Abstract
OBJECTIVE
To examine the effects of baseline and incident diabetes on change in cognitive function over 12 years.
RESEARCH DESIGN AND METHODS
A sample of 1,290 individuals aged ≥40 years at baseline, participating in the Maastricht Aging Study, were cognitively tested at baseline, after 6 years, and after 12 years. Of these, 68 participants had type 2 diabetes at baseline, and 54 and 57 had incident diabetes at the 6- and 12-year follow-up, respectively. Changes in performance on tests of information-processing speed, executive function, and verbal memory from baseline to 6- and 12-year follow-up were compared between groups using linear mixed models. Effects of diabetes on cognitive decline were adjusted for demographic variables, history of smoking, alcohol intake, and comorbid conditions, including hypertension, cardiovascular disease, BMI, and depression.
RESULTS
Participants with baseline diabetes showed larger decline in information-processing speed (estimate −7.64; P < 0.01), executive function (21.82; P < 0.01), and delayed word recall (−1.35; P < 0.05) over the 12-year follow-up compared with control subjects. No significant difference in decline was observed for immediate word recall. Compared with control subjects, participants with incident diabetes showed subtle early decline in information-processing speed only. Interestingly, they did not show larger decline in any other cognitive domain.
CONCLUSIONS
Individuals with baseline type 2 diabetes show accelerated cognitive decline, particularly in information-processing speed and executive function, compared with individuals without diabetes. In incident diabetes, decline in speed becomes detectable first, and cognitive decline seems to increase with increasing exposure time.
Type 2 diabetes is associated with an increased risk of cognitive impairment and dementia (1–3). Mechanisms underlying these associations are not well-understood, but macro- and microvascular disease might play an important role given their prevalence in diabetes (1) and dementia (4). Despite the increased risk of dementia and cognitive impairment, studies into the cognitive trajectories of patients with type 2 diabetes have been inconclusive.
Although some studies showed that cognitive decline in diabetic patients is largely within the range of normal aging (5–7), others have shown a greater cognitive decline in diabetic patients compared with control subjects (8–10). However, in most studies, the observed differences were generally small, and the follow-up duration was relatively short, with an average of 5 years (11). It might be that diabetes-associated cognitive dysfunction takes several years to emerge and that the follow-up durations of most previous studies are too short to detect substantial differences in cognitive decline. In addition, studies focused on baseline diabetes and hence the effect of incident diabetes on cognitive change over time is not adequately addressed. It is important to study this effect to increase the understanding of cognitive changes early in the disease.
Therefore, this study presents 12-year follow-up data from a population-based, prospective cohort study to investigate the effects of baseline and incident type 2 diabetes on decline in several cognitive domains. We hypothesized that 1) participants with baseline type 2 diabetes would show a larger cognitive decline than participants without diabetes; 2) given their shorter exposure time, the rate of decline of those with incident diabetes at follow-up would lie between that of participants with baseline diabetes and those without diabetes; and 3) associations between diabetes and cognitive decline could not be fully explained by risk factors that are potentially related to both diabetes and cognitive function.
RESEARCH DESIGN AND METHODS
Sample
The current study was performed as part of the Maastricht Aging Study (MAAS), a longitudinal study of determinants of cognitive aging (12). A total of 10,396 people were sampled from the Registration Network Family Practices (RNH), a patient register of collaborating general practitioners in the province of Limburg, the Netherlands (13). Patients in the RNH register are considered representative of the Limburg and Dutch population with respect to demographic characteristics (age, sex, and educational level) (12). Of these 10,396 people, 4,490 (43.2%) were willing to participate, 3,531 (34%) refused participation, and 2,375 (22.8%) did not respond to the written request. Medical exclusion criteria for baseline assessment were defined as active or inactive medical conditions in the RNH problem list that could interfere with normal cognitive function, including coma (only active), cerebrovascular pathology, a tumor of the nervous system, congenital malformation of the nervous system, multiple sclerosis, Parkinsonism, epilepsy (all types), dementia, organic psychosis, schizophrenia, affective psychosis, and mental retardation. Before participation in MAAS, all 4,490 participants were screened by telephone for the following medical conditions not documented in the RNH database: history of transient ischemic attacks, brain surgery, hemodialysis for renal failure, electroconvulsive therapy, and regular use of psychotropic drugs. Based on this interview, 301 participants were excluded. Of the remaining 4,189 participants, 1,823 (43.5%) were randomly selected, stratified for age (12 discrete age groups from 24–81 years), sex, and level of general ability (low/high) and equally distributed over four demographically identical test panels. The local ethics committee approved the study, and all participants gave informed consent. At baseline, these 1,823 participants underwent a comprehensive assessment of medical status, lifestyle, and anthropomorphic and cognitive functioning measures. The baseline assessment took place from 1993–1996 and was repeated 6 and 12 years after baseline. Only participants aged ≥40 years were included in the current study in order to make the sample more homogeneous. In addition, we excluded four participants with type 1 diabetes. The final study sample therefore consisted of 1,290 participants.
