Abstract
OBJECTIVE
Increased levels of vascular endothelial growth factor (VEGF) in human plasma samples have suggested that circulating VEGF is a cause of endothelial dysfunction in diabetes mellitus. However, artificial release of VEGF from platelets as a source of VEGF in plasma samples, as also occurs in serum samples, has not been ruled out in these studies.
RESEARCH DESIGN AND METHODS
We determined VEGF levels in plasma collected in both citrate and PECT, a medium that inactivates platelets, in a cross-sectional cohort of 21 healthy subjects and 64 patients with type 1 diabetes. In addition, we evaluated whether VEGF levels in both types of plasma correlated with the presence of diabetes, glycemic control, markers of in vivo or ex vivo platelet activation, and degree of diabetic retinopathy and nephropathy.
RESULTS
VEGF levels were invariably low in PECT plasma of both nondiabetic and diabetic subjects and were unrelated to any other diabetes-related variable studied. In contrast, VEGF levels in citrate plasma were 150% higher in diabetic patients than in control subjects and correlated with diabetes-related variables. Multiple linear regression analysis showed that levels of platelet factor 4, a marker for ex vivo platelet activation, and HbA1c were the independent predictors of VEGF levels in citrate plasma. Platelet activation, in vivo and ex vivo, was similar in diabetic persons and control subjects.
CONCLUSIONS
Like serum, citrate plasma is not suitable for reliable measurements of circulating VEGF. The low levels of VEGF in vivo, as represented by measurements in PECT plasma in our study, do not support a role of circulating VEGF in endothelial dysfunction in type 1 diabetes. Higher levels of VEGF in citrate plasma samples of diabetic persons do not represent the in vivo situation, but mainly originate from higher artificial ex vivo release from platelets correlating with the degree of glycemic control.
Type 1 diabetes mellitus (DM) induces systemic endothelial dysfunction, even before clinical microvascular or macrovascular angiopathies become manifest (1–5). It is characterized by increased vascular permeability and elevated plasma levels of endothelium-derived proteins like von Willebrand factor (vWF) (2,3). Endothelial dysfunction is related to increased glycemia, the most important risk factor for clinical diabetic angiopathies (3–5), but its pathogenesis is unclear.
Vascular endothelial growth factor (VEGF)-A is a potent angiogenic cytokine, released by hypoxic cells, activated platelets, leukocytes, and cancer cells (6–13). It increases vascular permeability in vivo and activates endothelial cells in vitro, leading to protein kinase C activation and release of several proteins for which plasma levels are elevated in vivo due to diabetic endothelial dysfunction (6,14). In cultured endothelial cells, VEGF production is induced by high levels of glucose and advanced glycation end products (15–18). Taken together, VEGF may play an important role in the pathogenesis of endothelial dysfunction (14,16,18). In support of this notion, VEGF plasma levels were found to be higher in diabetic persons than in control subjects (19–22), and a correlation of plasma VEGF levels with both diabetic nephropathy (DN) and proliferative retinopathy has been reported (23–25).
As VEGF is released by activated platelets (7–10), as occurs in serum samples, platelet activation during blood collection could be an artificial source of VEGF in plasma samples as well, but this possibility was not explored in the studies mentioned (19,23,24). VEGF levels in plasma samples may correlate with blood platelet activation at the time of blood sampling rather than represent the steady-state plasma levels of VEGF in vivo. This may explain conflicting results in studies of VEGF plasma levels in DM and other conditions with endothelial dysfunction (14,20–22,26–29).
Artificial ex vivo platelet activation during blood collection procedures can be largely inhibited by using anticoagulants to which a mixture of prostaglandin E1 and theophylline has been added (e.g., PECT medium) (30). When combined with measurements for platelet factor 4 (PF4), a marker for ex vivo artificial platelet activation in blood samples (31), and β-thromboglobulin (β-TG), a marker for in vivo platelet activation (31), PECT plasma allows a more accurate estimation of the actual levels of VEGF circulating in vivo. Using this approach, we have shown in a recent study that VEGF levels in the circulation of patients with metastasized cancer are very low, in contrast to many previous reports that were based on VEGF levels in citrate or EDTA plasma analyses (32).
