Abstract
OBJECTIVE
Inflammatory processes contribute to both diabetes and cardiovascular risk. We wanted to investigate whether circulating concentrations of proinflammatory immune mediators and adiponectin in diabetic patients are associated with incident cardiovascular events.
RESEARCH DESIGN AND METHODS
In 1,038 participants with diabetes of the population-based ESTHER study, of whom 326 showed signs of renal dysfunction, Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for the association of increasing concentrations of C-reactive protein (CRP), interleukin-6 (IL-6), IL-18, macrophage migration inhibitory factor (MIF), adiponectin, and leptin with cardiovascular events (myocardial infarction, stroke, or fatal cardiovascular event) during a follow-up period of 8 years.
RESULTS
During follow-up, 161 subjects with diabetes experienced a primary cardiovascular event. Proinflammatory markers were not associated with a higher risk for primary cardiovascular events in the total study population after adjustment for multiple confounders. However, IL-6 and MIF were associated with cardiovascular events in subjects with renal dysfunction (HR for the comparison of top vs. bottom tertile 1.98 [95% CI 1.12–3.52], P [trend] = 0.10 for IL-6; 1.48 [0.87–2.51], P [trend] = 0.04 for MIF). Adiponectin levels were associated with cardiovascular events in the total population (1.48 [1.01–2.21], P [trend] = 0.03), and the association was even more pronounced in the subgroup with renal dysfunction (1.97 [1.08–3.57], P [trend] = 0.02).
CONCLUSIONS
In particular, the absence of an association between CRP and a U-shaped association of adiponectin levels with incident cardiovascular events show that associations between circulating immune mediators and cardiovascular risk differ between diabetic patients and subjects of the general population.
Increased circulating concentrations of mostly proinflammatory immune mediators reflect inflammation-related processes that contribute to the development of type 2 diabetes and cardiovascular events. The proinflammatory cytokine interleukin-6 (IL-6) and the acute-phase protein C-reactive protein (CRP) are the most frequently investigated markers of subclinical inflammation in the context of cardiovascular risk (1–3). Both markers of subclinical inflammation were reported to predict cardiovascular events in a range of studies (1–3). However, these studies were mainly population based or consisted of individuals with a history of cardiovascular disease, whereas data for large populations of diabetic patients, who are characterized by an increased risk of myocardial infarction and stroke, are relatively rare (4–8). Thus, it is still unclear whether further immune activation in patients with type 2 diabetes, a proinflammatory state itself, additionally contributes to the increased cardiovascular risk of diabetic patients. A previous study indicated that circulating IL-6 and IL-18, but not CRP, are more strongly associated with incident cardiovascular events in individuals with high, compared with low, fasting glucose levels (9).
In addition to reports linking proinflammatory immune mediators and cardiovascular risk, there are also numerous studies on adiponectin in this context. Adiponectin is an adipocyte-derived hormone with anti-inflammatory, insulin-sensitizing, and atheroprotective properties at least in rodents, but this is less clear in humans. In epidemiological studies conducted in the general population, there is usually no significant association or an inverse association between circulating adiponectin concentrations and cardiovascular events or mortality. However, increased adiponectin levels are often associated with increased cardiovascular risk in individuals with certain pre-existing conditions, including comorbidities that are prevalent in diabetic patients such as renal dysfunction and chronic heart failure (10,11). Data on the direction of the association between adiponectin and risk of cardiovascular events in cohorts of diabetic patients are still scarce.
Our study in a large cohort of patients with diabetes had the following aims. First, we wanted to characterize the relationship between proinflammatory immune mediators (CRP, IL-6, IL-18, leptin, macrophage migration inhibitory factor [MIF]), adiponectin, and other cardiovascular risk factors. Second, we wanted to assess the risk for incident primary cardiovascular events with increasing proinflammatory immune mediators and adiponectin. Due to the link of kidney diseases to both immune activation and increased cardiovascular risk, the third aim was to explore these associations in diabetic patients with prevalent renal dysfunction at baseline.
RESEARCH DESIGN AND METHODS
Study population
This investigation is based on the ESTHER study, an ongoing cohort study including 9,949 subjects, 50–74 years of age at baseline. Study participants were recruited by their general practitioners during a routine health check-up between 2000 and 2002 in Saarland, a federal state of Germany (12–14). The distributions of sociodemographic baseline characteristics and common prevalent chronic diseases were similar to the distribution in respective age categories in the German National Health Survey, a representative sample of the German population (12). The ESTHER study was approved by the ethics committees of the Medical Faculty of the University of Heidelberg and the Medical Association of Saarland and is conducted in accordance with the Declaration of Helsinki.
The inclusion criterion of physician-diagnosed diabetes at baseline was met by 1,375 subjects. The exclusion criteria were a diabetes diagnosis prior to the 40th birthday (n = 53; to exclude subjects with potential type 1 diabetes), a missing or inappropriate serum sample (n = 39), self-reported history of myocardial infarction or stroke at baseline (n = 211), and loss to follow-up (n = 34), which left 1,038 individuals for analysis.
Data collection
At baseline, data on height, weight, sociodemographic factors, lifestyle, chronic diseases with date of onset, hormone replacement therapy (HRT), and history and family history of myocardial infarction or stroke were obtained by a standardized questionnaire (12). Systolic blood pressure, HDL cholesterol serum levels, and medical conditions were documented by general practitioners on the standardized health check-up form. They additionally reported currently prescribed drugs on a separate sheet.
Study participants were recorded as diabetic patients if this was documented on the health check-up form by the treating physician or if antidiabetic drugs were prescribed. The same principle was followed to define subjects with hypertension. Renal dysfunction was recorded by micro- or macroalbuminuria (urinary albumin ≥20 mg/L) or chronic kidney disease (glomerular filtration rate [GFR] <60 mL ⋅ min−1 ⋅ 1.73 m−2; GFR = 127.7 [cystatin C (mg/L)]−1.17 age−0.13 [0.91 if female] [1.06 if black]) (15).