Diabetes status
Presence of type 2 diabetes was based on a diagnosis made by a physician (as reported by the study participant) and current medication use for diabetes reported in a questionnaire. Diagnosis of type 2 diabetes based on self-report has previously been used in large cohort studies (10,14) and has been shown to be a reliable estimate of the actual prevalence of diabetes (15). To make a distinction between type 1 and type 2 diabetes, participants were considered to have type 2 diabetes when they used oral antidiabetic drugs, started using insulin at or after the age of 40 years, or were diagnosed with diabetes at or after the age of 40 years. Diabetes status was assessed at baseline and at the 6- and 12-year follow-ups.
Covariates
Several potentially health-related conditions that might influence the association between type 2 diabetes and cognitive change over time were taken into account. These included self-report of present or past history of smoking (yes/no), alcohol intake (according to World Health Organization guidelines, less/more than 21 standard consumption per week for females, less/more than 35 consumptions per week for males) (16), a history of cardiovascular disease (yes/no) as reported in a questionnaire, and BMI (weight in kg/length in m2). Blood pressure was measured three times at 5-min intervals on the left arm using an automatic recording device (Critikon Dinamap 8100; Critikon, Tampa, FL). Hypertension (yes/no) was defined as a mean systolic blood pressure of ≥140 mmHg, a mean diastolic blood pressure of ≥90 mmHg (17), or current use of antihypertensive medication as reported in a questionnaire. Depressive symptoms were assessed with the 16-item depression subscale of the revised Symptom Checklist-90 (score range 16–80) (18). Apolipoprotein (APOE) genotyping was determined on genomic DNA extracted from EDTA-anticoagulated blood using a PCR technique (19). The presence (yes/no) of the APOE ε4 (APOE-e4) risk allele, which is associated with an increased risk of dementia (20), was used in the current study. Furthermore, age, sex, and educational level [ranging from primary education, 1, to university degree, 8] (21) were included as demographic covariates. Three groups were made for educational level: low (levels 1 to 2), middle (levels 3–5), and high (levels 6–8). Hypertension, a history of cardiovascular disease, BMI, and depression were measured at baseline and each follow-up assessment.
Assessment of cognitive function
Cognitive function was measured at baseline and the 6- and 12-year follow-ups with a battery of neuropsychological tests administered by psychologists and trained test assistants. An a priori selection of these neuropsychological tests was used to assess cognitive function in the current study. The Visual Verbal Learning Test (22) was used to assess verbal memory. In this test, 15 nonrelated monosyllabic words are presented in five subsequent trials on a computer screen, followed by a recall phase immediately after each trial and a delayed recall phase 20 min after the test. The total number of correctly reproduced words in five trials was measured (immediate word recall), together with the number of correctly reproduced words 20 min after the last trial (delayed word recall). The Concept Shifting Test (CST) (23) was used to measure executive function. In three trials, the participants have to cross out as fast as possible 16 digits in ascending order (CST part A), 16 letters in alphabetical order (CST part B), and finally 8 digits and 8 letters in alternating order (CST part C). The shifting score is calculated by subtracting the average time needed to finish part A and B from the time needed to finish part C. The Letter Digit Substitution Test (24) was used to assess information-processing speed. Participants were instructed to match digits to letters, according to a key of letter/digit combinations at the top of the sheet, as quickly as possible within 90 s. The Mini–Mental State Examination (MMSE) (25) broadly assesses several domains of cognitive functioning and was used to measure global cognitive functioning.
Statistical analysis
Differences between group characteristics at baseline were tested using independent sample t tests for quantitative variables (age, BMI, depression score, MMSE score, and baseline scores on cognitive tests), χ2 tests for qualitative variables (sex, history of cardiovascular disease, hypertension, history of smoking, alcohol intake, and APOE-e4 allele), and the Mann-Whitney U test for the ordinal variable educational level. Linear regression was used to estimate the effect of baseline type 2 diabetes on cognitive performance at baseline. Next, separate linear mixed models (random-effects models) were run to measure the crude and adjusted effect of baseline diabetes on change in cognition from baseline to 6-year (F1) and to 12-year follow-up (F2). An advantage of mixed models over ANOVA with repeated measures is that it models time trajectories without restricting analyses to study completers. The model included terms for a random intercept and a random slope. An unstructured covariance structure provided the best model fit. Terms for the fixed effects included the intercept, baseline diabetes, age, age2 (in order to control for nonlinear trends), sex, educational level, baseline history of smoking, baseline alcohol intake, hypertension, history of cardiovascular disease, BMI, depression score, type 2 diabetes, time (F1, F2), as well as the interaction terms between F1 and diabetes and F2 and diabetes. APOE-e4 allele status (risk allele present/absent) was included as a covariate in an additional analysis, as this was only measured in a subsample.
