cells: two sides of the same coin, or two different coins? Atherosclerosis 2006;188:12–18 [DOI] [PubMed] [Google Scholar]
- 32.Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond) 2011;120:263–283 [DOI] [PubMed] [Google Scholar]
- 33.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I, International Society of Hematotherapy and Graft Engineering The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother 1996;5:213–226 [DOI] [PubMed] [Google Scholar]
- 34.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 2004;109:2058–2067 [DOI] [PubMed] [Google Scholar]
- 35.Müller-Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J 2008;29:1560–1568 [DOI] [PubMed] [Google Scholar]
- 36.Westerweel PE, Visseren FL, Hajer GR, et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy. Eur Heart J 2008;29:2808–2817 [DOI] [PubMed] [Google Scholar]
- 37.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 2001;103:2885–2890 [DOI] [PubMed] [Google Scholar]
- 38.Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension 2005;45:526–529 [DOI] [PubMed] [Google Scholar]
- 39.Danne T, Battelino T, Jarosz-Chobot P, et al. PedPump Study Group Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia 2008;51:1594–1601 [DOI] [PubMed] [Google Scholar]
- 40.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fadini GP, Baesso I, Agostini C, et al. Maternal insulin therapy increases fetal endothelial progenitor cells during diabetic pregnancy. Diabetes Care 2008;31:808–810 [DOI] [PubMed] [Google Scholar]
- 42.Humpert PM, Neuwirth R, Battista MJ, et al. SDF-1 genotype influences insulin-dependent mobilization of adult progenitor cells in type 2 diabetes. Diabetes Care 2005;28:934–936 [DOI] [PubMed] [Google Scholar]
- 43.Federici M, Menghini R, Mauriello A, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002;106:466–472 [DOI] [PubMed] [Google Scholar]
- 44.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes 2006;55:2231–2237 [DOI] [PubMed] [Google Scholar]
- 45.George J, Goldstein E, Abashidze S, et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J 2004;25:1003–1008 [DOI] [PubMed] [Google Scholar]
- 46.Lambiase PD, Edwards RJ, Anthopoulos P, et al. Circulating humoral factors and endothelial progenitor cells in patients with differing coronary collateral support. Circulation 2004;109:2986–2992 [DOI] [PubMed] [Google Scholar]
- 47.Kunz GA, Liang G, Cuculi F, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J 2006;152:190–195 [DOI] [PubMed] [Google Scholar]
- 48.Schlager O, Hammer A, Willfort-Ehringer A, et al. Microvascular autoregulation in children and adolescents with type 1 diabetes mellitus. Diabetologia 2012;55:1633–1640 [DOI] [PubMed] [Google Scholar]
- 49.Petrelli A, Maestroni A, Fadini GP, et al. Improved function of circulating angiogenic cells is evident in type 1 diabetic islet-transplanted patients. Am J Transplant 2010;10:2690–2700 [DOI] [PubMed] [Google Scholar]
- 50.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 2008;31:714–719 [DOI] [PubMed] [Google Scholar]