Laboratory measurements
Blood samples were taken during the health check-up from 9,834 study participants (8,452 fasting), centrifuged, shipped to the study center, and stored at −80°C until analysis. CRP was measured by immunoturbidimetry with the wrCRP antibody (Bayer, Leverkusen, Germany) on the ADVIA 2400 (Siemens Healthcare Diagnostics, Eschborn, Germany). Serum concentrations of IL-6, MIF, and total adiponectin (i.e., all isoforms of adiponectin) were determined using the Quantikine HS (IL-6) and the Quantikine (MIF and adiponectin) ELISA kits (R&D Systems, Wiesbaden, Germany) (16,17). Leptin and IL-18 serum concentrations were measured simultaneously by bead-based multiplex assay using a Bioplex analyzer (Bio-Rad, Munich, Germany) as described previously (16). Fluorescent xMAP COOH microspheres were purchased from Luminex. Recombinant proteins and antibody pairs were obtained from MBL (Nagoya, Japan) (IL-18) and R&D Systems (leptin). Intra/interassay coefficients of variation for CRP, IL-6, IL-18, MIF, adiponectin, and leptin were 2.4/5.3, 6.7/11.4, 3.3/18.6, 3.1/6.8, 4.7/6.9, and 2.6/15.4%, respectively.
HbA1c was measured from whole-blood samples with high-performance liquid chromatography on the Variant II (Bio-Rad). Total cholesterol and triglycerides were determined from serum samples by a high-performance liquid chromatography method calibrated with the Synchron LX multicalibrator system (Beckman Coulter, Galway, Ireland). Serum cystatin C concentrations and urinary albumin concentration in spot urine samples were measured by immunonephelometry on a Behring Nephelometer II (Siemens Dade-Behring, Marburg, Germany).
Cardiovascular outcomes
Study participants reported on the occurrence of incident myocardial infarctions and strokes in standardized questionnaires at 2-, 5-, and 8-year follow-up. Self-reported cases were validated by standardized questionnaires sent to the study participants’ general practitioners. Information on fatal cases was inquired at a central register of residents’ registration offices of Saarland on 31 December 2010. The vital status and date of death for the deceased could be ascertained for 99.8% of the analyzed cohort of subjects with diabetes. ICD-10 codes for the leading cause of death could be obtained for all deceased subjects with diabetes except one (completeness, 99.5%). All deaths coded with ICD-10 codes I21-I23 and I60-I69 were recorded as myocardial infarctions and strokes, respectively. Furthermore, all deaths coded with ICD-10 code I00-I99 were considered cardiovascular deaths. A combined end point of major cardiovascular events was created from the first occurrence of an incident myocardial infarction, incident stroke, or cardiovascular death. In summary, from 161 incident cardiovascular events in the analysis population, 152 cases (94.4%) were based on physician diagnoses either from validated nonfatal cases or death certificates. The nine self-reported cases, for which validation from physicians could not be obtained, were not excluded because confirmation rates for self-reported myocardial infarctions and strokes in the total ESTHER study were judged to be reliable (70.7 and 81.1% for myocardial infarction and stroke, respectively).
Statistical analyses
Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for the association of increasing proinflammatory immune mediator and adiponectin tertiles with cardiovascular events and to perform a trend test. We used an age- and sex-adjusted simple model and a “fully” adjusted model. Covariates of the full model were chosen from a list of factors potentially associated with cardiovascular diseases and subclinical inflammation by backward selection with P < 0.25 to stay in the model. The list of potential covariates comprised age, sex, smoking status, physical activity, high alcohol consumption, BMI, HbA1c, diabetes duration, systolic blood pressure, GFR, HDL cholesterol, non-HDL cholesterol, and use of insulin, oral antidiabetic drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), statins, HRT, or antihypertensive drugs blocking the renin-angiotensin-aldosterone system (modeled continuously or in the categories shown in Table 1). The final covariates of the full model, associated with cardiovascular events with P < 0.25, were age, sex, smoking status, physical activity, HbA1c, systolic blood pressure, non-HDL cholesterol, GFR, and use of NSAIDs. We additionally adjusted for those variables that did not enter the full model in sensitivity analyses. Dose-response relationships were assessed in fully adjusted Cox models by restricted cubic spline curves with a priori defined knots at the 5th, 33rd, 66th, and 95th percentiles of the distributions of the proinflammatory markers or adiponectin.
