Abstract
OBJECTIVE
The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) trial showed superiority of metformin plus rosiglitazone (M+R) over metformin alone (M), with metformin plus lifestyle (M+L) intermediate in maintaining glycemic control. We report here treatment effects on measures of body composition and their relationships to demographic and metabolic variables including glycemia.
RESEARCH DESIGN AND METHODS
Measures of adiposity (BMI, waist circumference, abdominal height, percent and absolute fat, and bone mineral content [BMC] and density [BMD]) were analyzed as change from baseline at 6 and 24 months.
RESULTS
Measures of fat accumulation were greatest in subjects treated with M+R and least in M+L. Although fat measures in M+L were less than those of M+R and M at 6 months, differences from M were no longer apparent at 24 months, whereas differences from M+R persisted at 24 months. The only body composition measure differing by race and/or ethnicity was waist circumference, greater in M+R than either M or M+L at both 6 and 24 months in whites. BMD and BMC increased in all groups, but increased less in M+R compared with the other two groups by 24 months. Measures of adiposity (increases in BMI, waist circumference, abdominal height, and fat) were associated with reduced insulin sensitivity and increased hemoglobin A1c (HbA1c), although effects of adiposity on HbA1c were less evident in those treated with M+R.
CONCLUSIONS
Despite differential effects on measures of adiposity (with M+R resulting in the most and M+L in the least fat accumulation), group differences generally were small and unrelated to treatment effects in sustaining glycemic control.
The recent increase in obesity among children and adolescents has been associated with an increased prevalence of type 2 diabetes in this population (1). The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored a multicenter, randomized clinical trial to address treatment and progression of youth-onset type 2 diabetes. The primary objective of Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) was to compare the ability of three treatments to maintain glycemic control. Treatment with metformin plus rosiglitazone (M+R) was superior to metformin alone (M) in preventing loss of glycemic control in youth with type 2 diabetes (2). Metformin plus an intensive lifestyle program (M+L) was not different from M or M+R. Neither BMI at baseline nor BMI over time was a determinant of treatment failure, although BMI (up to 60 months of treatment) increased most in M+R and least in M+L (P < 0.001). Analyses of the differential effects of treatment on fat and lean mass showed that subjects treated with M+R had the greatest increase in fat mass between baseline and 24 months. The current report presents a more in-depth analysis of BMI and other markers of adiposity by treatment group, including subgroup comparisons by sex and race/ethnicity. The analysis addresses whether changes in measures of body composition, including adiposity and bone, were associated with changes in measures of durable glycemic control in response to treatment.
RESEARCH DESIGN AND METHODS
TODAY sample and study design
The TODAY study rationale, design, and methods have been reported in detail (3) and are described briefly. Between July 2004 and February 2009, 699 youth were enrolled that met the following criteria: 10–17 years of age, diagnosed with type 2 diabetes <2 years according to American Diabetes Association (ADA) diagnostic glucose criteria (4), BMI ≥85th percentile, and negative for diabetes autoantibodies (5). Subjects also had to successfully complete a 2–6 month prerandomization run-in period in which they demonstrated mastery of standard diabetes education, were weaned from nonstudy diabetes medications, demonstrated tolerance of metformin up to a dose of 1,000 mg twice daily but no less than 500 mg twice daily, attained glycemic control (HbA1c <8% [64 mmol/mol] monthly for at least 2 months) on metformin alone, and demonstrated adherence to study medication and visit attendance (6). The primary objective was to compare the three treatment arms (M, M+R, and M+L) on time to treatment failure, i.e., loss of glycemic control defined as either HbA1c ≥8% (64 mmol/mol) over a 6-month period or inability to wean from temporary insulin therapy within 3 months after acute metabolic decompensation. After treatment failure, rosiglitazone was stopped, if applicable, and participants continued on metformin plus insulin for diabetes management. The protocol was approved by an external evaluation committee convened by the NIDDK and the institutional review board of each participating institution. All participants provided both informed parental consent and minor child assent.
