Abstract
Sphingolipids are emerging as important mediators of immune and inflammatory responses. We have previously demonstrated that sphingosine-1-phosphate (S1P) and its synthetic enzyme sphingosine kinase-1 (SK1) play an important role in inflammatory bowel disease. S1P generation is dependent on SK phosphorylation of sphingosine. Generation of sphingosine results only from the breakdown of ceramide by ceramidases (CDase). In this study, we set out to determine the role of neutral CDase (nCDase) in S1P generation and inflammatory bowel disease. To this end, we established nCDase expression is increased in patients with ulcerative colitis. Using the dextran sulfate sodium (DSS)-induced colitis model, we determined nCDase activity increased in colon epithelium, but not submucosa, in wild-type (WT) mice. Following DSS, ceramide levels were elevated in colon epithelium from WT and nCDase−/− mice, while S1P levels were significantly elevated only in the epithelium of nCDase−/− mice. Similarly, cyclooxygenase-2 (Cox-2) levels were significantly elevated only in the epithelium of nCDase−/− mice. Neutral CDase−/− mice also exhibited higher endotoxin levels in circulation, as well as higher circulating levels of S1P. This increase in S1P in nCDase−/− mice was accompanied by a marked leukocytosis, most notably circulating neutrophils and lymphocytes. Taken together these data demonstrate that loss of nCDase results in an unexpected increase in S1P generation in inflammation, and suggests that nCDase may actually protect against inflammation.
Keywords: ceramide, ceramidase, sphingosine-1-phosphate, colitis, neutrophil, inflammation
INTRODUCTION
Ceramidases (CDases) are a family of lipid metabolizing enzymes that degrade ceramide. There are five known CDases, namely neutral, acid and 3 alkaline CDases based on their pH optimum (reviewed in [1]). In addition to pH, location is an important factor in the activity of these enzymes. Acid CDase (aCDase) is localized to the lysosome [2] and can be secreted extracellularly [3], alkaline ceramidases (ACER1, 2 and 3) are localized to the ER and Golgi [4, 5], while neutral CDase (nCDase) mainly localizes to the plasma membrane [6] and likely mitochondria [7]. These enzymes degrade ceramide, a pivotal lipid in the sphingolipid metabolism pathway. De-acylation of ceramide produces sphingosine, which can then be phosphorylated by a sphingosine kinase (SK) to form sphingosine-1-phosphate (S1P), a potent biologically active lipid. Therefore, both a ceramidase and a sphingosine kinase are required for the generation of S1P. Ceramide has been implicated in the regulation of cellular responses to stress, including apoptosis [8] and senescence [9]. On the other hand, S1P has been shown to serve quite the opposite functions. S1P binds to five different G-protein coupled receptors to induce proliferation and migration [10–13], as well as regulate inflammation, immune cell functions and trafficking [14, 15].
Moreover, our laboratory and others have shown that SK, specifically SK1, and S1P play an essential role in chronic inflammatory diseases such as inflammatory bowel disease (IBD) [16], and rheumatoid arthritis [17]. This is due in part through activation of SK1 by cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL1-β) [18, 19]. Inhibition of SK or S1P receptors have been shown to prevent inflammation in numerous animal models including asthma [20, 21], lupus nephritis [22], arthritis [23, 24] and IBD [25–28].
While much is known about the regulation and activation of SK1, little is known about the potential roles of CDases, upstream of SK that generate sphingosine. The alkaline CDases have been implicated to be important mainly in skin, and aCDase has been implicated to be regulated by TNFα [29]; however, little is known about the role and regulation of CDases in the intestinal tract. Interestingly nCDase is expressed in the brush border of the intestinal tract and is involved in the breakdown of dietary ceramide [30]. Additionally, loss of this enzyme also leads to a decrease in sphingosine levels in the colon [30]; however, little is known about the role of this enzyme in inflammation or as a source of S1P.