A second set of linear mixed models tested the crude and adjusted effect of incident type 2 diabetes on change in cognitive performance over time, excluding participants with baseline diabetes. This yielded three groups for comparison (1, no diabetes [reference]; 2, incident diabetes at F1; and 3, incident diabetes at F2). Hypertension, past or present cardiovascular disease, BMI, and depression score were now treated as time-dependent covariates, thereby allowing them to covary with diabetes status over time. To test whether loss to follow-up could be predicted by the observed variables, a logistic regression with lost to follow-up (yes/no) as a dependent variable was performed. A P value <0.05 (two-sided) was considered statistically significant. All analyses were done in IBM SPSS Statistics 19 (SPSS, Chicago, IL).
RESULTS
At baseline, 1,290 participants (aged 40.0–82.7 years) were tested, of whom 68 (5.3%) had diabetes. At F1, 925 (75.7%) participants without type 2 diabetes at baseline and 41 (60.3%) participants with diabetes at baseline were still in the study. At F2, the sample sizes were 761 (62.3%) and 21 (30.9%) for the two groups, respectively. The mean follow-up time was 8.7 years. Of the 1,222 participants without type 2 diabetes at baseline, 54 (4.4%) participants developed type 2 diabetes between baseline and F1, and 57 (4.7%) participants developed type 2 diabetes between F1 and F2. Having type 2 diabetes at baseline, being older, having a history of smoking, cardiovascular disease, higher BMI, or lower baseline MMSE score increased the risk of study dropout.
Baseline differences
Baseline characteristics stratified by diabetes status are shown in Table 1. Compared with participants without diabetes, participants with diabetes were older, had a lower educational level, had a higher BMI, were more likely to have hypertension and a history of cardiovascular disease, and performed worse on all cognitive measures. These between group differences in cognitive performance were still significant after adjustment for demographic variables, history of smoking, alcohol use, and comorbid conditions. There were no statistically significant differences in sex, alcohol intake, history of smoking, presence of APOE-e4 allele, and depression score between groups.
Table 1.
Baseline characteristics of the study group, stratified by diabetes status at baseline and follow-up
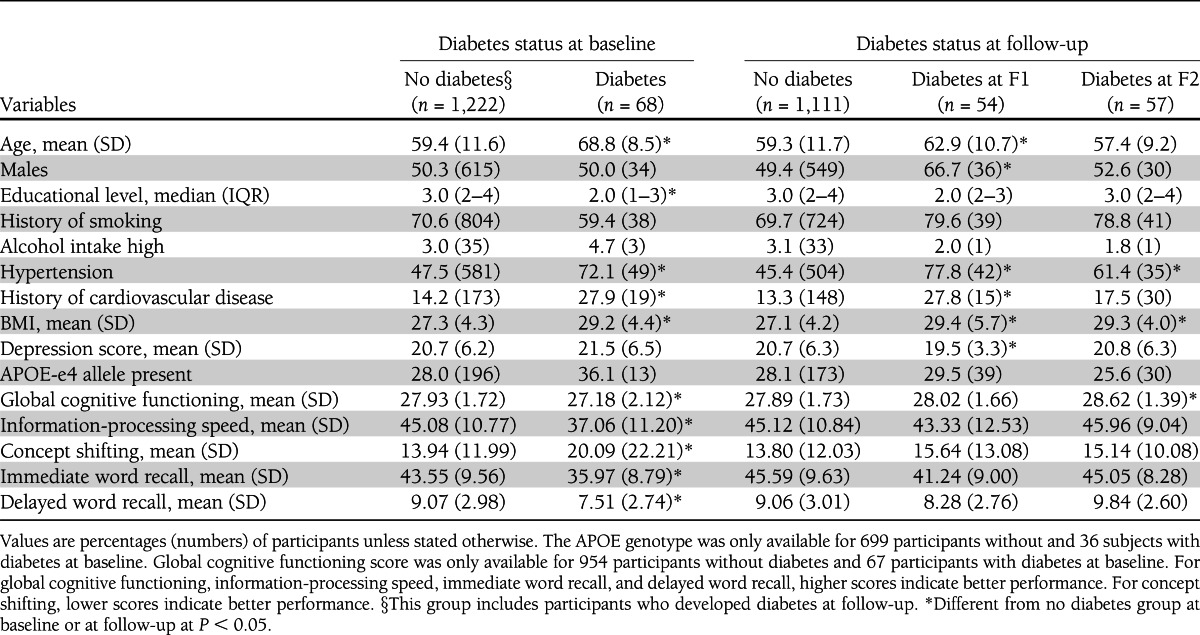
Participants with incident diabetes at F1 were more likely to be male, were significantly older, had a higher BMI, were more likely to have a history of cardiovascular disease and hypertension, and had a lower depression score than participants who were free of diabetes during the whole follow-up period. Participants with incident diabetes at F2 were more likely to have hypertension and had a significantly higher BMI than participants without diabetes during the whole follow-up period. At baseline (i.e., before a diagnosis of diabetes was made), there were no significant differences in measures for information-processing speed (P = 0.24), concept shifting (P = 0.28), immediate word recall (P = 0.08), and delayed word recall (P = 0.06) between participants with incident diabetes at F1 and control subjects. Likewise, there were no significant differences in information-processing speed (P = 0.57), concept shifting (P = 0.35), immediate word recall (P = 0.26), and delayed word recall (P = 0.06) between participants with incident diabetes at F2 and control subjects. However, participants with incident diabetes at F2 had a significantly higher MMSE score than participants without diabetes.