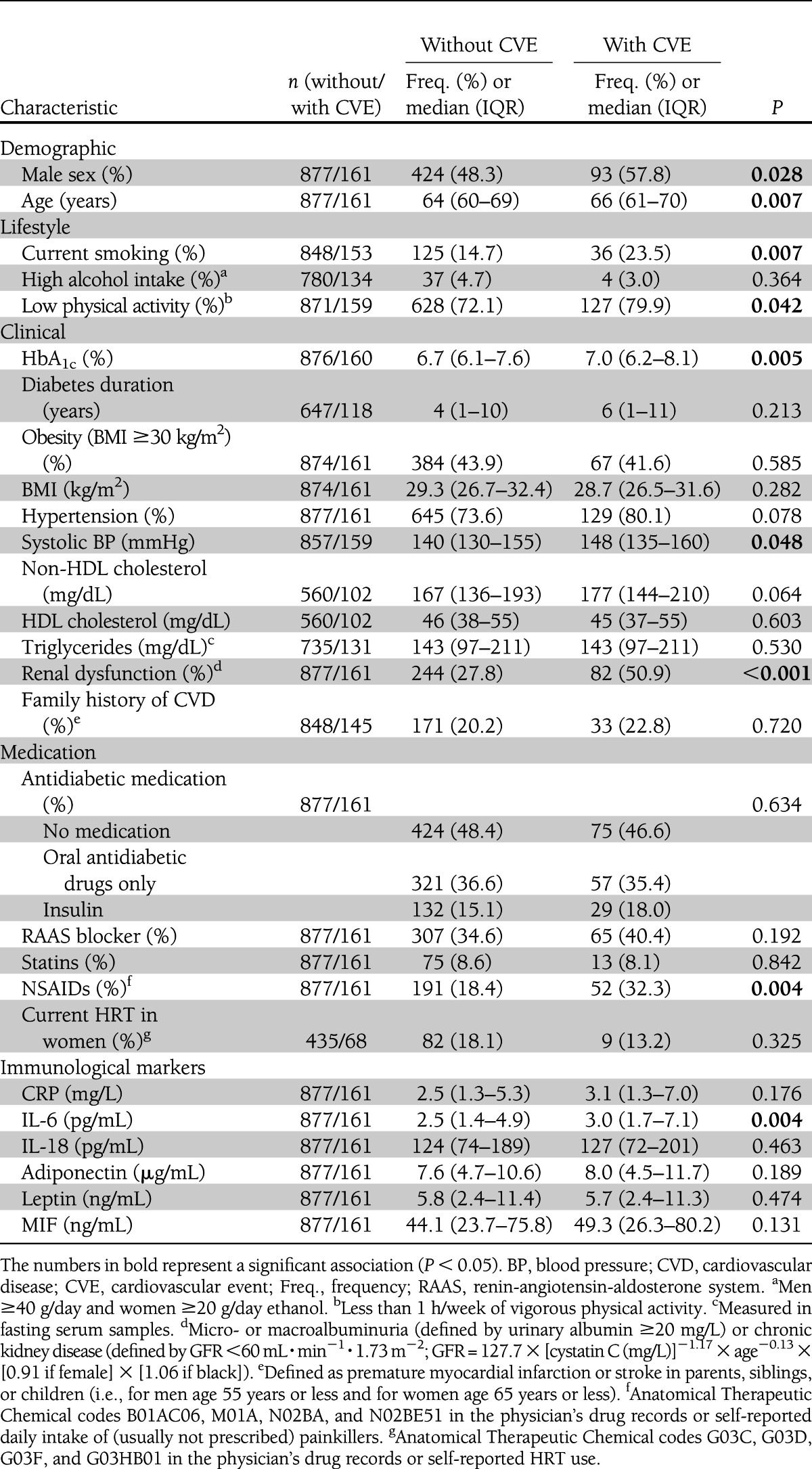
Table 1.
Baseline characteristics of the study population stratified by incident cardiovascular event status
Interactions of the proinflammatory markers and adiponectin among each other and interactions of these biomarkers with the variables of the full model were tested. In the case of interactions among the biomarkers of interest or independent associations of several of the markers with the cardiovascular end point (at least with an HR of 1.20 for the comparison of top and bottom biomarker tertile), we aimed for a joint risk assessment. Due to the strong association between kidney diseases, inflammation, and cardiovascular risk (18,19), all analyses were also repeated in a subgroup of subjects with renal dysfunction.
To adequately deal with missing covariate values, 20 datasets were created by multiple imputation. A detailed description is given in the Supplementary Appendix. All statistical tests were two sided, using an α level of 0.05, and all analyses were conducted with the software package SAS, version 9.2 (Cary, NC).
RESULTS
Correlations and associations of proinflammatory markers and adiponectin with conventional cardiovascular risk factors are shown in the Supplementary Data (Supplementary Tables 1–3).
Risk for cardiovascular events in the total population
During follow-up, 63 study participants with diabetes had an incident primary myocardial infarction (6.1%; incidence rate [IR] = 9.2 per 1,000 person-years [pPY]), 72 had a stroke (6.9%; IR = 10.7 pPY), and 77 had a fatal cardiovascular event (7.4%; IR = 8.4 pPY), leading to 161 subjects who reached the composite cardiovascular end point (15.5%, IR = 23.9 pPY) that only recorded first events in the case of multiple events. Male sex, higher age, current smoking, low physical activity, higher HbA1c, higher systolic blood pressure, renal dysfunction, use of NSAIDs, and higher IL-6 at baseline were significantly associated with incident cardiovascular events (Table 1). CRP, adiponectin, and MIF were slightly (P < 0.2) increased in subjects with incident cardiovascular events, whereas IL-18 and leptin were rather similar in both groups.