Anthropometric outcomes
Staff were trained and certified to perform all measures. Height and weight were collected at all visits and waist circumference and abdominal height annually. Height measurements were made using a clinical stadiometer, and weight was measured in duplicate using a Seca scale (model 882; seca, Hanover, MA) with a third measurement obtained if the first measurements differed >0.2 kg. Scales and stadiometers were calibrated at least every 8 weeks, and scale accuracy was checked against 150-lb weights every 4 weeks. BMI was calculated as weight (kg)/height2 (m2). Waist circumference (cm) was measured at the iliac crest during maximum exhalation using a nonstretch, nontension fiberglass Gulick II tape measure positioned horizontally to the floor according to the U.S. Department of Health and Human Services, Public Health Service, National Health and Nutrition Examination Survey III Anthropometric Procedures (http://www.ncbi.nlm.nih.gov/books/NBK2004/). Abdominal height (cm) was measured laterally with the patient supine using a Holtain Kahn abdominal caliper (8). Measurements were taken midabdominally between the right and left iliac crest.
Dual X-ray absorptiometry outcomes
Dual X-ray absorptiometry (DXA) scans were performed using the existing densitometry system at each clinical center (models QDR4500A, Discovery A, Discovery W/Wi, Delphi W, and Delphi A from Hologic Inc., Bedford, MA; models Prodigy and iDXA from GE Lunar Corp, Madison, WI; model DPX-IQ from Lunar Corp, Madison, WI). All scans were performed according to study-specific guidelines for subject positioning standardized across the different DXA systems. DXA quality assurance procedures included phantom cross-calibration and longitudinal monitoring (9,10). Although there are well-known systematic differences in the absolute values of these systems, the change in the Hologic and GE-Lunar body composition values (i.e., relative results to baseline) is comparable because of the demonstrated linear relationship across a wide range of ages for both sexes (11). All scans were analyzed centrally at the TODAY DXA Central Reading Center (University of California at San Francisco) by study-trained personnel using software according to manufacturer guidelines. The software versions for analyzing the scans were Hologic Discovery 12.3 for the Hologic scans, Prodigy 11.4 for the Prodigy and iDXA scans, and GE-Lunar 4.7e for the DPX-L scans. About one-quarter of the DXA scans could not be used in the analysis; scans were invalidated due to either weight and size limitations set by the equipment manufacturers or because a body part (e.g., arm or leg) was completely or partially off the scanner, there was hand-hip overlap, or there was movement during the scan (specific reasons for missing scan data were not recorded during the trial).
Statistical methods
Analyses were performed on data collected at baseline (n = 699), month 6 (n = 596), and month 24 (n = 403), which were the major outcome data collection visits including DXA. Only data collected prior to the occurrence of the primary outcome (failure to maintain glycemic control) were included since management with insulin, which was started after treatment failure, is known to change measures of body composition.
A mixed effects repeated measures analysis that appropriately accounted for the longitudinal data structure was used to analyze change from baseline. The method used all data available for each participant at time points prior to treatment failure. The model included treatment, visit, and treatment-by-visit interaction, with the value of the outcome at baseline as a covariate. Models of bone mineral content (BMC) and bone mineral density (BMD) also adjusted for height and age at the time of visit. If the treatment-by-visit interaction was significant, we examined pairwise treatment differences at 6 and 24 months. Model-adjusted means (least squares means [LSmeans]) and SEs of the change from baseline are reported in the tables and the graphs. Subgroup differences across treatments were modeled, including an interaction term for treatment by sex or treatment by race/ethnicity. Results are given for the three categories of race/ethnicity with acceptable sample size (non-Hispanic black [NHB], Hispanic [H], and non-Hispanic white [NHW]). The relationships between change from baseline insulin sensitivity (1/fasting insulin in μU/mL) or HbA1c and measures of body composition included the baseline value of either insulin sensitivity or HbA1c as a covariate.
All analyses were considered exploratory, with statistical significance defined as P < 0.05 and no adjustment for multiple testing; the study was powered for the primary study outcome only. The Statistical Analysis Software package (SAS, version 9.2, 2008; SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
The TODAY cohort at baseline (7) is described as follows: average age 14.0 (SD 2.0) years, duration of type 2 diabetes 7.8 (SD 5.9) months, mostly pubertal (88.7% Tanner stage 4 or 5), 64.7% female, 32.5% NHB, 39.7% H, 20.3% NHW, 5.9% American Indian, and 1.6% Asian. Almost half of the cohort (n = 319, 45.6%) reached the primary outcome after an average follow-up duration of 3.9 years (range, 2–6.5 years); the M+R combination was superior to M (P = 0.006), and M+L was intermediate but not different from M or M+R (2).