In the current study, we compared VEGF measurements in blood samples of diabetic persons and control subjects collected in citrate and PECT medium. We investigated: 1) whether VEGF levels found in citrate plasma levels were different from those found in PECT plasma; and 2) whether VEGF levels in citrate and PECT plasma correlate with parameters of diabetes and endothelial dysfunction such as HbA1c levels, glucose levels, vWF levels, or with the presence of diabetic retinopathy (DR) and DN.
RESEARCH DESIGN AND METHODS
Subjects
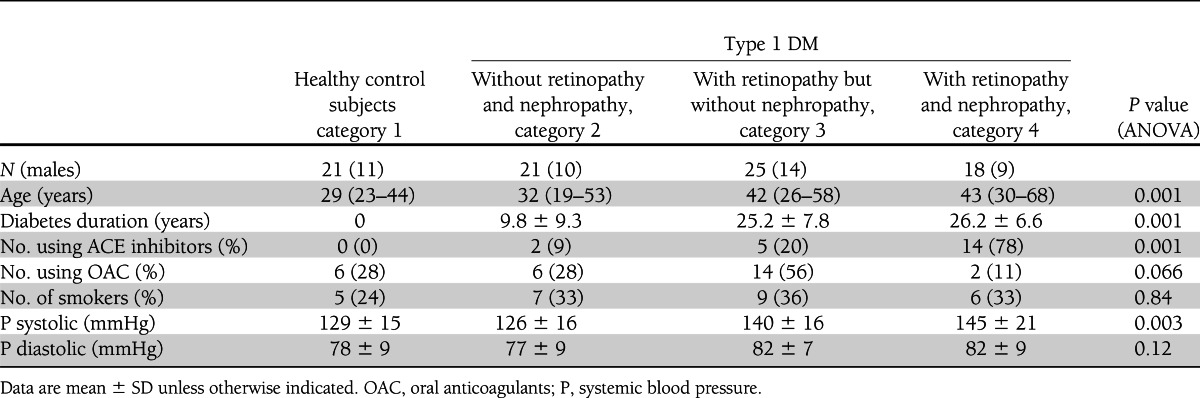
Twenty-one healthy subjects (category 1) and 64 consecutive patients with type 1 DM, without DR and DN (category 2; n = 21), with DR but without DN (category 3; n = 25), or with both DR and DN (category 4; n = 18) participated in the study. Retinopathy was defined according to the definitions of the Early Treatment Diabetic Retinopathy Study Research Group (33); DN was defined as such when albuminuria >30 mg/24 h, when not explained by other causes than DM. The study was approved by the Medical Ethical Committee of our institute, and patients gave their written informed consent (34).
After an overnight fast and abstaining from vigorous physical activity during the previous 24 h, patients presented at the outpatient clinic between 8:00 and 10:00 a.m., bringing their 24-h urine collection for measurement of urinary creatinine and albumin excretion. Demographic and relevant medical history data were recorded: age, sex, diabetes duration, insulin dose, comorbidity, medication, and smoking habits (Table 1). Blood pressure was measured with a sphygmomanometer in the sitting position: the median of three successive measurements was noted. Height and weight were measured. The degree of DR was scored by an experienced ophthalmologist (R.O.S.) by fundoscopic examination and examination of the clinical charts as no DR (A), nonproliferative DR (B), preproliferative DR (C), or proliferative DR (D), and previous panretinal photocoagulation therapy was noted. Demographic and clinical data are given in Table 1 according to the division in categories.
Table 1.
Demographic and clinical parameters according to category
Anticoagulants
From each patient or volunteer, venous blood was taken with a microperfuser (diameter 1 mm; Microflex; Vycon, Ecouen, France) and divided into different tubes. Plastic (polypropylene) blood-collection tubes were filled with 400 µL of a solution containing: prostaglandin E1 (94 nmol/L), Na2CO3 (0.63 mmol/L), EDTA (90 mmol/L), and theophyllin (10 mmol/L) (PECT medium). Blood samples (4 mL) were collected in these PECT tubes in an open system, drop by drop, without using a tourniquet to (maximally) avoid platelet activation ex vivo. Blood was also collected in tubes filled with citrate (BD Vacutainers Systems, Breda, the Netherlands). Blood collected in the PECT and citrate tubes was immediately placed on ice. Platelet-depleted PECT plasma was prepared by spinning for 60 min at 1,700g at 4°C within 1 h after collection. The citrate blood samples were centrifuged within 30 min for 15 min at 1,000g to obtain plasma.