Baseline diabetes and 12-year cognitive decline
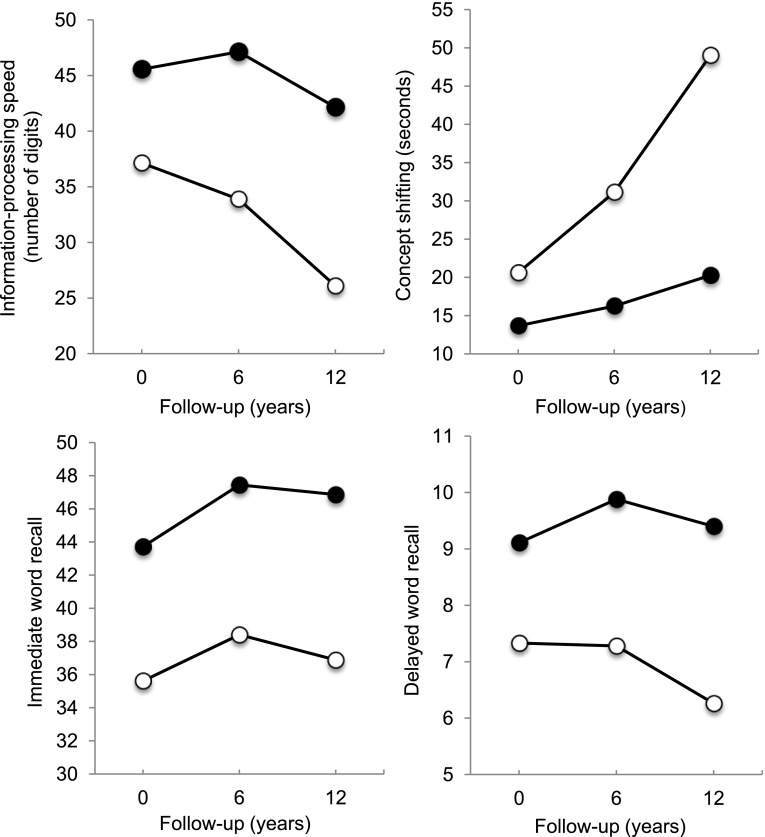
Results of the association between baseline diabetes and cognitive change over time are presented in Table 2. In crude analyses (model 1), participants with diabetes showed a significantly larger decline in information-processing speed and concept shifting from baseline to F1 and to F2 compared with those without diabetes. This was virtually unchanged after adjusting for demographic variables, history of smoking, and alcohol intake (model 2) and after further adjustments for comorbid conditions (model 3). The association between diabetes and change in delayed word recall from baseline to F1 and to F2 was not significant in models 1 and 2, but became modestly significant in the fully adjusted model 3. In contrast, there were no associations with decline in immediate word recall. To examine the effect of carrying the APOE-e4 allele on the association between diabetes and cognitive change over time, APOE-e4 was included as a covariate in a restricted subgroup of 699 individuals without and 36 with baseline diabetes. Results were similar to the analyses in the full sample (data not shown), except that the effect of diabetes on decline in delayed word recall from baseline to F1 was not statistically significant anymore (P = 0.14). However, the magnitude of this effect was similar to the analysis without APOE-e4. The results per cognitive outcome domain of the fully adjusted models (without APOE-e4) are presented in Fig. 1. Decline in information-processing speed from baseline to F2 was three times larger for participants with diabetes compared with those without and decline in set shifting was four times larger. Although participants without diabetes did not decline in delayed word recall, participants with diabetes declined by 14%. In contrast, there were no group differences in change in immediate word recall.
Table 2.