Supplementary Table 1 includes raw means and SDs for the five adiposity measures (BMI, waist circumference, abdominal height, percent, and absolute fat mass) and the two DXA bone measures (BMC and BMD) by study visit (baseline and 6 and 24 months) and treatment group; part A includes output for the overall sample and by sex and part B by racial/ethnic subgroup for NHB, H, and NHW.
The outcomes were moderately to highly intercorrelated: r > 0.8 among BMI, waist circumference, absolute fat mass, and abdominal height; r ≈ 0.5 for percent fat mass with BMI, waist circumference, and abdominal height; r ≈ 0.7 for percent fat mass with absolute fat mass; and r > 0.8 between BMC and BMD. In addition, despite statistical significance, the absolute magnitude was extremely small for most of the body composition measures reported.
Treatment effects on indices of adiposity
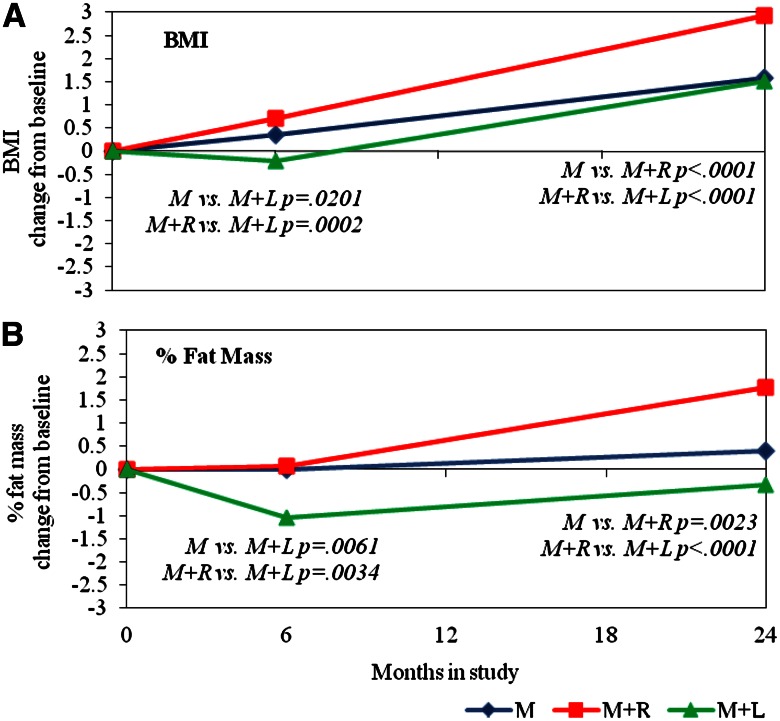
Table 1 includes change from baseline data by treatment group assignment; Fig. 1 shows the trends for BMI and percent fat graphically. All changes in measures of adiposity with treatment, and all differences between treatment groups, even if statistically significant, were small. During the first 6 months of treatment, all measures of adiposity declined in the M+L group but increased slightly in the other groups. Change in the M+L group was significantly lower than in M+R for four of the five measures (BMI, percent fat mass, abdominal height, and absolute fat mass) and lower than in M for three (BMI, percent fat mass, and absolute fat mass); M was not significantly different from M+R. By 24 months, all measures of adiposity had increased relative to baseline in all treatment groups except for percent fat mass in M+L; all changes were significantly greater in M+R than in either M or M+L, and there were no statistically significant differences between M and M+L.
Table 1.
