Adiponectin (AdpN) is an abundant protein that circulates in blood in microgram quantities. Although AdpN is primarily produced by adipose tissue, the paradox of AdpN is that plasma concentrations decrease with increasing adiposity (1,2). AdpN concentrations are decreased in obesity (3) and obesity-related complications—for example, diabetes, cardiovascular diseases, nonalcoholic fatty liver disease, and in pregnancy (4,5). AdpN exerts pleiotropic actions including potent insulin sensitizing properties. Studies in obesity, type 2 diabetes, and gestational diabetes mellitus (GDM) suggest that the deficiency of AdpN triggers the progression to insulin resistance and the development of metabolic inflammation (recently reviewed in ref. 6). This has encouraged numerous groups to investigate whether AdpN could serve as a systemic biomarker that would predict the risk of diabetes.
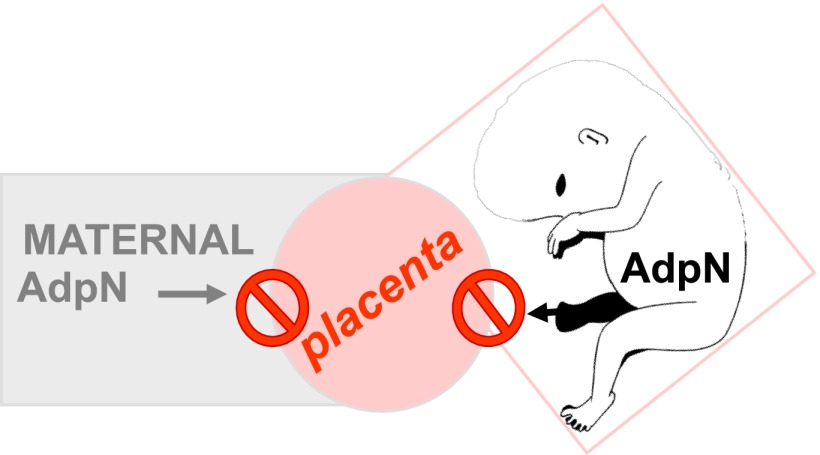
A link between hypoadiponectinemia and reduced insulin action also exists in pregnancy. In healthy women, AdpN levels reach their lowest concentrations in the third trimester when maternal insulin resistance is greatest (5,7). The low AdpN concentrations in the third trimester are further accentuated in GDM (8). Total AdpN and high molecular weight AdpN and the high molecular weight-to-AdpN ratio are inversely related to insulin resistance as estimated by clamp studies in early and late pregnancy (5). Maternal AdpN does not cross the placenta and consequently does not contribute to fetal adiponectinemia (9). Contrary to other cytokines, AdpN is not expressed nor produced by the placenta (10–12). Similar to the nonpregnant state, these characteristics underscore that maternal adipose tissue is the major source of maternal plasma AdpN, which thus can be assessed as an independent maternal biomarker (Fig. 1).
Figure 1.
AdpN is an independent marker of maternal adipose homeostasis. The placenta does not express AdpN, nor does fetal AdpN contribute to maternal circulating AdpN.
In this issue of Diabetes Care, Lacroix et al. (13) evaluate the risk of developing GDM in women with lower plasma AdpN concentrations in early pregnancy. The investigators report on a prospective cohort study of 445 pregnant women, 39 of whom later developed GDM. Decreased systemic AdpN levels in the first trimester had a significant positive correlation with GDM diagnosed in the second trimester. Lacroix et al. propose that AdpN in the first trimester may serve as an early marker of GDM susceptibility. The authors diagnosed GDM in the second trimester using a positive 50-g glucose challenge test (GCT) followed by a 75-g oral glucose tolerance test (OGTT) based on International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria (14). This would only lead to an exclusion of GDM if the GCT was above a defined threshold. A potentially better design for a study of this nature would be to perform a 75-g OGTT in early gestation and at 28 weeks in order to examine the predictors of progression to GDM in those not already meeting the criteria at the time of the first evaluation.
However, previously there have been inconsistencies in trying to establish an association between low AdpN levels and increased GDM risk. While some studies have documented that low AdpN levels are associated with increased GDM risk (15,16), others showed no association (17,18). These discrepancies underscore that association does not necessarily define a biomarker, but require further evaluation such as determining the sensitivity, specificity, and positive and negative predictive value of AdpN to serve as an early biomarker of GDM. Another concern is the high intersubject variability in basal AdpN concentration as reported by Lacroix et al. (13) and other groups (17,19).
It has long been recognized that maternal characteristics and circulating biomarkers are valuable tools in predicting the outcome of metabolically compromised pregnancies. Over the years, clinical practice has proven that fasting glucose and increased maternal prepregnancy BMI are strong predictors for fetal adiposity (20,21). Savvidou et al. (22) recently reported that in the first trimester, advanced maternal age, gestational age, higher BMI, Asian ethnicity, history of GDM, and family history of type 2 diabetes were useful in identifying women who went on to later develop GDM. This combination of factors demonstrated an area under the receiver operating curve of 0.824, which increased to 0.861 with the addition of the metabolic markers HDL cholesterol and tissue plasminogen activator. These results confirm that using a constellation of clinical plus metabolic markers provides a useful tool to predict the risk of GDM utilizing true positive and false positive rates. Of interest is that AdpN concentrations did not improve the model. A recent study by Skvarca et al. (23) reported that concentrations of AdpN, leptin, resistin, visfatin, and RBP4 were also not associated with the degree of glucose tolerance in pregnancy. The discrepancy in these reports may result from both physiological and technical issues. First, cytokine levels are much lower in meta-inflammation than in sepsis. Hence, the circulating concentrations of these adipocytokines or the sensitivity of detection may be too low to detect significant changes. Despite the huge and still growing number of commercial kits based on ELISA techniques, the sensitivity of assays is usually low and varies among vendors. The specificity of the immobilized antibody on the ELISA plate is certainly one major limitation when assaying small numbers of samples.