Change in cognitive performance (95% CI) from baseline to 6-year follow-up (F1) and to 12-year follow-up (F2) in participants with baseline diabetes relative to participants without diabetes before and after adjustment for various covariates
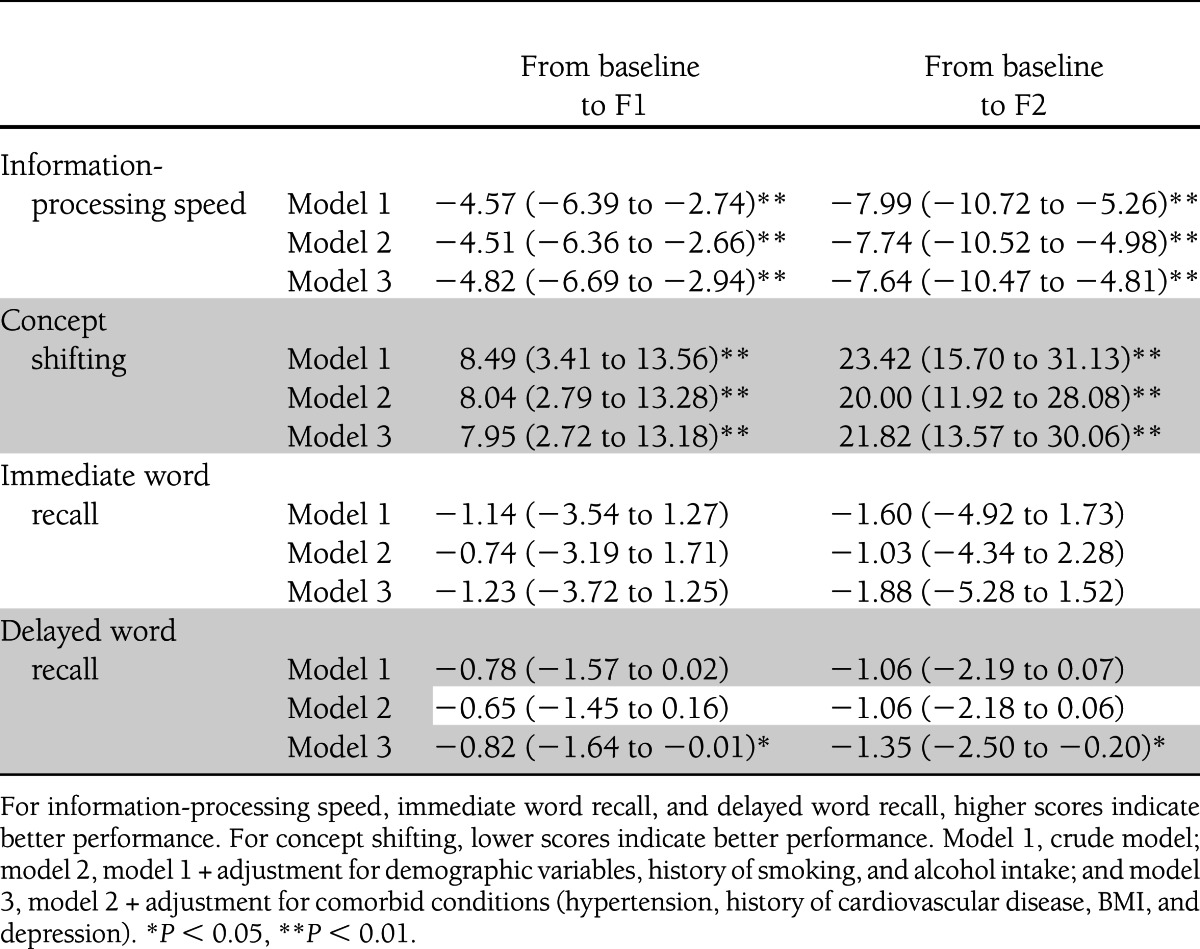
Figure 1.
Cognitive performance (mean domain score adjusted for demographics, history of smoking, alcohol intake, and comorbid conditions) for participants with baseline type 2 diabetes (white circles) and participants without baseline diabetes (black circles) at baseline (0), 6-year follow-up (6), and 12-year follow-up (12). For information-processing speed and immediate and delayed word recall, a higher score indicates better performance. For concept shifting, a lower score indicates better performance.