Body composition model-adjusted mean* (SE) change from baseline and significant treatment group comparisons
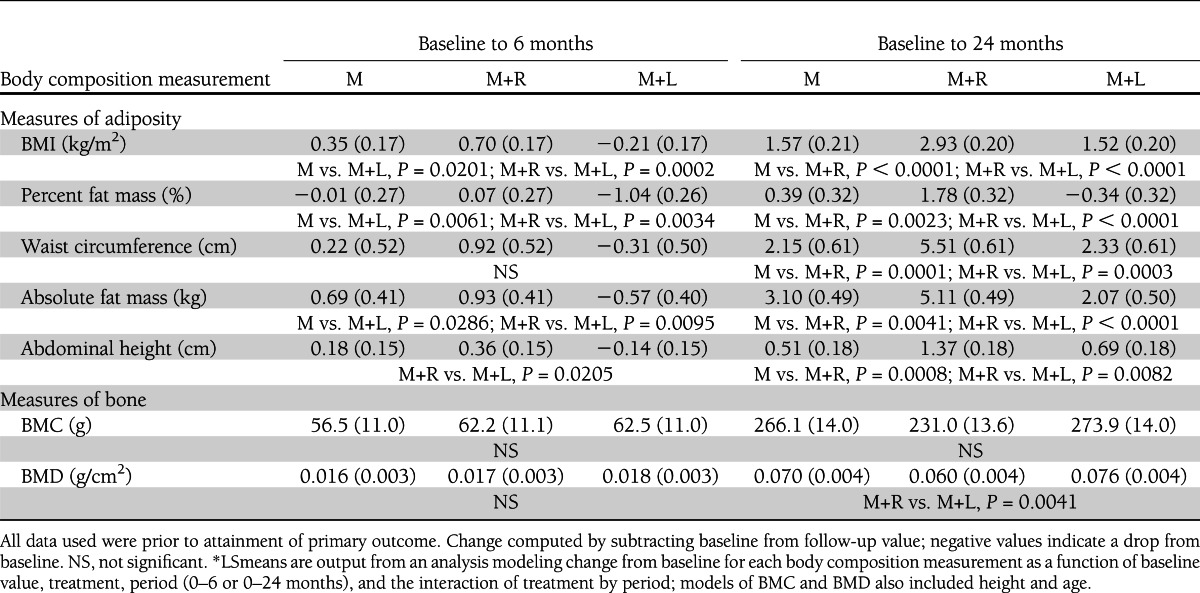
Figure 1.
LSmean change from baseline for BMI (A) and percent fat mass (B) at 6 and 24 months, by treatment. Treatment groups are denoted by M for metformin alone, M+R for metformin + rosiglitazone, and M+L for metformin + intensive lifestyle intervention. LSmeans and P values are output from an analysis modeling BMI and percent fat mass change from baseline (6 − 0 months and 24−0 months) as a function of baseline value, treatment, period (0–6 or 0–24 months), and the interaction of treatment by period. Significant treatment group differences for the 6- and 24-month change from baseline are indicated within the figure.
Treatment effects by sex and race/ethnicity
There was no treatment-by-sex interaction for any adiposity indicator. The only measure of adiposity that showed a significant treatment effect by race/ethnicity was waist circumference (P = 0.0205) (Supplementary Fig. 1). In NHW, the increase in waist circumference for M+R was significantly greater compared with that of M and M+L at both 6 (P = 0.0006 and P = 0.0041) and 24 months (P < 0.0001 and P = 0.0006; M and M+L, respectively). Within subjects treated with M+R, the increase in waist circumference was significantly greater in NHW compared with H at both 6 (P = 0.0404) and 24 (P = 0.0454) months; within subjects treated with M, significant differences between NHW and H (P = 0.0095) and NHW and NHB (P = 0.0106) at 6 months had disappeared by 24 months.
Treatment effects on bone
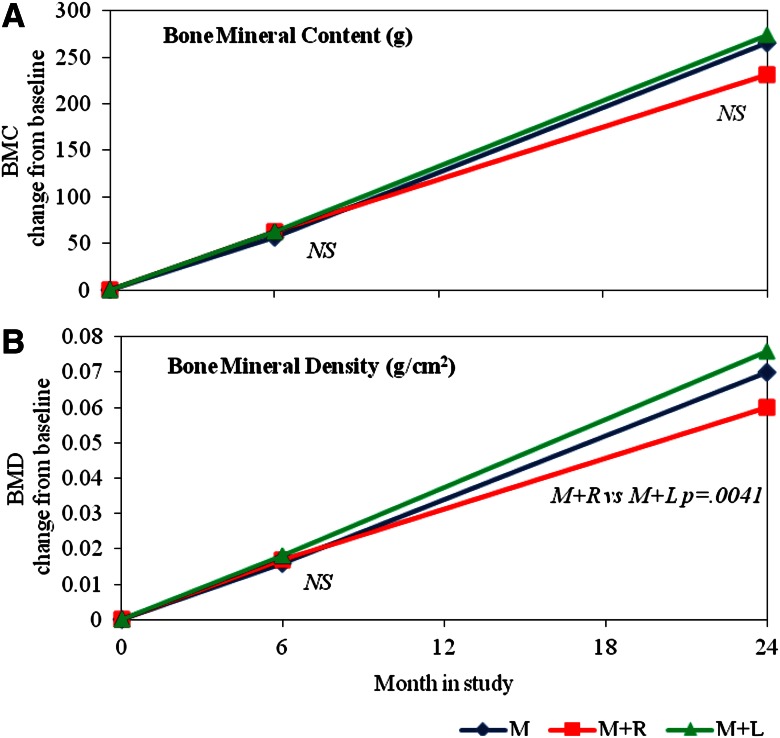
Both BMD and BMC increased from baseline in all groups (Table 1 and Fig. 2), but by 24 months, the increase in BMD in the M+R group was significantly less than that of the M+L group (P = 0.0041). The trend was similar in BMC, although it did not reach statistical significance (P = 0.0670).