Therefore in this study we set out to investigate the role of nCDase in the generation of S1P and the effects of loss of this enzyme in IBD. Human tissue samples from ulcerative colitis patients demonstrated a slight increase in expression of nCDase, specifically in the epithelium. Additionally, we show that nCDase activity is significantly increased in colon tissue, specifically in the epithelium, in an animal model of IBD. Interestingly nCDase−/− mice were not protected from this disease model and in fact demonstrated increases in local and systemic inflammatory responses. Perhaps most interesting is that the nCDase−/− mice actually generate more S1P in colon tissue and in circulation, than do WT mice following DSS administration. The implications of these results for our understanding of sphingolipids in IBD and possible roles for nCDase are discussed.
MATERIALS AND METHODS
Human tissue array
A human tissue array from Cooperative human tissue network (CHTN), Colorectal Carcinoma Progression TMA (CHTN2003CRCprog), was used for immunohistochemistry. An antibody against nCDase (gift from Dr. Richard Proia NIDDK) was used to detect the presence or absence of nCDase in normal colon mucosa or tissue from patients with ulcerative colitis (UC).
Mice
C57BL/6 wild type (WT) mice were purchased from Harlan Laboratories, and nCDase knockout mice (nCDase−/−) were from Dr. Rick Proia at NIDDK and had been backcrossed at least 6 generations to C57BL/6 [30]. Animals were maintained under standard laboratory conditions. All animal procedures were approved by the Ralph H. Johnson VA Medical Center Institutional Animal Care and Use Committee and followed the guidelines of the American Veterinary Medical Association.
Induction of colitis
Acute colitis was induced by adding 5% (w/v) DSS (MP Biomedicals, Inc., Solon, OH, USA) to drinking water for 5 days. DSS solutions were monitored to ensure equal consumption between WT and nCDase−/− mice. Untreated mice were given regular drinking water.
In vitro ceramidase activity assay
Ceramidase activity was determined as described previously [31, 32]. Following euthanasia, colons were removed, rinsed with PBS, and snap frozen in liquid nitrogen. Colon tissue was homogenized on ice using a rotor homogenizer in buffer containing 20 mM Tris-HCl, pH 7.4 and complete protease inhibitors. After brief sonication and determination of protein concentration, 50μg of proteins were subjected to reactions of acid or nCDase activities. NCDase activity was measured using detergent/lipid mixed micelles with D-erythro-C12-NBD-ceramide, at a concentration of 50 μM in a 200 mM Tris buffers (pH 7.0) (containing 1 mM CaCl2, 0.3% (w/v) Triton X-100 final concentration, with a total volume of 100 μL). The reaction mixtures were incubated at 37 °C for 1 hour. Conversion of NBD-C12 ceramide to NBD-dodecanoic acid was detected by spotting the organic phase on a TLC plate. Results were quantified by densitometric analysis using ImageQuant software.
Histology score
Colons were removed, rinsed with PBS, opened longitudinally, and fixed with 10% formalin. Sections were embedded in paraffin, fixed to glass slides, and stained with hematoxylin and eosin (H&E). The entire colon was microscopically examined for damage, and scored in a blinded fashion. Scores were assigned as previously described [26], briefly: 0—normal colon mucosa; 1—shortening of basal 1/3 of crypts with slight edema and lymphocytic infiltration; 2—loss of the basal 2/3 of crypts with moderate inflammation in the lamina propria; 3—total loss of crypts with severe inflammation in the lamina propria, but with surface epithelium still remaining; 4—loss of all crypts and surface epithelium with sever inflammation in the mucosa, muscularis propria, and submucosa. The total histology score was determined by multiplying the above score by the percent of area involved, thus, the minimal score is 0 and the maximal score is 40.
Lipid analysis
Advanced analyses of sphingosine and ceramide species were performed by the Lipidomics Core at MUSC on a Thermo Finnigan TSQ 7000, triple-stage quadrupole mass spectrometer operating in a Multiple Reaction Monitoring (MRM) positive ionization mode as described [33].
Complete Blood Counts
Whole blood was collected from mice following euthanasia into EDTA coated tubes. Blood counts were completed by Antech Diagnostics.
Harvesting of RNA
Following euthanasia, colons were removed, rinsed with PBS, epithelium and sub-mucosa were separated using a razor blade and snap frozen in liquid nitrogen. Next, tissue was homogenized, and total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA, USA) per the manufacturer’s instructions. RNA purity and yield were determined by spectroscopic analysis.