Measurements of metabolic markers
Plasma creatinine levels were measured using an automated spectrophotometrical assay using creatininase, glucose with automated spectrophotometrical assay using glucose dehydrogenase, and glycated HbA1c with high-performance liquid chromatography. In 24-h urine samples, creatinine was measured by the Jaffé method, albumin with an immunonephelometric assay, and renal creatinine clearance was calculated.
Measurements of markers of platelet activation and endothelial dysfunction and VEGF
Platelet count was determined by automated optical scatter detection. Levels of vWF antigen (antibodies from DakoCytomation, Glostrup, Denmark), β-TG (Asserachrom β-TG; Diagnostica Stago, Roche, Almere, the Netherlands), PF4 (Asserachrom PF 4; Diagnostica Stago), and VEGF (R&D Systems, Abingdon, U.K.) were determined by ELISA.
Statistical analysis
Statistical analysis was performed with the computer program SPSS version 12.0 (SPSS, Gorinchem, the Netherlands). Values are given as mean (± SD). Differences between subject categories were analyzed by ANOVA. Comparison of means for variables between non-DM and DM patient groups were performed with the Student t test for unpaired data. Pearson correlation coefficients were calculated between VEGF plasma levels and clinical indicators of microvascular complications, markers of glycemia, and platelet activation. Forward multiple linear regression analysis was performed with VEGF plasma levels as the dependent variable and those determinants that correlated in the univariate analysis with P < 0.10 as independent variables. With multiple comparisons, the level of significance was set at P < 0.01.
RESULTS
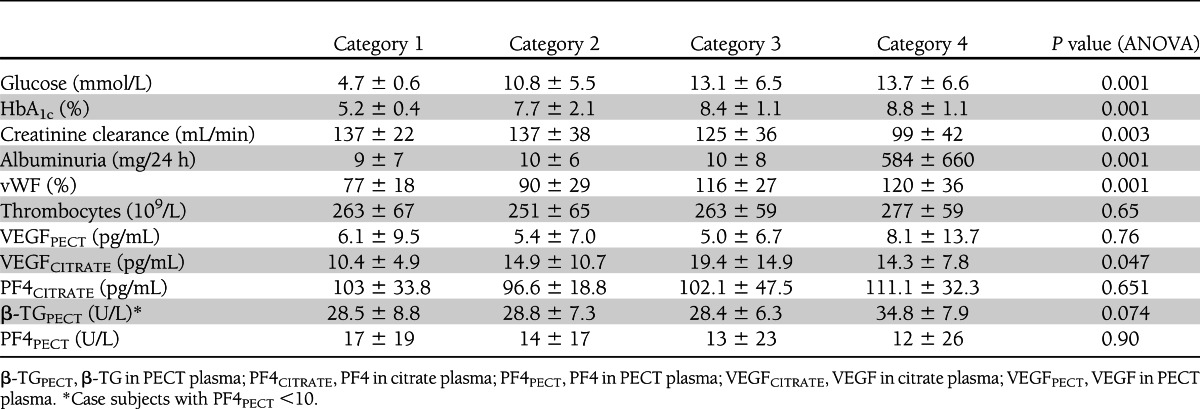
Table 1 shows that microvascular complications were related to duration of DM and age. Systolic blood pressure and use of ACE inhibitors per category increased with microvascular complications. Glycemic control and fasting glucose levels increased with DM categories (Table 2), and creatinine clearance, increasing albuminuria, and increased vWF plasma levels were related with DM and endothelial dysfunction (Table 2).
Table 2.
Metabolic and platelet activation parameters according to category
VEGF levels in blood samples
In PECT plasma, VEGF levels were invariably low in both non-DM and DM subjects [mean ± SD (range): 6.1 ± 9.4 (0–35) vs. 6.1 ± 9.5 (0–45) pg/mL]. VEGF levels in PECT plasma did not correlate with any markers of DM, endothelial dysfunction, in vivo or ex vivo platelet activation, DR, and DN.