Given the results of this and other studies, the question is, how do we proceed clinically to better evaluate the risk of GDM in early pregnancy? In the article by Lacroix et al. (13), AdpN concentrations in the first trimester accounted for only 8% of the variance in the Matsuda index of insulin sensitivity in the second trimester. Direct measures of insulin resistance with either a fasting homeostasis model assessment (24) or OGTT using the Matsuda index (25) may more accurately identify the risk of later GDM. However, even direct measures of insulin resistance have limitations since the pathophysiology of GDM is not only a disorder of insulin resistance but also of inadequate insulin response (26). Assuming we have information regarding maternal glucose metabolic status in early pregnancy, how would this information be of clinical use in preventing GDM? Is there a role for pharmacologic preventive therapy? Unfortunately there may be very little we can do during pregnancy to improve β-cell function. Although glyburide has been used in the treatment of GDM, there is limited evidence of improvement of β-cell function during pregnancy (27). Relating to insulin sensitizers, metformin has been used to treat women with GDM but crosses the placenta in significant amounts, and long-term safety has not been demonstrated. Furthermore, metformin treatment of women with polycystic ovary syndrome in early pregnancy did not decrease the risk of later GDM (28).
In conclusion, although low AdpN concentrations may be present in women eventually developing GDM, at this time the use of AdpN may not be of clinical value in predicting GDM as a routine clinical tool. Based on the article by Savvidou et al. (22), however, there are currently simple, inexpensive historical factors that may help us identify women at risk for the development of GDM. While we do not know if any pharmacologic preventive therapy would significantly decrease the risk of GDM or ameliorate some of the adverse outcomes of GDM, lifestyle interventions including a prudent, low simple sugar/saturated fat diet coupled with moderate exercise for the vast majority of women would appear as a reasonable first step. Our challenge is to recognize that GDM, while clinically manifest in mid to late pregnancy, has clinical and subclinical manifestations of metabolic dysfunction prior to conception (i.e., maternal obesity, meta-inflammation, insulin resistance, and impaired β-cell function). A holistic life-course approach of lifestyle interventions before, during, and after pregnancy may be our best and most cost-effective means to treat and more importantly prevent GDM in women as well as improve long-term outcomes for their offspring.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See Lacroix et al., p. 1577
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–26749 [DOI] [PubMed] [Google Scholar]
- 2.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996;271:10697–10703 [DOI] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano PM, Hoegh M, Minium J, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 2006;49:1677–1685 [DOI] [PubMed] [Google Scholar]
- 6.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 2012;11:8–20 [DOI] [PubMed] [Google Scholar]
- 7.Mazaki-Tovi S, Kanety H, Pariente C, et al. Maternal serum adiponectin levels during human pregnancy. J Perinatol 2007;27:77–81 [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care 2004;27:799–800 [DOI] [PubMed] [Google Scholar]
- 9.D’Ippolito S, Tersigni C, Scambia G, Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors 2012;38:14–23 [DOI] [PubMed] [Google Scholar]
- 10.Corbetta S, Bulfamante G, Cortelazzi D, et al. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab 2005;90:2397–2402 [DOI] [PubMed] [Google Scholar]
- 11.Pinar H, Basu S, Hotmire K, et al. High molecular mass multimer complexes and vascular expression contribute to high adiponectin in the fetus. J Clin Endocrinol Metab 2008;93:2885–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazaki-Tovi S, Kanety H, Pariente C, et al. Determining the source of fetal adiponectin. J Reprod Med; 2007;52:774–778 [PubMed] [Google Scholar]
- 13.Lacroix M, Battista M-C, Doyon M, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care 2013;36:1577–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab 2004;89:2306–2311 [DOI] [PubMed] [Google Scholar]
- 16.Georgiou HM, Lappas M, Georgiou GM, et al. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol 2008;45:157–165 [DOI] [PubMed] [Google Scholar]
- 17.Lain KY, Daftary AR, Ness RB, Roberts JM. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf) 2008;69:407–411 [DOI] [PubMed] [Google Scholar]
- 18.Paradisi G, Ianniello F, Tomei C, et al. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol 2010;26:539–545 [DOI] [PubMed] [Google Scholar]
- 19.Jarvie E, Hauguel-de Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119:123–129 [DOI] [PMC free article] [PubMed]
- 20.Catalano PM, Hauguel-de Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011;204:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano PM, McIntyre HD, Cruickshank JK, et al. HAPO Study Cooperative Research Group The Hyperglycemia and Adverse Pregnancy Outcome Study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes 2010;59:3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skvarca A, Tomazic M, Krhin B, Blagus R, Janez A. Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. J Int Med Res 2012;40:583–589 [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 26.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebert MF, Ma X, Naraharisetti SB, et al. Obstetric-Fetal Pharmacology Research Unit Network Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009;85:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–E455 [DOI] [PubMed] [Google Scholar]