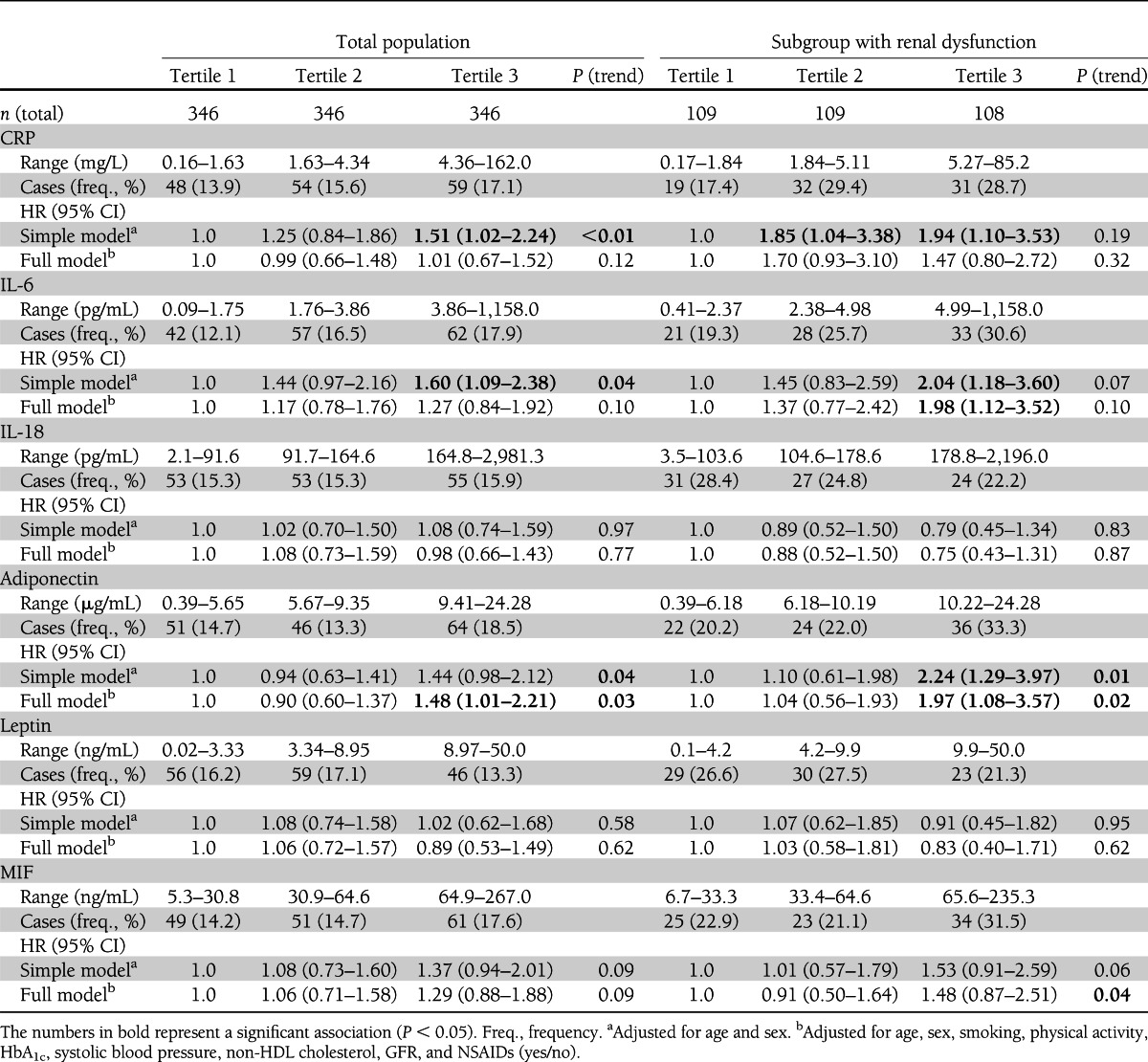
In the total population, fully adjusted Cox regression models showed an ∼1.5-fold increased risk of primary cardiovascular events for subjects in the top tertile of adiponectin levels compared with individuals in the bottom tertile (Table 2). Furthermore, a nonsignificant (about 1.3-fold) increased risk in the top IL-6 and MIF tertiles was observed. IL-18 and leptin tertiles showed no association with cardiovascular events, and the increased risk in the top CRP tertile observed in the simple model did not persist after adjustment for the covariates of the full model.
Table 2.
HRs for cardiovascular events according to immune mediator tertiles in the total population and in subjects with renal dysfunction
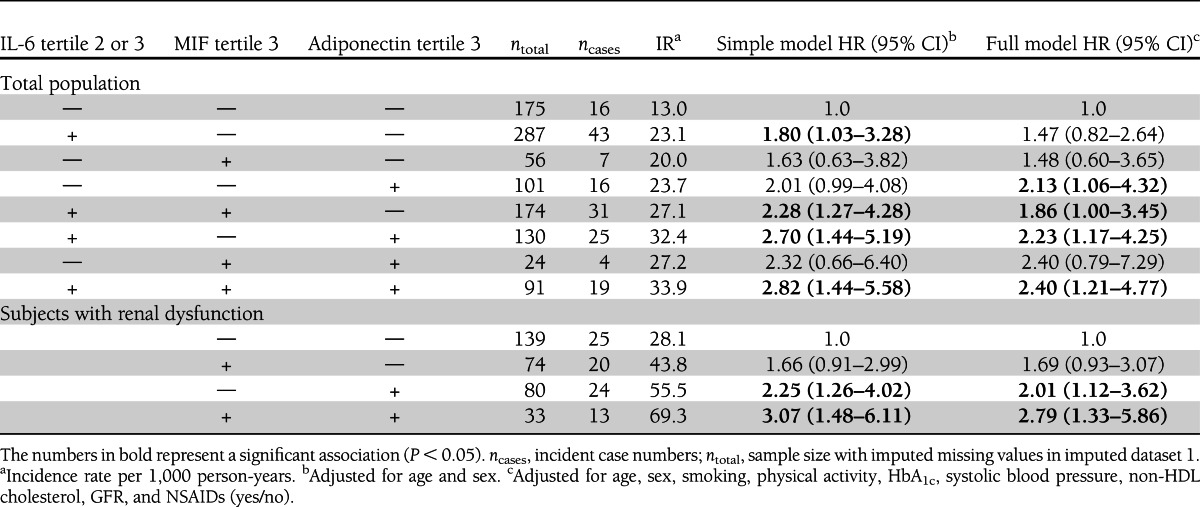
A combined assessment of all proinflammatory markers and adiponectin in the full model showed almost no attenuations in HRs for IL-6, MIF, and adiponectin tertile comparisons, raising the possibility of independent associations of these biomarkers with incident cardiovascular events (data not shown). In joint risk assessment, subjects who were in MIF tertile 3, IL-6 tertile 2 or 3, and adiponectin tertile 3 had a more than doubled risk for cardiovascular events (HR 2.40 [95% CI 1.21–4.77]) compared with subjects that met none of these criteria (Table 3).
Table 3.