Real-time RT-PCR
RNA extracted from colon epithelium was reverse-transcribed into cDNA using 0.5 μg RNA. OligodT primers and SuperScript III were from Invitrogen (Carlsbad, CA, USA). Real-time RT-PCR was performed on a Bio-Rad iCycler to quantify mRNA levels of TNFα and Cox-2. Real-time RT-PCR reaction volumes were 25μl, including 12.5 μl SYBR Green PCR reagents (Biorad, Hercules, CA), 5μl cDNA template, 1 μl forward primer (10 μM), 1 μl reverse primer (10 μM), and 5.5 μl water. The primers for real-time were used as previously described [16]; briefly, TNFα forward primer 5′-AATGGCCTCCCTCTCATCAGTT-3′ and reverse primer 5′-CCACTTGGTGGTTTCCTACGA-3′; Cox-2 forward primer 5′-TGAGTACCGCAAACGCTTCTC-3′, and reverse primer 5′-TGCAGCCATTTCTTTCTCTCCT-3′; β-actin forward primer 5′-TAAGGCCAACCGTGAAAAGATG-3′, and reverse primer 5′-CTGGATGGCTACGTACATGGCT-3′. The RT-PCR steps were as follows: 2 min at 95 °C, followed by 40 cycles of 15s at 95 °C, 60s at 60 °C and a final step of 1-min incubation at 60°C. All reactions were performed in triplicate. The data were analyzed using Q-Gene software [34] and expressed as fold-change of mean normalized expression from untreated value. This value is directly proportional to the amount of RNA of the target gene relative to the amount of RNA of the reference gene, β-actin.
Endotoxin Measurements
Whole blood was collected from mice following euthanasia in eppendorf tubes and allowed to clot. Serum was analyzed for endotoxin levels by Charles River Laboratories.
Data analysis
Statistical analyses were performed using two-way ANOVA or students t-test for strain and treatment effects. P-values <0.05 were considered significant. In the figures, *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
Elevated nCDase expression in intestinal epithelial cells from patients with ulcerative colitis
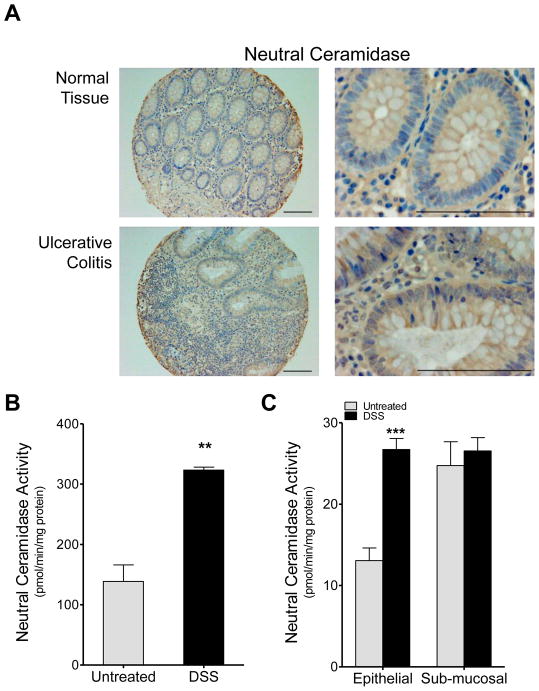
Neutral CDase has been shown to be expressed in the brush border of the intestinal epithelium and to be involved in intestinal degradation of ceramide [35]. Therefore, we set out to examine the role of nCDase in UC, and began by examining nCDase expression levels in human samples. We examined nCDase expression in a human tissue array for colon carcinoma progression from the CHTN. Expression of nCDase was examined in seven samples from normal colon mucosa and six samples from ulcerative colitis. Neutral CDase expression was present primarily in the epithelium in both normal tissue and in tissue from patients with ulcerative colitis with similar overall patterns (Fig. 1A left panels); however, a some increase in expression could be discerned in ulcerative colitis specifically in the epithelium (Fig. 1A right panels).
Figure 1. nCDase expression and activity increases in colon epithelium in ulcerative colitis.