Incident diabetes and 12-year cognitive decline
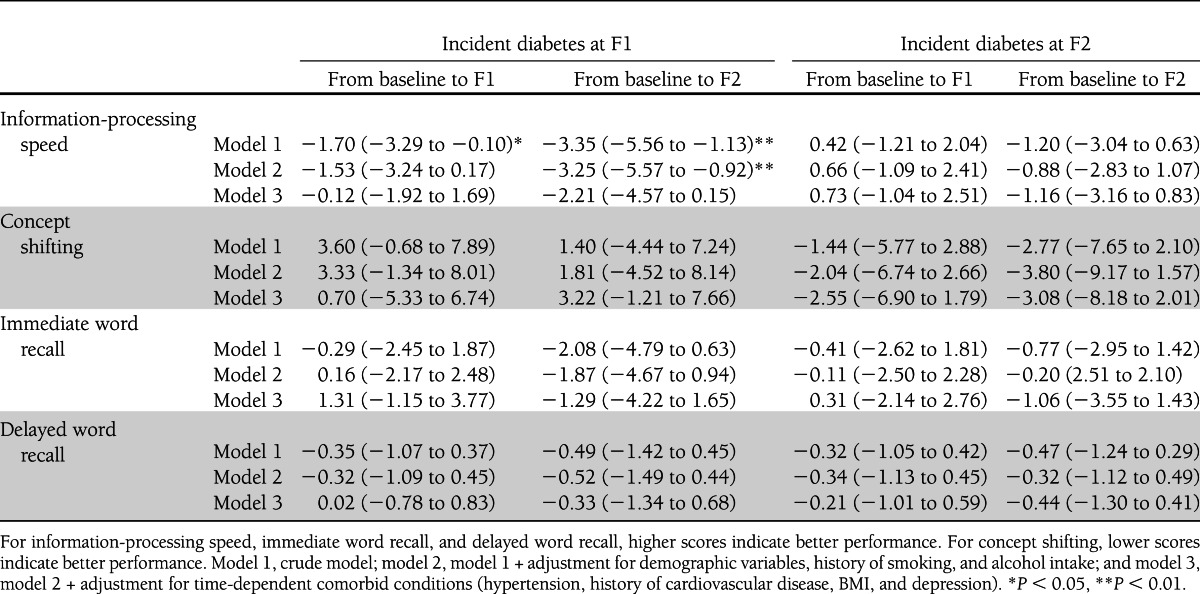
Results of the longitudinal analyses are presented in Table 3. In crude analyses, participants with incident diabetes at F1 showed a larger decline in information-processing speed from baseline to F1 and to F2 (Table 3, model 1). This effect was considerably attenuated in the fully adjusted model (model 3). No differences were observed between participants who developed diabetes at follow-up and control subjects.
Table 3.
Change in cognitive performance (95% CI) from baseline to 6-year follow-up (F1) and to 12-year follow-up (F2) in participants with incident diabetes relative to participants without diabetes before and after adjustment for various covariates
Post hoc analyses
To better understand the effects of incident diabetes on cognitive decline, we performed two post hoc analyses. In the first analysis, the two groups with participants with incident diabetes were pooled (N = 111) to increase power. In the fully adjusted model, the incident diabetes group showed a significantly larger decline in information processing speed from baseline to F2 compared with the control group (estimate −1.62; P = 0.04). There was no significant difference in decline in the other cognitive measures. Next, we explored whether disease-exposure time plays a role in the development of cognitive decline. We performed a post hoc analysis including only diabetic patients and measured the effect of disease duration on cognitive decline. Data on disease duration (based on self-report) were available at baseline for 67 participants with baseline diabetes, at follow-up for 44 participants with incident diabetes at F1, and for 53 participants with incident diabetes at F2. Missing values were replaced with the strata-specific mean of the observed values of disease duration within each of the three diabetes strata (baseline, at F1, and at F2). Disease duration was calculated relative to the year of baseline assessment. On average, diabetes had been diagnosed 1.01 ± 9.15 years (mean ± SD) after baseline assessment. In line with our expectation, we found that, after adjustment for all covariates described previously, increasing disease duration had a significant effect on decline in information-processing speed from baseline to 12-year follow-up (estimate −0.19; P < 0.05). We did not find a significant effect of disease duration on immediate recall (estimate 0.04; P = 0.72), delayed recall (estimate 0.03; P = 0.41), or concept shifting (estimate 0.59; P = 0.05).
CONCLUSIONS
This study examined the effect of baseline and incident diabetes on change in multiple cognitive functions over a long follow-up period of 12 years. Participants with baseline diabetes performed worse on all cognitive measures at baseline and also showed a three times larger decline in information-processing speed and a four times larger decline in executive function than participants without diabetes. The effect of diabetes on memory was less pronounced. The effects on speed and executive function were largely independent of APOE-e4 genotype status.
Interestingly, no significant effect of incident diabetes at F1 and F2 on cognitive decline was observed, although the coefficients suggested a small effect on decline in information-processing speed from baseline to 12-year follow-up. Few previous studies examined the effect of incident diabetes on cognitive decline (26,27). The study by Nooyens et al. (26) was performed in a restricted sample of middle-aged individuals followed for 5 years. The recent study by Yaffe et al. (27) used two cognitive measures and was performed in older adults followed for 9 years. Though both studies did not find an independent effect of incident diabetes on cognitive decline, both point out that the decline in the incident diabetes group was intermediate between the control group and the prevalent diabetes group. In a post hoc analysis in which we pooled the two groups with incident diabetes at F1 and F2, incident diabetes indeed had a significant effect on decline in information-processing speed. This might indicate that our initial model had missed the small differences in cognitive decline between incident diabetic patients and control subjects. So, diabetes might have a subtle effect on cognition even in the early stages of the disease.