Figure 2.
LSmean change from baseline for BMC (g) (A) and BMD (g/cm2) (B) at 6 and 24 months, by treatment. Treatment groups are denoted by M, M+R, and M+L. LSmeans and P values are output from an analysis modeling BMC and BMD change from baseline (6–0 and 24–0) as a function of baseline value, treatment, period (0–6 or 0–24 months), height, and age at the time of visit, and the interaction of treatment by period. Significant treatment group differences for the 6- and 24-month change from baseline are indicated within the figure (nonsignificant differences are indicated by NS).
Associations with insulin sensitivity and HbA1c
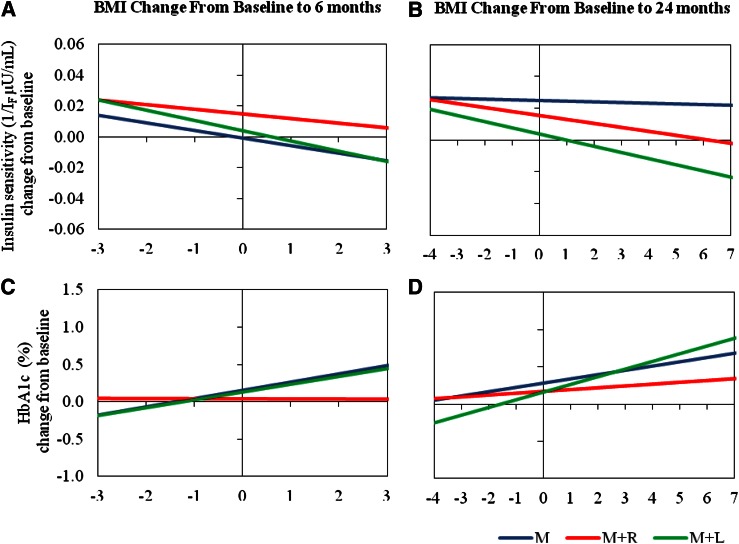
BMI change from baseline (Fig. 3) was associated with significant and consistent changes in a positive direction for HbA1c (as BMI increases so does HbA1c) and in a negative direction for insulin sensitivity (as BMI increases, insulin sensitivity decreases). In general, in the M+R group, fat accumulation had less of an effect on increasing HbA1c compared with the other treatment groups, as treatment with M+R appeared to ameliorate the effect of increased adiposity on HbA1c. As shown in Fig. 3, M+R blunted the effect of adiposity (as indicated by BMI) on HbA1c relative to that seen with M and M+L. This effect was significant at 6 months (P = 0.0109, M+R vs. M+L; P = 0.0253, M+R vs. M) but not at 24 months.
Figure 3.
Regression lines of change from baseline for insulin sensitivity (1/IF µU/mL) versus BMI change, from baseline to 6 months (A) and baseline to 24 months (B); and regression lines of change from baseline for HbA1c (%) vs. BMI change, from baseline to 6 months (C) and baseline to 24 months (D), by treatment. Treatment groups are denoted by M, M+R, and M+L. The horizontal axis scales represent the 5th–95th percentiles of the distribution of BMI change from baseline (−3 to 3 at 6 months and −4 to +7 at 24 months). Note that the 5th–95th percentile range of BMI change from baseline is greater for the 24-month period than for the 6-month one. Slopes indicate change in insulin sensitivity or HbA1c per unit increase in BMI change and were evaluated from models including the baseline value of either insulin sensitivity or HbA1c as a covariate.