Joint risk assessment for incident cardiovascular events of MIF, adiponectin, and IL-6 in the total population and of MIF and adiponectin in subjects with renal dysfunction
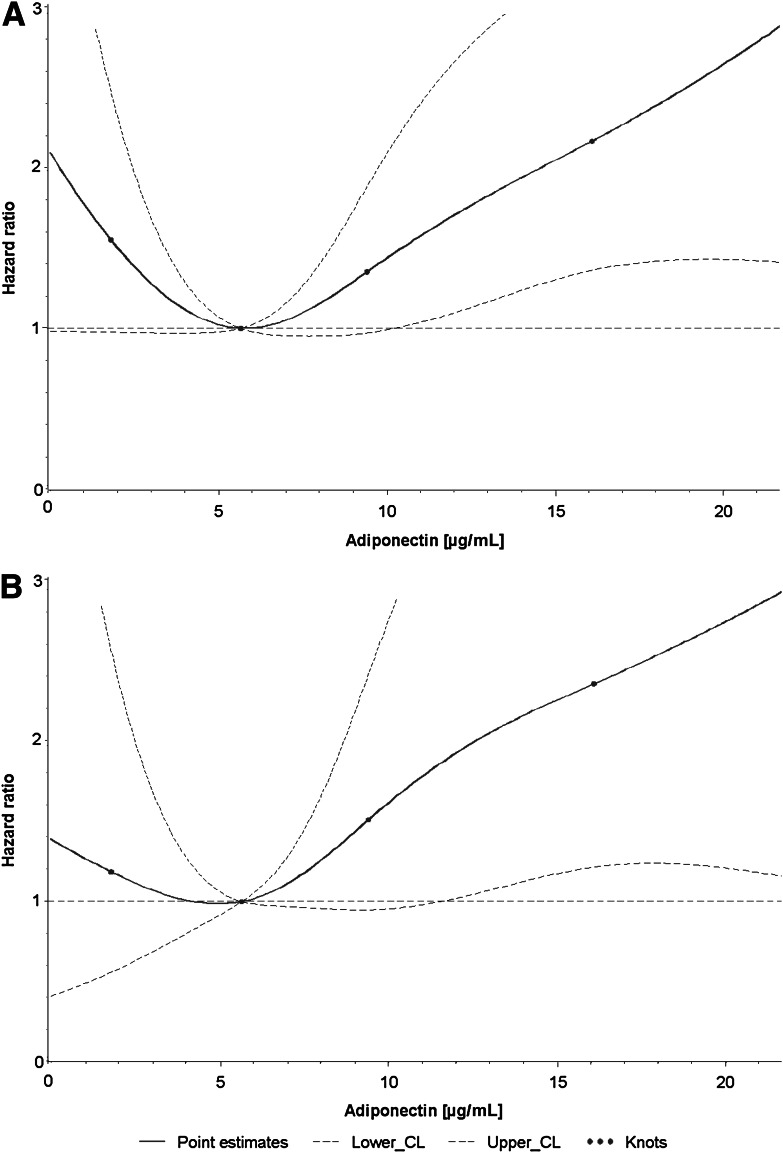
The dose-response relationships between all analyzed proinflammatory markers and incident cardiovascular events were approximately linear. However, there is a noteworthy flattening of the IL-6 dose-response curves at higher serum levels (Supplementary Fig. 1). In contrast, the dose-response curve for adiponectin and cardiovascular event risk showed a U-shaped association in the fully adjusted model (Fig. 1A).
Figure 1.
Dose-response relationship of adiponectin levels with risk for incident cardiovascular events in subjects with diabetes (A) and subjects with diabetes and renal dysfunction (B) in fully adjusted models.
Three significant interaction terms (P < 0.05) of 1) adiponectin and HbA1c levels, 2) leptin levels and systolic blood pressure, and 3) MIF levels and smoking were detected. Subjects with an HbA1c >8% were at higher cardiovascular risk if they were in the top adiponectin tertile (HR for comparison with bottom adiponectin tertile 2.15 [95% CI 1.04–4.44], P [trend] = 0.002) than subjects with an HbA1c ≤8% (1.30 [0.80–2.11], P [trend] = 0.719). Subjects with systolic blood pressure >150 mmHg were at higher cardiovascular risk if they were in the upper two leptin tertiles (HR for comparison with bottom leptin tertile 2.12 [1.01–4.44], P [trend] = 0.120) than subjects who had a systolic blood pressure ≤150 mmHg (0.96 [0.61–1.52], P [trend] = 0.942). Finally, current smokers were at higher cardiovascular risk if they were in the top MIF tertile (HR for comparison with bottom MIF tertile 3.54 [1.27–9.86], P [trend] = 0.025) than subjects who did not smoke (1.02 [0.66–1.57], P [trend] = 0.606).
Risk for cardiovascular events in subgroup with renal dysfunction
In the subgroup of 326 subjects with renal dysfunction, the incidence of cardiovascular events was particularly high: n = 82 (25.2%; IR = 41.7 pPY). In this subgroup, hypertension and higher adiponectin levels were associated with incident cardiovascular events (Supplementary Table 4). Furthermore, CRP, IL-6, and MIF were slightly increased (P < 0.2) among cases, whereas IL-18 and leptin were not.
In subjects with renal dysfunction, the HRs for primary cardiovascular events were more strongly increased (about doubled) in subjects within the top tertile of IL-6 levels and adiponectin levels compared with individuals in the bottom tertile (Table 2). The same pattern was observed for CRP and MIF, with an ∼1.5-fold increased risk in the top tertile, but the estimate did not reach statistical significance except for the trend test for increasing MIF concentrations (P [trend] = 0.04). In agreement with the results from the total population, IL-18 and leptin tertiles showed no association with cardiovascular events. A combined assessment of all proinflammatory markers in the full model showed attenuated HRs for CRP and IL-6 and only adiponectin and MIF remained significantly associated with the outcome in the full model (both P [trend] = 0.01). Subjects who were in tertile 3 of both MIF and adiponectin levels had an ∼2.8-fold increased risk for cardiovascular events compared with subjects that were in lower tertiles (Table 3).