A) Immunohistochemistry was performed on human tissue arrays, staining for nCDase, the upper panels for represent normal colon tissue samples and the lower panels represent samples from patients with ulcerative colitis. Bars indicate 50μm. B & C) WT mice were administered 5% DSS in their drinking water for 5 days and colon nCDase activity was assessed in the B) whole colon and C) epithelial and sub-mucosal layers. Data represent mean ± SD; n≥3 mice per treatment group, **p<0.01, ***p<0.001 as compared to untreated.
Neutral CDase activity increases in colon epithelium of mice with DSS-induced colitis
Next we examined nCDase activity in an animal model of IBD to determine if activity mirrored the changes we saw in protein expression from human patients. We employed the DSS-induced colitis mouse model a well-established model for IBD. WT mice were administered regular drinking water (untreated) or drinking water containing 5% DSS (w/v) for five days. Following DSS administration, mice were euthanized, and colon tissue was collected and assayed for nCDase activity. Neutral CDase activity increased significantly in colon tissue from WT mice following DSS administration (Figure 1B). When we separated the epithelial layer from the underlying musculature (stroma), increased nCDase activity of approximately two-fold was found to be primarily in the epithelial layer and not in the sub-mucosal elements (Figure 1C). These data demonstrate that nCDase expression and activity increase in colon epithelium during IBD, suggesting that this change may play a role in the pathogenesis of disease.
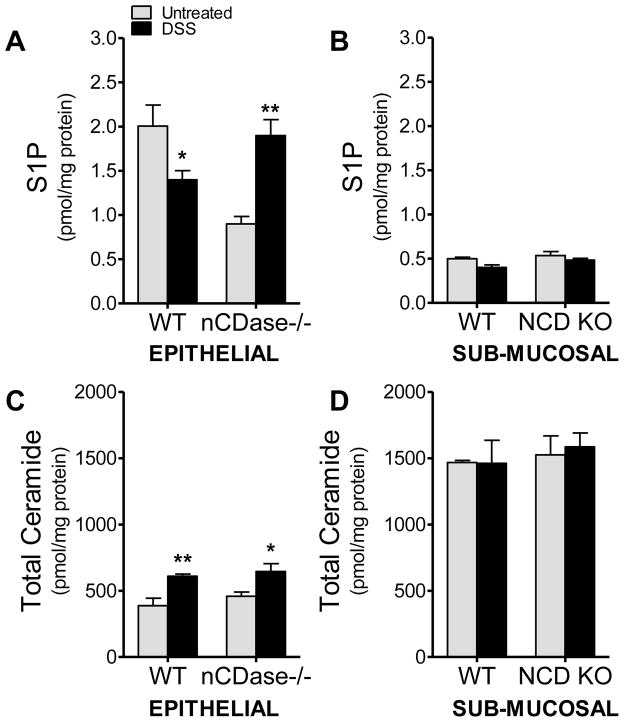
Loss of nCDase increases S1P levels in DSS-induced colitis
Due to the observed increases in nCDase activity in colon epithelium following DSS-induced colitis, we set out to determine if this increase in activity altered colon sphingolipid levels. We examined sphingolipid changes in colon tissue from WT and nCDase−/− mice following 5 days of 5% DSS administration. Given that we had observed differential activity in the epithelium when compared to the sub-mucosa, we also separated the epithelial layer from the sub-mucosa for tissue sphingolipid measurements. As shown in Figure 2A, total ceramide levels in the epithelium increased in both strains of mice following DSS administration, which was somewhat unexpected given the increase in ceramidase activity observed with DSS-induced colitis. The observed increase in ceramide was specific to the colon epithelium and was chain length specific. There were no significant differences in ceramide levels basally between colon epithelium from WT or nCDase−/− mice. Following DSS administration C16, C18 and C24 ceramide species increased significantly in colon epithelium from WT mice; however, only C18 increased significantly in colon epithelium from nCDase−/− mice (data not shown). There were no significant changes in ceramide levels in the colon sub-mucosal layer from either strain (Figure 2B). More notable was the significant increase in S1P levels in colon epithelium (Figure 2C) and lack of change in S1P levels in sub-mucosa (Figure 2D) from nCDase−/− mice. It is also of note here that S1P levels were higher in the epithelium while ceramide levels are higher in the sub-mucosal layer. These data demonstrate that loss of nCDase activity in DSS-induced colitis unexpectedly alters epithelial sphingolipid levels, specifically increasing S1P levels.