CONCLUSIONS
During the design of the TODAY trial, we hypothesized that treatment with M+L would result in the greatest reductions in adiposity, that treatment with M+R would result in the greatest weight gain, and that increased adiposity would be associated with a deterioration in glycemic control. Data presented herein indicate that the treatment group differences reported (2) in the primary outcome analysis (superiority of M+R in sustaining glycemic control compared with M, with M+L intermediate) are not explained by concordant differences in BMI or whole-body adiposity. These data also indicate that treatment with M+R was associated not merely with weight gain but also with more fat deposition than M or M+L treatment. Although treatment with M+L was associated with reductions in most measures of adiposity at 6 months after the start of treatment, these effects generally were modest and no longer detected at 24 months.
It was not surprising that increases in measures of adiposity were observed in the M+R group during the course of treatment, since similar effects of thiazolidinediones (TZDs, the drug class that encompasses rosiglitazone) are well described in adults (12–16). These observations in adults suggest that the beneficial effects of TZDs on glycemia are not mediated through an improvement in insulin sensitivity expected from reduced adiposity. Indeed despite its superiority in maintaining durable glycemic control, treatment with M+R in this study resulted in substantially more adiposity, as indicated by a variety of measures (with an absolute mean increase in BMI of 2.93 kg/m2 in the M+R group over 24 months), compared with treatment with the other modalities. It should be noted, however, that increased weight and fat gain with TZD treatment have not been universally found in children (17–19).
Nor was it surprising that improved indices of body composition in the M+L group were most evident during the first 6 months of treatment, a time of frequent and close contact between the participant and the TODAY lifestyle intervention team. These data are similar to those from other adult and pediatric cohorts in which lifestyle treatment has been shown to be most effective during the initial, high-contact phase (20,21).
It is less clear why the significant improvements in most measures of body composition during M+L did not translate into sustained glycemic control. As noted, the changes in all measures of adiposity were small, and the favorable changes in the M+L group at 6 months were no longer significantly different at 24 months. It is possible that the favorable changes in body composition observed in the M+L group simply were not of sufficient magnitude or duration to be translated into maintenance of glycemic control. Although the amount of weight loss required to effect glycemic control in growing adolescents is not known, the observed losses in measures of adiposity in the M+L treatment group were below thresholds reported to have an effect on HbA1c in the adult literature (22).
BMD and BMC accretion occurred in all treatment groups over time as expected in this age-group (23). Worth emphasizing, however, is the fact that the change in BMD from baseline to 24 months was less in the M+R group than in the M+L group, with a similar but nonsignificant trend seen in BMC. Since TZD treatment has been associated with increased rate of bone loss and fractures (24), it is possible that these relative reductions in BMD, albeit small, may translate over time to reduced bone strength. It is unknown whether TZD effects on bone are confined to the period of treatment, or whether effects continue after TZD exposure is stopped.
Indices of adiposity generally were associated with inverse changes in insulin sensitivity, confirming that adiposity is a key modulator of insulin sensitivity. Indices of adiposity also were associated with concordant changes in HbA1c, effects that could be partially overcome by M+R treatment. Whether these relationships between treatment and adiposity are common to all youth with type 2 diabetes or reflect in some way the extreme adiposity of our cohort (mean 97.7 BMI percentile at baseline) remains unclear.
Several limitations in our data are worth noting. First, all indices of adiposity measured were moderately to strongly correlated; thus it was not surprising that most treatment group effects were consistent for all measures. However, the various measures selected do provide slightly different reflections of adiposity, and this analysis gives no clear indication of selective regional effects. Second, it is well known that visceral adiposity is more predictive of cardiovascular risk and insulin resistance (25,26) than total body fat, and visceral fat was not measured directly in this study. However two surrogate markers (waist circumference and abdominal height) (27–29) were measured and both increased the most in the M+R group. In this regard, our data closely resemble those from A Diabetes Outcome Progression Trial (ADOPT) study in which rosiglitazone treatment was associated with better glycemic control than metformin alone, despite increases in total body weight, body fat, and waist circumference (30). Although speculative, a redistribution of abdominal fat by TZD treatment from visceral to subcutaneous fat depots, as suggested in previous studies (14,31), cannot be discounted. Third, ∼25% of DXA scans were invalid or could not be performed for a variety of reasons, including weight limitations, thus possibly introducing some degree of sampling bias. It is likely, however, that such bias would only have reduced our ability to discriminate between treatment group differences in body composition, especially since the M+R participants were heavier, and thus more likely to have been excluded from DXA measurements because of weight restrictions. Finally, it is important to consider the magnitude of the differences noted in this manuscript. Although statistically significant, the absolute magnitude of all changes reported is very small, which raises the question of biological relevance despite statistical significance in these measures of body composition.