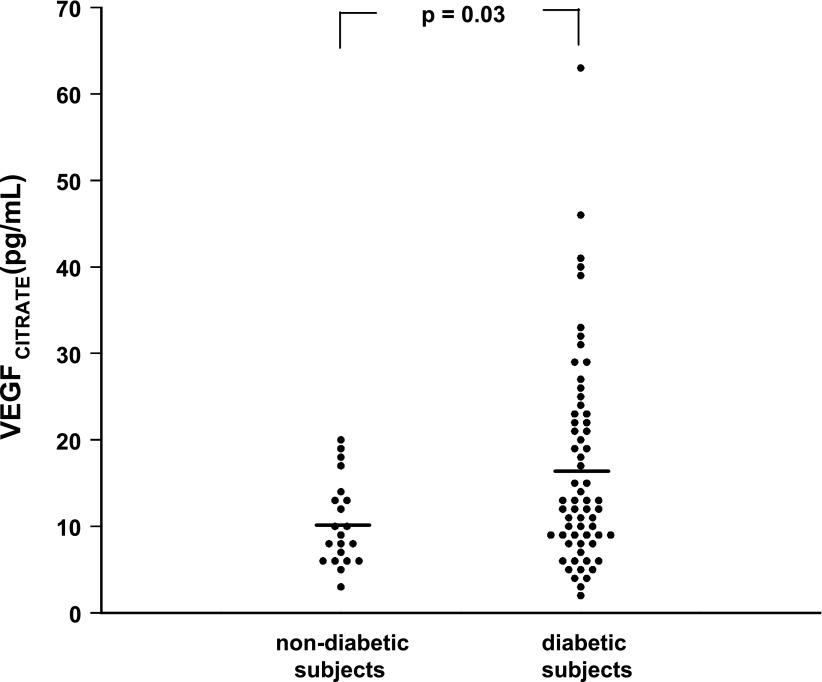
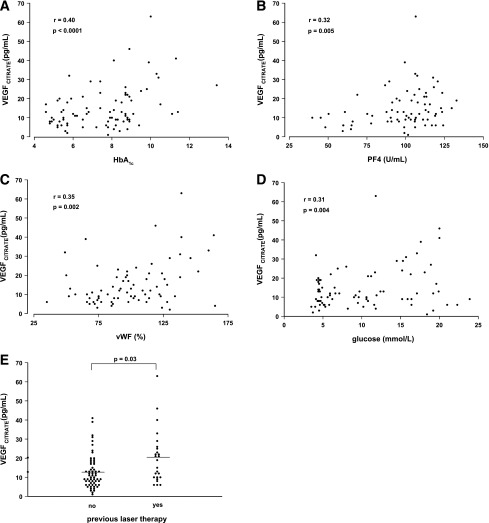
In contrast, VEGF levels in citrate plasma were significantly higher in DM patients as compared with control subjects [16.5 ± 12.0 (1–63) vs. 10.4 ± 4.9 (3–20) pg/mL; P = 0.03; Fig. 1] and correlated positively with HbA1c levels (r = 0.40; P < 0.0001), fasting glucose levels (r = 0.31; P < 0.004), vWF levels (r = 0.35; P < 0.002), PF4 levels (r = 0.32; P < 0.005), DR grade (r = 0.29; P < 0.007), and previous retinal photocoagulation (r = 0.34; P < 0.003) (Fig. 2). VEGF levels in citrate plasma showed no relation with VEGF levels in PECT plasma of the same patients, in vivo platelet activation, platelet count, smoking, blood pressure, or albuminuria.
Figure 1.
Distribution of VEGF levels in citrate plasma (VEGFCITRATE; mean indicated by horizontal lines) in healthy subjects (N = 21) and patients with type 1 DM (N = 64).
Figure 2.
The relationship between VEGF levels in citrate plasma (VEGFCITRATE) and its putative determinants in healthy subjects and patients with type 1 DM: glycated HbA1c (A), PF4 (B), vWF (C), fasting glucose (D), and previous treatment with panretinal laser photocoagulation (E).
Multiple linear regression analysis indicated that only PF4 and HbA1c levels were independent predictors of VEGF levels in citrate plasma (regression equation of citrate plasma levels was VEGF = −7.58 + 1.9 HbA1c + 0.08 PF4; r = 0.47; P = 0.0002). The residual sum of the squares indicated that 22% of the variation in VEGF levels in citrate plasma was explained by HbA1c and PF4 levels in citrate plasma. This indicates that VEGF in citrate plasma samples is derived from ex vivo platelet activation, but also that in patients with poor glycemic control, more VEGF is released ex vivo.