The dose-response relationships between all analyzed proinflammatory markers and incident cardiovascular events were also approximately linear in subjects with renal dysfunction (not shown). However, the U-shaped curve between adiponectin levels and cardiovascular events observed in the total population was not found in subjects with renal dysfunction (Fig. 1B). Instead, a rather J-shaped curve was observed because cardiovascular risk at low adiponectin levels (<5 µg/mL) was only marginally increased.
CONCLUSIONS
In this large cohort of diabetic patients, high adiponectin concentrations were associated with higher risk for primary cardiovascular events after adjustment for multiple confounders, whereas no associations were found for CRP, IL-18, and leptin. A weak association was observed for IL-6 and MIF that was not statistically significant. These associations were more pronounced in a subgroup with renal dysfunction in which results for IL-6 and MIF also reached statistical significance. The aforementioned associations of proinflammatory immune mediators and adiponectin with cardiovascular events were independent from each other and enabled risk stratification by several of these biomarkers to identify patients at particularly high cardiovascular risk. The lack of an association of CRP with cardiovascular events and the association of high adiponectin levels with incident cardiovascular events that was particularly pronounced in subjects with diabetes and renal dysfunction indicated differences from observations from the general population.
Our findings are in line with studies that showed that the association between CRP and cardiovascular risk was much weaker in individuals with high glucose levels or with diabetes than in subjects with lower glucose levels or without diabetes (4,5,9) and with an Italian study that found no association of CRP and cardiovascular disease in diabetic patients without known cardiovascular disease (7). In contrast, CRP was associated with incident cardiovascular events and/or mortality in cohorts of diabetic patients from Korea and Finland (6,8). Furthermore, a large pooled analysis from four U.K. cohorts observed significant associations of CRP with cardiovascular and all-cause mortality in both subjects with and without diabetes, and diabetes was not an effect modifier (20). Our study also included less severe, nonfatal, cardiovascular events, which make the results not directly comparable. Larger, adequately powered studies are required to investigate the association of CRP with myocardial infarction, stroke, and cardiovascular mortality separately in subjects with and without diabetes to shed light on the conflicting results in the literature. We conclude that our study supports the results of the studies that observed an interaction between elevated blood glucose levels and CRP with regard to cardiovascular risk with an attenuation of the association in diabetic patients.
Adiponectin and cardiovascular event risk showed a U-shaped association in our cohort of diabetic patients. The risk was particularly strongly increased at high adiponectin levels if diabetes was poorly controlled (HbA1c >8.0%). These data are novel because most previous studies analyzing this association were population based or based on patients with high cardiovascular risk or pre-existing cardiovascular conditions. The population-based studies observed no statistically significant (21,22) or inverse associations (23,24) of adiponectin with cardiovascular outcomes, which is in line with our finding at low adiponectin levels. In contrast, studies in high-risk populations or with pre-existing cardiovascular conditions reported statistically nonsignificant or positive associations (25–27), which is in line with our finding at high adiponectin levels. Our subgroup analysis in diabetic patients with renal dysfunction, which is a strong predictor of cardiovascular events, also points in this direction and is supported by previous reports from other populations with kidney diseases (28,29). It is known that patients with certain proinflammatory conditions like coronary heart disease, heart failure, and chronic kidney disease have increased adiponectin levels (10,11). This could serve as an explanation for these findings if increased adiponectin levels indicate pre-existing cardiovascular conditions, although they might not be on the causal pathway to cardiovascular events. On the contrary, subjects with well-controlled diabetes without comorbidities do not have increased adiponectin levels. However, these relatively healthy subjects can nonetheless be obese, which is associated with lower adiponectin levels and an increased cardiovascular risk in the general population (24). This mixture of relatively healthy obese subjects with low adiponectin levels and comorbid subjects with high adiponectin levels in our cohort of older subjects with diabetes can explain the observed U-shaped curve of adiponectin levels and cardiovascular events.
In contrast to adiponectin, leptin is a proinflammatory adipokine. Fewer studies for cardiovascular outcomes have been published for leptin than for adiponectin and most of them showed no independent association in general population samples (22). Thus, findings on leptin in our diabetic study population did not deviate from findings in the general population.
The proinflammatory cytokine IL-18 is associated with an increased risk of type 2 diabetes (30), but it is not clear whether it is also associated with incident cardiovascular events. Previous population-based cohorts (31–34) and cohorts with acute coronary syndromes or high-risk populations for cardiovascular diseases (9,35–38) yielded inconsistent results. Increased IL-18 concentrations were associated with a higher risk of cardiovascular events in some studies (9,32,35,36,38), whereas no statistically significant association was evident in others after adjustment for confounders (31,33,34,37). Differences in study characteristics or the definition of cardiovascular outcomes among these studies cannot explain the conflicting results. Neither in our cohort of diabetic patients nor in our subgroup with diabetes and renal dysfunction did IL-18 show a significant association with the incidence of cardiovascular events, which supports the view that IL-18 levels are not associated with cardiovascular outcomes.
Furthermore, there is no consensus about the association of circulating concentrations of the proinflammatory cytokine MIF and cardiovascular events. Population-based studies observed a weak (39) or no association (17) of increasing MIF levels with cardiovascular outcomes. We observed that risk for incident cardiovascular events was weakly, but not statistically significantly, increased in subjects with high MIF concentrations in the total study population, and that risk was significantly increased in the subgroup with renal dysfunction in the fully adjusted model extended by adiponectin. These are the first data for subjects with diabetes from a large population-based cohort. They are supported by the results from a smaller cohort of patients with stable coronary artery disease in which MIF was positively associated with incident coronary events in patients with impaired glucose tolerance or type 2 diabetes but not in those without these conditions (40). Additional studies are warranted that investigate whether MIF contributes to cardiovascular risk independently of other proinflammatory markers in different populations (especially in subjects with diabetes and renal dysfunction) and to explore potential pathways.