Figure 2. S1P is elevated in colon epithelium from nCDase−/− mice following DSS.
Colon tissue from WT and nCDase−/− mice removed following 5 days of 5% DSS or regular drinking water and the epithelial and sub-mucosal layers were analyzed separately. A & B) S1P, and C & D) ceramide levels were analyzed by the Lipidomics Core at MUSC on samples normalized for protein concentration. Data represent mean ± SEM., n=5 mice per treatment group, *p<0.05, **p<0.01 as compared to strain untreated.
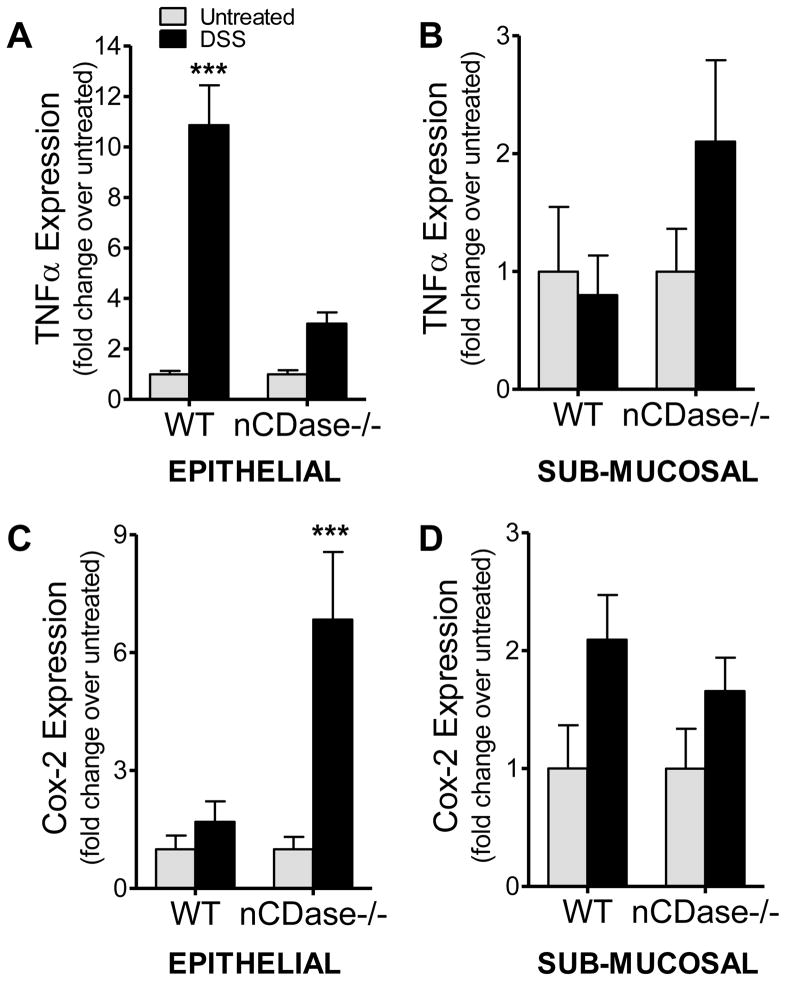
Cox-2 induction in colon epithelium is exaggerated in nCDase−/− mice following DSS-induced colitis
Tumor necrosis factor alpha (TNFα) has been demonstrated to activate sphingosine kinase-1 and induce production of Cox-2. This S1P mediated induction of Cox-2 has been demonstrated previously by our laboratory and others in cells [19, 36] and in vivo [16]. Therefore, we examined TNFα and Cox-2 expression in colon tissue in epithelial and sub-mucosal layers. TNFα expression was significantly increased (10-fold) in the epithelium from WT mice (Figure 3A), while only modestly induced in epithelium from nCDase−/− mice treated with DSS. This increase was observed only in the epithelium, and not in the sub-mucosal layer (Figure 3B). Interestingly, Cox-2 expression increased modestly in the epithelial layer from WT mice, while expression increased almost 7-fold in the epithelium of nCDase−/− mice following DSS administration (Figure 3C). These data demonstrate that loss of nCDase is associated with increased activation of the S1P/Cox-2 pathway in the colonic epithelium of DSS-treated mice.