Platelet activation in vivo and in blood samples ex vivo
To further explore the cause of elevated VEGF in citrate plasma samples in type 1 DM, we investigated the contribution of in vivo platelet activation to VEGF levels in plasma. β-TG levels, as a measure of in vivo platelet activation (measured in case subjects without any ex vivo platelet activation as indicated by PF4 level <10 IU), were similar in DM subjects compared with non-DM subjects, indicating that platelet activation in vivo is not altered in type 1 DM.
In citrate plasma samples, PF4 was used as parameter for ex vivo platelet activation. PF4 levels were not higher in DM subjects as compared with non-DM subjects (Table 2), indicating that platelets of DM and non-DM subjects are activated to a similar extent by blood-collection procedures. Taken together, our findings indicate that the higher levels of VEGF in citrate plasma samples in type 1 diabetes are caused by higher release of VEGF by individual platelets upon activation by the blood-harvesting procedure, in particular in patients with poorer glycemic control.
Although not correlated with type 1 DM per se, β-TG levels in PECT plasma correlated positively with albuminuria (r = 0.38; P = 0.005), indicating that DN is associated with increased platelet activation in vivo.
CONCLUSIONS
The most important finding reported in this article is that free VEGF is only present at low levels in the circulation, both in DM and non-DM subjects, when platelet activation is inhibited during blood-sampling procedures by the use of PECT. This argues against a role of circulating VEGF in endothelial dysfunction in type 1 DM. Previous studies have suggested such a role on the basis of VEGF levels found in citrate or EDTA plasma (19,23,24). We demonstrate in this study that the two- to threefold higher VEGF levels in citrate plasma compared with PECT plasma, as were reported in the literature (19,23,24) and confirmed in the current study, originate from artificial ex vivo release of VEGF in plasma samples. The main sources of VEGF in blood are activated platelets and leukocytes (10,11), whereas in PECT medium, platelet activation but not leukocyte activation is inhibited (30). Therefore, our results suggest that activated platelets are the main source of VEGF in citrate plasma samples. The strong correlation between citrate plasma levels of VEGF and PF4, a marker for ex vivo platelet activation (31), confirms this conclusion.
These observations are in line with results from our recent study (32) in cancer patients in whom levels of circulating VEGF, reported by others as a biomarker for angiogenic activity (35), were studied in PECT plasma compared with citrate or EDTA plasma (32). This study indicated that levels of freely circulating VEGF are not elevated in the majority of cancer patients with widespread metastases. Only patients with renal cell carcinoma, a cancer type characterized by excessive VEGF production due to a specific genetic defect, showed elevated circulating VEGF levels. In addition, this study indicated that the elevated plasma VEGF levels in cancer patients previously reported by others can be explained by release from platelets with an increased VEGF content, activated during the blood-harvest procedure (32,36).
As in cancer patients, levels of VEGF in citrate plasma samples were significantly higher in patients with type 1 DM than in non-DM subjects. In multivariate analysis, only HbA1c and PF4 were found to be independent determinants of VEGF levels in citrate plasma. As platelet activation in vivo was not increased in our DM subjects as compared with non-DM subjects, the correlation of VEGF levels with PF4 indicates that ex vivo release of VEGF from platelets activated during blood sample collection is an important source of VEGF in citrate plasma samples. The independent correlation between VEGF and HbA1c, which has not been reported previously, suggests that in type 1 diabetes, poor glycemic control alters individual platelets in such a way that more VEGF is released upon activation. This is in line with our study in cancer, which showed that elevated levels of VEGF in citrate plasma samples could be explained by an increased VEGF content of individual platelets in patients with metastatic cancer (36). We can only speculate what these results mean for the understanding of a possible role of VEGF in endothelial dysfunction in type 1 DM (4,5), but the possibility of higher release of VEGF from individual platelets in tissue capillaries in patients with poor glycemic control should be explored as a potential contributor to endothelial dysfunction in diabetic patients (37).
However, the low levels of VEGF in PECT plasma indicate that efficient mechanisms exist in the circulation to remove or inactivate this potent cytokine (38). One of these may be elevated levels of the soluble VEGF receptor sFLT-1 in DM (21), but platelets may also have a role in this respect. In fact, studies of VEGF in blood in cancer patients not only suggest that platelets are a carrier of VEGF, but also that platelets may act as scavengers of free VEGF in the circulation (8–10,36). Another explanation of our results is therefore that individual platelets in type 1 DM carry more VEGF that has been scavenged after being produced in tissues, possibly induced by high plasma levels of glucose and/or advanced glycation end products (15–18,39). This explanation is supported by our finding and that of others that in patients with proliferative DR, in whom high amounts of VEGF are produced in the eye (6), particularly high VEGF levels were found in citrate plasma (25) but not in PECT plasma and by our observation that increased in vivo platelet activation in patients with established DN was not associated with an increase in VEGF levels in PECT plasma.