We had previously observed and reported a strong association of high IL-6 levels and primary cardiovascular events in diabetic patients in this cohort with a smaller number of events until the 5-year follow up (13). This finding was only replicated with the extended follow-up data in the subgroup with renal dysfunction after adjusting for GFR, which was not included in the model of the previous analysis. Modeling IL-6 as a log-transformed term, as suggested by the dose-response curves, would have yielded a significant association in the subgroup and the total population (P [trend] = 0.03 in both groups). However, formal testing did not reveal a statistically significant deviation from linearity and, therefore, these results are not shown in the main tables. Nevertheless, taken together, our results support suggestions that IL-6 plays a role in the development of cardiovascular diseases in subjects with diabetes.
Besides IL-6, MIF was also only significantly associated with cardiovascular events in subjects with diabetes and renal dysfunction but both were useful for risk stratification in all subjects with diabetes. Subjects who were in the IL-6 middle or top tertile, MIF top tertile, and adiponectin top tertile had a more than doubled risk for cardiovascular events compared with subjects that met none of these criteria. Diabetic patients are per se at an increased risk for cardiovascular events, but IL-6, MIF, and adiponectin could be of value for further risk stratification. The observed interactions of adiponectin with HbA1c and MIF with smoking could especially add predictive value to cardiovascular risk models for subjects with diabetes that contain these conventional cardiovascular risk factors. Overall, the correlations and/or associations of proinflammatory markers and adiponectin among each other and with conventional cardiovascular risk factors were weak, which is another argument for the potential value of IL-6, MIF, and adiponectin in cardiovascular risk assessment in subjects with diabetes. Moreover, the independence of these associations and the interactions seen here are potentially interesting for the pathophysiology of cardiovascular disease and need to be further explored in more mechanistic studies.
The strengths of our study include the selection of diabetic patients from a cohort that is representative for this patient group in Germany, which supports the external validity of the results, its large sample size, the high response rates in follow-up examinations, the detailed assessment of renal dysfunction by serum cystatin C and urinary albumin levels, and the availability of multiple biomarkers of subclinical inflammation. The study also has limitations. The results can only be generalized for older (50–74 years of age) Caucasian adults with type 2 diabetes (only 0.05% of study participants were born in a non-European country). The assumption that subjects who reported disease onset before 40 years of age have type 1 diabetes is imprecise and was chosen due to the lack of autoantibody measurements. Furthermore, we relied on single measurements of biomarkers, whereas serial measurements may have yielded a higher precision (2). Further limitations are that sample size restrictions did not allow myocardial infarction and stroke to be analyzed as separate end points and that we had to rely on BMI to adjust for obesity because data for waist circumference were not available.
In conclusion, in this large cohort of diabetic patients, high adiponectin concentrations were associated with a higher risk for primary cardiovascular events, whereas no significant associations were found for the proinflammatory markers CRP, IL-6, IL-18, leptin, and MIF after adjustment for confounders. However, besides adiponectin, IL-6 and MIF were also strongly associated with cardiovascular events in a subgroup with renal dysfunction. Our data indicate that the associations between circulating immune mediators and cardiovascular risk differ between diabetic patients and subjects of the general population. Furthermore, the associations of increasing IL-6, MIF, and adiponectin levels with cardiovascular events were independent from each other and enabled risk stratification, which has implications for the potential use of these biomarkers in cardiovascular risk models specifically designed for diabetic patients.
Acknowledgments
The ESTHER study was funded by the Ministry of Science, Research, and Arts of the State of Baden-Württemberg (Stuttgart, Germany); the Federal Ministry of Education and Research (Berlin, Germany); and the Federal Ministry of Family Affairs, Senior Citizens, Women, and Youth (Berlin, Germany). The German Diabetes Center is funded by the German Federal Ministry of Health (Berlin, Germany) and the Ministry of Innovation, Science, and Research of the State of North Rhine-Westphalia (Düsseldorf, Germany). This study was supported in part by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD e.V.).
No potential conflicts of interest relevant to this article were reported.
B.S. planned the analyses, performed statistical analyses, and wrote the manuscript. C.H. planned the analyses, obtained funding, supervised laboratory measurements, and wrote the manuscript. D.R. was involved in the design and conduction of the ESTHER study, obtained funding for clinical data used in this study, contributed to discussion, and edited the manuscript. M.R. contributed to discussion and edited the manuscript. H.K. obtained funding, contributed to discussion, and edited the manuscript. H.M. is responsible for the data management of the ESTHER study, contributed to discussion, and edited the manuscript. H.B. is responsible for the design and conduct of the ESTHER study, obtained funding for the ESTHER study, contributed to discussion, and edited the manuscript. B.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Gabi Gornitzka, Ulrike Poschen, and Karin Röhrig (German Diabetes Center) for their excellent technical assistance and Sonja Wolf, Gregor Thal, Tatjana Demtschuk (Saarland Cancer Registry), and Volker Herrmann (German Cancer Research Center) for assistance in conducting the ESTHER cohort study.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1416/-/DC1.