In summary, these findings indicate that the variable times to glycemic failure by treatment group observed in the TODAY trial were not explained by concordant treatment effects on measures of body composition. In the primary outcome analysis, the treatment failure rate for NHB (52.9%) was greater than that for NHW (36.9%) or H (44.8%), with the highest failure rates observed in NHB participants treated with M alone (2). From the current analysis, it is evident that race/ethnicity differences in changes in adiposity measures do not account for these race/ethnicity differences in treatment responses, since the rates of fat accumulation in NHB participants, including those treated with M, were no greater than those of the other race/ethnic groups. It can be concluded that additional factors other than reductions in BMI or adiposity are responsible for some of the differences observed between treatment groups in the TODAY study in maintaining glycemic control. We have shown previously that adherence to treatment did not account for the primary outcome results (2). Future studies, including the long-term follow-up of the TODAY cohort, may help clarify whether the differential effects of diabetes treatment seen in this study are most related to the basic underlying mechanisms of the drugs used, or to genetic, social, biological, or lifestyle factors.
Acknowledgments
This work was completed with funding from the NIDDK of the National Institutes of Health (grants U01-DK-61212, U01-DK-61230, U01-DK-61239, U01-DK-61242, and U01-DK-61254), the National Center for Research Resources (NCRR) General Clinical Research Centers Program (grants M01-RR-00036 [Washington University School of Medicine], M01-RR-00043-45 [Children’s Hospital Los Angeles], M01-RR-00069 [University of Colorado Denver], M01-RR-00084 [Children’s Hospital of Pittsburgh], M01-RR-01066 [Massachusetts General Hospital], M01-RR-00125 [Yale University], and M01-RR-14467 [University of Oklahoma Health Sciences Center]), and the NCRR Clinical and Translational Science Awards (grants UL1-RR-024134 [Children’s Hospital of Philadelphia], UL1-RR-024139 [Yale University], UL1-RR-024153 [Children’s Hospital of Pittsburgh], UL1-RR-024989 [Case Western Reserve University], UL1-RR-024992 [Washington University in St. Louis], UL1-RR-025758 [Massachusetts General Hospital], and UL1-RR-025780 [University of Colorado Denver]).
K.C.C. receives a consulting fee and honorarium as a member of the Steering Committee for a research study conducted by Daiichi Sankyo. No other potential conflicts of interest relevant to this article were reported.
K.C.C., J.H., and K.H. researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. L.E. and M.G. researched data and reviewed and edited the manuscript. L.D. and A.M.K. contributed to the discussion and reviewed and edited the manuscript. T.H.L. contributed to the discussion and wrote, reviewed, and edited the manuscript. L.P. and J.S. researched data, contributed to the discussion, and reviewed and edited the manuscript. L.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan; Pfizer; and Sanofi. The TODAY Study Group also gratefully acknowledges the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
APPENDIX
The members of the writing group are as follows: Kenneth C. Copeland (chair), MD, University of Oklahoma Health Sciences Center; Janine Higgins, PhD, University of Colorado Health Sciences Center; Laure El ghormli, MS, George Washington University Biostatistics Center; Linda Delahanty, MS, RD, Massachusetts General Hospital Diabetes Center; Margaret Grey, PhD, Yale University School of Nursing; Andrea M. Kriska, PhD, Children's Hospital of Pittsburgh; Terri H. Lipman, PhD, Children's Hospital of Philadelphia; Laura Pyle, PhD, George Washington University Biostatistics Center; John Shepherd, PhD, University of California, San Francisco; and Kathryn Hirst, PhD, George Washington University Biostatistics Center.
Footnotes
Clinical trial reg. no. NCT00081328, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2534/-/DC1.