In conclusion, 1) the low levels of VEGF in vivo as determined in PECT plasma do not support a role of circulating VEGF in endothelial dysfunction in type 1 DM; 2) platelet activation in PECT plasma (ex vivo) or in vivo is not increased in DM in general, but platelet activation in vivo is associated with DN; 3) VEGF in citrate plasma mainly originates from artificial ex vivo release by activated platelets, and the higher levels previously reported in DM appear due to increased release of VEGF by platelets related to poor glycemic control; and 4) VEGF plasma levels have to be measured while avoiding ex vivo platelet activation.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
R.O.S. initiated the study; contributed to the study design, data collection, and data analysis; and wrote the manuscript. C.J.F.V.N. contributed to the data analysis and reviewed and edited the manuscript. M.J.M.D. initiated the study, contributed to the study design and data collection, and reviewed and edited the manuscript. A.T. contributed to data collection and reviewed and edited the manuscript. J.C.M.M. supervised all of the laboratory work, data collection, and statistic analysis and edited the manuscript. P.K. contributed to the study design, data collection, and analysis and reviewed and edited the manuscript. W.M.W. contributed to the study design and reviewed and edited the manuscript. R.O.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the patients and control participants for help and generosity; the personnel of the Department of Experimental Vascular Medicine, Academic Medical Center; Erna Peters from the Gaubius Laboratory TNO-PG for help in these studies; and Monique Arendse from the Department of Cell Biology and Histology, Academic Medical Center, for help with preparing the manuscript.
References
- 1.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989;32:219–226 [DOI] [PubMed] [Google Scholar]
- 2.Stehouwer CDA, Fischer HR, van Kuijk AWR, Polak BCP, Donker AJM. Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes 1995;44:561–564 [DOI] [PubMed] [Google Scholar]
- 3.Stehouwer CDA, Lambert J, Donker AJM, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res 1997;34:55–68 [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 2009;120:1266–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 2009;335:165–189 [DOI] [PubMed] [Google Scholar]
- 6.Schlingemann RO, van Hinsbergh VW. Role of vascular permeability factor/vascular endothelial growth factor in eye disease. Br J Ophthalmol 1997;81:501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möhle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 1997;94:663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verheul HM, Hoekman K, Luykx-de Bakker S, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res 1997;3:2187–2190 [PubMed] [Google Scholar]
- 9.Banks RE, Forbes MA, Kinsey SE, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 1998;77:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunsilius E, Petzer A, Stockhammer G, et al. Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology 2000;58:169–174 [DOI] [PubMed] [Google Scholar]
- 11.Webb NJ, Myers CR, Watson CJ, Bottomley MJ, Brenchley PE. Activated human neutrophils express vascular endothelial growth factor (VEGF). Cytokine 1998;10:254–257 [DOI] [PubMed] [Google Scholar]
- 12.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003;22:1–29 [DOI] [PubMed] [Google Scholar]
- 13.Siemerink MJ, Augustin AJ, Schlingemann RO. Mechanisms of ocular angiogenesis and its molecular mediators. Dev Ophthalmol 2010;46:4–20 [DOI] [PubMed] [Google Scholar]
- 14.Asselbergs FW, de Boer RA, Diercks GF, et al. Vascular endothelial growth factor: the link between cardiovascular risk factors and microalbuminuria? Int J Cardiol 2004;93:211–215 [DOI] [PubMed] [Google Scholar]
- 15.Stephan CC, Chang KC, LeJeune W, et al. Role for heparin-binding growth factors in glucose-induced vascular dysfunction. Diabetes 1998;47:1771–1778 [DOI] [PubMed] [Google Scholar]