References
- 1.Kaptoge S, Di Angelantonio E, Lowe G, et al. Emerging Risk Factors Collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med 2008;5:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteley W, Jackson C, Lewis S, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med 2009;6:e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curb JD, Abbott RD, Rodriguez BL, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation 2003;107:2016–2020 [DOI] [PubMed] [Google Scholar]
- 5.Best LG, Zhang Y, Lee ET, et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation 2005;112:1289–1295 [DOI] [PubMed] [Google Scholar]
- 6.Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 7.Bruno G, Fornengo P, Novelli G, et al. C-reactive protein and 5-year survival in type 2 diabetes: the Casale Monferrato Study. Diabetes 2009;58:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Kim IT, Park HB, et al. High-sensitivity C-reactive protein can predict major adverse cardiovascular events in Korean patients with type 2 diabetes. J Korean Med Sci 2011;26:1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trøseid M, Seljeflot I, Hjerkinn EM, Arnesen H. Interleukin-18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009;32:486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res 2007;39:1–2 [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Nelson SM. Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J Clin Endocrinol Metab 2008;93:3299–3301 [DOI] [PubMed] [Google Scholar]
- 12.Raum E, Rothenbacher D, Löw M, Stegmaier C, Ziegler H, Brenner H. Changes of cardiovascular risk factors and their implications in subsequent birth cohorts of older adults in Germany: a life course approach. Eur J Cardiovasc Prev Rehabil 2007;14:809–814 [DOI] [PubMed] [Google Scholar]
- 13.Herder C, Schöttker B, Rothenbacher D, et al. Interleukin-6 in the prediction of primary cardiovascular events in diabetes patients: results from the ESTHER study. Atherosclerosis 2011;216:244–247 [DOI] [PubMed] [Google Scholar]
- 14.Schöttker B, Herder C, Müller H, Brenner H, Rothenbacher D. Clinical utility of creatinine- and cystatin C-based definition of renal function for risk prediction of primary cardiovascular events in patients with diabetes. Diabetes Care 2012;35:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 2011;58:682–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murdolo G, Nowotny B, Celi F, et al. Inflammatory adipokines, high molecular weight adiponectin, and insulin resistance: a population-based survey in prepubertal schoolchildren. PLoS ONE 2011;6:e17264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herder C, Illig T, Baumert J, et al. Macrophage migration inhibitory factor (MIF) and risk for coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Atherosclerosis 2008;200:380–388 [DOI] [PubMed] [Google Scholar]
- 18.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004;65:1009–1016 [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M, Muntner P, Lloyd A, et al. Alberta Kidney Disease Network Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012;380:807–814 [DOI] [PubMed] [Google Scholar]
- 20.Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care 2012;35:396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation 2006;114:623–629 [DOI] [PubMed] [Google Scholar]
- 22.Karakas M, Zierer A, Herder C, et al. Leptin, adiponectin, their ratio and risk of Coronary Heart Disease: results from the MONICA/KORA Augsburg Study 1984-2002. Atherosclerosis 2010;209:220–225 [DOI] [PubMed] [Google Scholar]
- 23.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab 2007;92:571–576 [DOI] [PubMed] [Google Scholar]
- 24.Pischon T, Hu FB, Girman CJ, et al. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis 2011;219:322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab 2008;93:1489–1496 [DOI] [PubMed] [Google Scholar]
- 26.Schnabel R, Messow CM, Lubos E, et al. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J 2008;29:649–657 [DOI] [PubMed] [Google Scholar]
- 27.Beatty AL, Zhang MH, Ku IA, Na B, Schiller NB, Whooley MA. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: data from the Heart and Soul Study. Atherosclerosis 2012;220:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2599–2606 [DOI] [PubMed] [Google Scholar]
- 29.Jorsal A, Tarnow L, Frystyk J, et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int 2008;74:649–654 [DOI] [PubMed] [Google Scholar]
- 30.Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes 2005;54:2932–2938 [DOI] [PubMed] [Google Scholar]
- 31.Welsh P, Woodward M, Rumley A, MacMahon S, Lowe GD. Does interleukin-18 or tumour necrosis factor-alpha have an independent association with the risk of coronary heart disease? Results from a prospective study in New Zealand. Cytokine 2010;50:94–98 [DOI] [PubMed] [Google Scholar]
- 32.Blankenberg S, Luc G, Ducimetière P, et al. PRIME Study Group Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2003;108:2453–2459 [DOI] [PubMed] [Google Scholar]
- 33.Koenig W, Khuseyinova N, Baumert J, et al. Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Arterioscler Thromb Vasc Biol 2006;26:2745–2751 [DOI] [PubMed] [Google Scholar]
- 34.Woodward M, Welsh P, Rumley A, Tunstall-Pedoe H, Lowe GD. Do inflammatory biomarkers add to the discrimination of cardiovascular disease after allowing for social deprivation? Results from a 10-year cohort study in Glasgow, Scotland. Eur Heart J 2010;31:2669–2675 [DOI] [PubMed] [Google Scholar]
- 35.Blankenberg S, Tiret L, Bickel C, et al. AtheroGene Investigators Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002;106:24–30 [DOI] [PubMed] [Google Scholar]
- 36.Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Athero Gene Investigators Impact of inflammatory markers on cardiovascular mortality in patients with metabolic syndrome. Eur J Cardiovasc Prev Rehabil 2008;15:278–284 [DOI] [PubMed] [Google Scholar]
- 37.Stott DJ, Welsh P, Rumley A, et al. Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol 2009;38:253–261 [DOI] [PubMed] [Google Scholar]
- 38.Hartford M, Wiklund O, Hultén LM, et al. Interleukin-18 as a predictor of future events in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol 2010;30:2039–2046 [DOI] [PubMed] [Google Scholar]
- 39.Boekholdt SM, Peters RJ, Day NE, et al. Macrophage migration inhibitory factor and the risk of myocardial infarction or death due to coronary artery disease in adults without prior myocardial infarction or stroke: the EPIC-Norfolk Prospective Population study. Am J Med 2004;117:390–397 [DOI] [PubMed] [Google Scholar]
- 40.Makino A, Nakamura T, Hirano M, et al. High plasma levels of macrophage migration inhibitory factor are associated with adverse long-term outcome in patients with stable coronary artery disease and impaired glucose tolerance or type 2 diabetes mellitus. Atherosclerosis 2010;213:573–578 [DOI] [PubMed] [Google Scholar]