The finding that baseline diabetes had an effect on cognitive decline is in line with numerous previous studies with shorter follow-up duration (8,9), although the rate of decline in our study was larger than reported before, and some studies did not find an effect of diabetes on cognitive decline (5–7). Given the differences in the effect of baseline diabetes and incident diabetes, it appears that effects of diabetes on cognition take time to evolve and that profound differences in cognition between people with diabetes and people without diabetes become apparent only in the long run. This is consistent with the finding that incident diabetes only had an effect on a measure of information-processing speed, which is known to be most sensitive to diabetes-related cognitive decline (28) and with the finding that disease duration had a significant effect on decline in information-processing speed. These results seem to indicate that duration of disease exposure plays an important role in the development of cognitive decline, which is in line with previous research showing that longer duration of diabetes is associated with increased odds of mild cognitive impairment (29), a possible prodromal stage of dementia.
Few studies examined the effect of baseline diabetes on cognitive decline over a comparable follow-up period (10,30–32). In the Baltimore Longitudinal Study of Aging, no effect of diabetes on cognitive decline over a 12-year follow-up period was found. However, this study only included men and assessed other cognitive domains, namely visual memory and vocabulary performance (31). The Study of Osteoporotic Fractures has shown that lack of diabetes was predictive of maintaining optimal cognitive function over a 15-year follow-up as opposed to minor cognitive decline in older women (32). The Indianapolis-Ibadan Dementia Project and The Atherosclerosis Risk in Communities Studies showed a modest effect of diabetes on change in cognition over a 15-year and 14-year follow-up period, respectively (10,30). Stronger associations might have been missed because measures of general cognitive functioning were used (10), which are less sensitive to change, and because the study sample was restricted to middle-aged individuals (30), in whom the association between diabetes and cognitive decline might be less obvious (26). In contrast, the current study had no upper age restriction and used a comprehensive and validated multicognitive domain test battery.
In line with previous studies (33–35), we found that diabetes was most strongly related to decline in information-processing speed and executive function, although in these studies, the follow-up durations were shorter and the age ranges were smaller than in our study. In the absence of major cerebrovascular disease or neurodegenerative processes, decline in these domains has been linked to cerebral small vessel disease (36,37). Indeed, patients with diabetes have been shown to have more white matter lesions, which have been related to cognitive functioning in these patients (38). Furthermore, microvascular disease seems to play a role in the development of dementia in diabetic patients (1,39). Other putative factors include hyperglycemia, which can have toxic effects on the brain by glycation of important proteins (1,2), and insulin, which inhibits degradation of amyloid-β peptide (the main product of the Alzheimer's disease process) by competition for insulin-degrading enzyme in the brain (1,2).
Our study has some limitations. First, the size of the diabetes groups was relatively small due to the population-based nature of MAAS, especially the incident diabetes groups. We are thus likely to have missed more subtle associations between incident diabetes and cognitive decline, especially for the group with incident diabetes at F1 and decline from baseline to F2. Next, participants with diabetes and poorer global cognition were more likely to drop out of the study, which might have led to selection bias. Hence, our results most likely are an underestimation of the true effect of diabetes on cognitive decline in the population. However, we partially accounted for this attrition by using mixed models, which does not restrict analysis to study completers. Furthermore, the diagnosis of diabetes was based on self-report or on antidiabetic medication use. As a diagnosis of diabetes is often missed (40), some participants with diabetes might have been wrongly assigned to the nondiabetic group. It is unknown how this would have affected our estimates, but it seems unlikely that this accounts for the robust differences in the rate of cognitive decline between groups. Finally, because we did not have data on HbA1C levels, we were unable to examine the effect of glucose control on cognitive decline.
This study also has a number of important strengths. These include multiple measures of cognitive functions, assessment of separate cognitive domains by a comprehensive neuropsychological test battery, inclusion of important potential confounders, and a long follow-up time. Furthermore, we had the opportunity to measure the effect of incident diabetes and comorbid conditions at each follow-up moment.
To conclude, this study indicates that patients with baseline type 2 diabetes show accelerated cognitive decline, particularly in information-processing speed and executive function, compared with individuals without diabetes and that patients with incident diabetes show signs of early decline in information-processing speed. It seems that disease-exposure time plays an important role in the development of cognitive decline. This might provide a window of opportunity for prevention and early treatment of diabetes-related cognitive deficits. For this, it is important to assess cognitive status at an early stage of the disease and on a regular basis. Studies that focus on the underlying mechanisms between diabetes and cognitive decline are highly desirable in order to develop adequate treatment for cognitive decline.
Acknowledgments
The Maastricht Aging Study is supported by the University of Limburg, the University Hospital of Maastricht, and the Dutch government through a grant from the Netherlands Program for Research on Aging.
No potential conflicts of interest relevant to this article were reported.
P.J.J.S. analyzed data, interpreted results, and wrote the manuscript. S.K. contributed to data analysis, discussion, and reviewed and edited the manuscript. F.R.J.V. and C.D.A.S. contributed to discussion and reviewed and edited the manuscript. M.P.J.v.B. contributed to study conception, study design, discussion, and reviewed and edited the manuscript. P.J.J.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented at the 48th European Association for the Study of Diabetes Annual Meeting, Berlin, Germany, 1–5 October 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0746/-/DC1.