- 16.Tilton RG, Kawamura T, Chang KC, et al. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest 1997;99:2192–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata C, Nakano K, Nakamura N, et al. Advanced glycation end products induce expression of vascular endothelial growth factor by retinal Muller cells. Biochem Biophys Res Commun 1997;236:712–715 [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Kuroki M, Amano S, et al. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest 1998;101:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren M, Elhadd TA, Greene SA, Belch JJ. Elevated plasma vascular endothelial cell growth factor and thrombomodulin in juvenile diabetic patients. Clin Appl Thromb Hemost 1999;5:21–24 [DOI] [PubMed] [Google Scholar]
- 20.Zakareia FA. Correlation of peripheral arterial blood flow with plasma chemerin and VEGF in diabetic peripheral vascular disease. Biomarkers Med 2012;6:81–87 [DOI] [PubMed] [Google Scholar]
- 21.Nandy D, Mukhopadhyay D, Basu A. Both vascular endothelial growth factor and soluble Flt-1 are increased in type 2 diabetes but not in impaired fasting glucose. J Investig Med 2010;58:804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaumdally RJ, Goon PK, Varma C, Blann AD, Lip GY. Effects of atorvastatin on circulating CD34+/CD133+/ CD45- progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med 2010;267:385–393 [DOI] [PubMed] [Google Scholar]
- 23.Hovind P, Tarnow L, Oestergaard PB, Parving HH. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl 2000;75:S56–S61 [PubMed] [Google Scholar]
- 24.Lip PL, Belgore F, Blann AD, Hope-Ross MW, Gibson JM, Lip GY. Plasma VEGF and soluble VEGF receptor FLT-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatment. Invest Ophthalmol Vis Sci 2000;41:2115–2119 [PubMed] [Google Scholar]
- 25.Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol 2012;153:307–313, e2 [DOI] [PubMed] [Google Scholar]
- 26.Abdel Aziz MY, Ben Gharbia O, el-Sayed Mohamed K, Muchaneta-Kubara EC, el Nahas AM. VEGF and diabetic microvascular complications. Nephrol Dial Transplant 1997;12:1538. [DOI] [PubMed] [Google Scholar]
- 27.Burgos R, Simó R, Audí L, et al. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia 1997;40:1107–1109 [DOI] [PubMed] [Google Scholar]
- 28.Sharp PS, Al-Mrayat M, Valabhji J, Kearney TM, Wright D. Serum levels of vascular endothelial growth factor in diabetic subjects: the relationship with blood pressure. Diabetologia 1998;41:984–985 [DOI] [PubMed] [Google Scholar]
- 29.Malamitsi-Puchner A, Sarandakou A, Tziotis J, Dafogianni C, Bartsocas CS. Serum levels of basic fibroblast growth factor and vascular endothelial growth factor in children and adolescents with type 1 diabetes mellitus. Pediatr Res 1998;44:873–875 [DOI] [PubMed] [Google Scholar]
- 30.Levi M, Biemond BJ, van Zonneveld AJ, ten Cate JW, Pannekoek H. Inhibition of plasminogen activator inhibitor-1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation 1992;85:305–312 [DOI] [PubMed] [Google Scholar]
- 31.Kaplan KL, Owen J. Plasma levels of platelet secretory proteins. Crit Rev Oncol Hematol 1986;5:235–255 [DOI] [PubMed] [Google Scholar]
- 32.Niers TM, Richel DJ, Meijers JC, Schlingemann RO. Vascular endothelial growth factor in the circulation in cancer patients may not be a relevant biomarker. PLoS ONE 2011;6:e19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Early Treatment Diabetic Retinopathy Study Research Group Effects of aspirin treatment on diabetic retinopathy. ETDRS report number 8. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98(Suppl.):757–765 [PubMed] [Google Scholar]
- 34.Roestenberg P, van Nieuwenhoven FA, Wieten L, et al. Connective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathy. Diabetes Care 2004;27:1164–1170 [DOI] [PubMed] [Google Scholar]
- 35.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer 2010;102:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson JE, Zurakowski D, Italiano JE, Jr, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012;15:265–273 [DOI] [PubMed] [Google Scholar]
- 37.Witmer AN, Dai J, Weich HA, Vrensen GF, Schlingemann RO. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem 2002;50:767–777 [DOI] [PubMed] [Google Scholar]
- 38.Vuorela-Vepsäläinen P, Alfthan H, Orpana A, Alitalo K, Stenman UH, Halmesmäki E. Vascular endothelial growth factor is bound in amniotic fluid and maternal serum. Hum Reprod 1999;14:1346–1351 [DOI] [PubMed] [Google Scholar]
- 39.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]