A complete list of the members of the TODAY Study Group can be found in the Supplementary Data online. The members of the writing group are listed in the appendix.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
References
- 1.The Writing Group for the SEARCH for Diabetes in Youth Study Group Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 2.Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeitler P, Epstein L, Grey M, et al. TODAY Study Group Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2005;28(Suppl. 1):S37–S42 [DOI] [PubMed] [Google Scholar]
- 5.Klingensmith GJ, Pyle L, Arslanian S, et al. TODAY Study Group The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010;33:1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laffel L, Chang N, Grey M, et al. ; TODAY Study Group. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland KC, Zeitler P, Geffner M, et al. TODAY Study Group Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouliot MC, Després JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994;73:460–468 [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Mathur AK, Blunt BA, et al. Dual X-ray absorptiometry quality control: comparison of visual examination and process-control charts. J Bone Miner Res 1996;11:626–637 [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Ye K, Mathur AK, Hui S, Fuerst TP, Genant HK. Comparative calibration without a gold standard. Stat Med 1997;16:1889–1905 [DOI] [PubMed] [Google Scholar]
- 11.Shepherd JA, Fan B, Lu Y, et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012; 27:2208–2216 [DOI] [PubMed]
- 12.Gross JL, Kramer CK, Leitão CB, et al. Diabetes and Endocrinology Meta-analysis Group (DEMA) Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann Intern Med 2011;154:672–679 [DOI] [PubMed] [Google Scholar]
- 13.Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007;30:1127–1142 [DOI] [PubMed] [Google Scholar]
- 14.Bell DS, Ovalle F. Long-term glycaemic efficacy and weight changes associated with thiazolidinediones when added at an advanced stage of type 2 diabetes. Diabetes Obes Metab 2006;8:110–115 [DOI] [PubMed] [Google Scholar]
- 15.Nam JS, Nam JY, Yoo JS, et al. The effect of rosiglitazone on insulin sensitivity and mid-thigh low-density muscle in patients with type 2 diabetes. Diabet Med 2010;27:30–36 [DOI] [PubMed] [Google Scholar]
- 16.Sharma A, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue – understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 2007;92:386–395 [DOI] [PubMed] [Google Scholar]
- 17.Narsing Rao L, Jacob JJ, Paul TV, Rajarathinam S, Thomas N, Seshadri MS. Effects of pioglitazone on menstrual frequency, hyperandrogenism and insulin resistance in adoloscents and young adults with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2009;22:91–95 [DOI] [PubMed] [Google Scholar]
- 18.Stone ML, Walker JL, Chisholm D, et al. The addition of rosiglitazone to insulin in adolescents with type 1 diabetes and poor glycaemic control: a randomized-controlled trial. Pediatr Diabetes 2008;9:326–334 [DOI] [PubMed] [Google Scholar]
- 19.Cali AM, Pierpont BM, Taksali SE, et al. Rosiglitazone improves glucose metabolism in obese adolescents with impaired glucose tolerance: a pilot study. Obesity (Silver Spring) 2011;19:94–99 [DOI] [PubMed] [Google Scholar]
- 20.Steinbeck K, Baur L, Cowell C, Pietrobelli A. Clinical research in adolescents: challenges and opportunities using obesity as a model. Int J Obes (Lond) 2009;33:2–7 [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Neiberg RH, Wing RR, et al. Look AHEAD Research Group Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shantha GP, Kumar AA, Kahan S, Cheskin LJ. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ 2012;38:417–426 [DOI] [PubMed] [Google Scholar]
- 23.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007;92:2087–2099 [DOI] [PubMed] [Google Scholar]
- 24.Bilik D, McEwen LN, Brown MB, et al. Thiazolidinediones and fractures: evidence from translating research into action for diabetes. J Clin Endocrinol Metab 2010;95:4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messiah SE, Arheart KL, Lipshultz SE, Miller TL. Body mass index, waist circumference, and cardiovascular risk factors in adolescents. J Pediatr 2008;153:845–850 [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr 2006;148:188–194 [DOI] [PubMed] [Google Scholar]
- 27.Sampaio LR, Simões EJ, Assis AM, Ramos LR. Validity and reliability of the sagittal abdominal diameter as a predictor of visceral abdominal fat. Arq Bras Endocrinol Metabol 2007;51:980–986 [DOI] [PubMed] [Google Scholar]
- 28.Gustat J, Elkasabany A, Srinivasan S, Berenson GS. Relation of abdominal height to cardiovascular risk factors in young adults: the Bogalusa heart study. Am J Epidemiol 2000;151:885–891 [DOI] [PubMed] [Google Scholar]
- 29.Ohrvall M, Berglund L, Vessby B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int J Obes Relat Metab Disord 2000;24:497–501 [DOI] [PubMed] [Google Scholar]
- 30.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 31.Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care 2006;29:510–514 [DOI] [PubMed] [Google Scholar]