References
- 1.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 2.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE 2009;4:e4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 2011;7:108–114 [DOI] [PubMed] [Google Scholar]
- 4.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 2001;357:169–175 [DOI] [PubMed] [Google Scholar]
- 5.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia 2006;49:2015–2023 [DOI] [PubMed] [Google Scholar]
- 6.Fischer AL, de Frias CM, Yeung SE, Dixon RA. Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. J Clin Exp Neuropsychol 2009;31:809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg E, Reijmer YD, de Bresser J, Kessels RP, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia 2010;53:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontbonne A, Berr C, Ducimetière P, Alpérovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001;24:366–370 [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 2004;63:658–663 [DOI] [PubMed] [Google Scholar]
- 10.Wessels AM, Lane KA, Gao S, Hall KS, Unverzagt FW, Hendrie HC. Diabetes and cognitive decline in elderly African Americans: a 15-year follow-up study. Alzheimers Dement 2011;7:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 2010;26:507–519 [DOI] [PubMed] [Google Scholar]
- 12.Jolles J, Houx PJ, van Boxtel MPJ, Ponds RWHM, Eds. Maastricht Aging Study: Determinants of Cognitive Aging. Maastricht, Neuropsych Publishers, 1995 [Google Scholar]
- 13.Metsemakers JF, Höppener P, Knottnerus JA, Kocken RJ, Limonard CB. Computerized health information in The Netherlands: a registration network of family practices. Br J Gen Pract 1992;42:102–106 [PMC free article] [PubMed] [Google Scholar]
- 14.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70-81 years. BMJ 2004;328:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol 2003;56:148–154 [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Committee on Problems Related to Alcohol Consumption Problems Related to Alcohol Consumption: Technical Report Series 650. Geneva, World Health Organization, 1980 [PubMed] [Google Scholar]
- 17.World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 1999;17:151–183 [PubMed] [Google Scholar]
- 18.Arrindell WA, Ettema JHM. SCL-90. Een Multidimensionele Psychopathologie Indicator. Lisse, Swets & Zeitlinger, 1986 [Google Scholar]
- 19.Bekers O, op den Buijsch RA, de Vries JE, Wijnen PA, van Dieijen-Visser MP. Capillary electrophoretic detection in apolipoprotein E genotyping. Electrophoresis 2002;23:1878–1881 [DOI] [PubMed] [Google Scholar]
- 20.Slooter AJ, Tang MX, van Duijn CM, et al. Apolipoprotein E epsilon4 and the risk of dementia with stroke. A population-based investigation. JAMA 1997;277:818–821 [DOI] [PubMed] [Google Scholar]
- 21.De Bie SE. Standaardvragen 1987: Voorstellen voor Uniformering van Vraagstellingen naar Achtergrondkenmerken en Interviews. Leiden, Leiden University Press, 1987 [Google Scholar]
- 22.Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 2005;11:290–302 [DOI] [PubMed] [Google Scholar]
- 23.Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Concept Shifting Test: adult normative data. Psychol Assess 2006;18:424–432 [DOI] [PubMed] [Google Scholar]
- 24.van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol 2006;28:998–1009 [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 26.Nooyens AC, Baan CA, Spijkerman AM, Verschuren WM. Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care 2010;33:1964–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012;69:1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 2005;26(Suppl. 1):26–30 [DOI] [PubMed] [Google Scholar]
- 29.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol 2008;65:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopman DS, Mosley TH, Catellier DJ, Coker LH, Atherosclerosis Risk in Communities Study Brain MRI Study Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 31.Robertson-Tchabo EA, Arenberg D, Tobin JD, Plotz JB. A longitudinal study of cognitive performance in noninsulin dependent (type II) diabetic men. Exp Gerontol 1986;21:459–467 [DOI] [PubMed] [Google Scholar]
- 32.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc 2007;55:259–264 [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Iseki C, Wada M, et al. Impaired glucose metabolism slows executive function independent of cerebral ischemic lesions in Japanese elderly: the Takahata study. Intern Med 2011;50:1671–1678 [DOI] [PubMed] [Google Scholar]
- 34.Bourdel-Marchasson I, Lapre E, Laksir H, Puget E. Insulin resistance, diabetes and cognitive function: consequences for preventative strategies. Diabetes Metab 2010;36:173–181 [DOI] [PubMed] [Google Scholar]
- 35.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–666 [DOI] [PubMed] [Google Scholar]
- 36.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041 [DOI] [PubMed] [Google Scholar]
- 37.Umemura T, Kawamura T, Umegaki H, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011;82:1186–1194 [DOI] [PubMed] [Google Scholar]
- 38.Manschot SM, Brands AM, van der Grond J, et al. Utrecht Diabetic Encephalopathy Study Group Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006;55:1106–1113 [DOI] [PubMed] [Google Scholar]
- 39.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol 2009;66:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]