Figure 3. Loss of nCDase results in increased local induction of Cox-2 in DSS-induced colitis.
Colon tissue from WT and nCDase−/− mice was removed after 5% DSS administration for 5 days and the epithelial and sub-mucosal layers were analyzed separately. Real-time RT-PCR was performed to determine expression of A) & B) TNFα and C) & D) Cox-2. Data represent fold change from untreated ± S.D., n=5 mice per treatment group, ***p<0.001 as compared to strain untreated.
After observing increased S1P and Cox-2 induction in colon epithelium from nCDase−/− mice following DSS-induced colitis, we began investigating if the local inflammatory response could affect the systemic inflammatory response. To this end, we measured endotoxin levels in serum from WT and nCDase−/− mice following 5 days of DSS in their drinking water. WT mice demonstrated no change in circulating endotoxin levels following 5 days of DSS whereas nCDase−/− mice exhibited a modest increase in circulating endotoxin levels that did not reach statistical significance (Table 1).
Table 1. Endotoxin levels are elevated in serum from nCDase−/− mice following DSS-induced colitis.
Serum was collected from WT and nCDase−/− mice following 5 days of either 5% DSS or regular drinking water. Endotoxin levels in serum were measured by Charles River Laboratories. Data represent mean ± S.D., n=5 mice per treatment group.
| Strain | Treatment | Endotoxin Level (Eu/ml) |
|---|---|---|
| WT C57BL6 | Water | 0.399±0.123 |
| WT C57BL6 | 5% DSS | 0.399±0.107 |
| nCDase−/− | Water | 0.357±0.174 |
| nCDase−/− | 5% DSS | 0.474±0.192 |
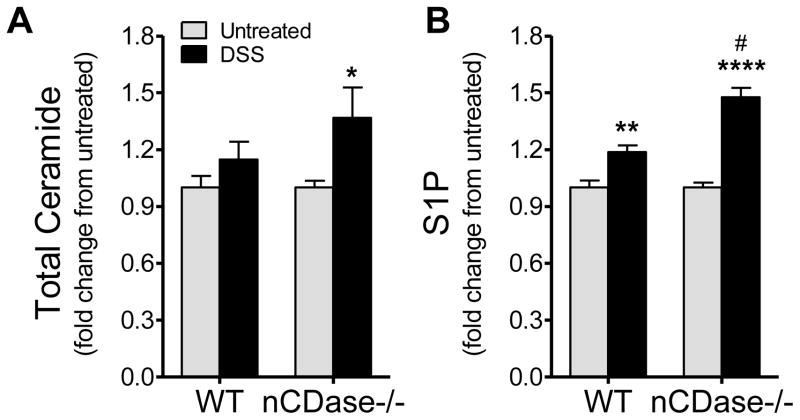
Loss of nCDase increases systemic inflammation and circulating S1P
After examining the effect of nCDase loss in the local inflammatory response and data suggesting decreased epithelial barrier function in nCDase−/− mice, we set out to determine if circulating sphingolipid levels or systemic inflammation were altered between strains of mice or with DSS-induced colitis. We have shown previously that the increase in circulating white blood cells that occurs during DSS-induced colitis is accompanied by changes in circulating sphingolipids, specifically S1P [16]. For that reason, we examined sphingolipid levels in circulation from WT and nCDase−/− mice following DSS-induced colitis. There was a small but significant increase in circulating ceramide levels in nCDase−/− mice following DSS-induced colitis (Figure 4A). Additionally, both strains of mice demonstrated significant increases in S1P. However, the increase in S1P from nCDase−/− was significantly higher than the increase in S1P measured in blood from WT mice (Figure 4B). Circulating sphingosine levels, while lower in nCDase−/− mice, were not significantly altered when considering strain or treatment (data not shown). These data demonstrate S1P is elevated in circulation despite the loss of nCDase, suggesting that nCDase-derived sphingosine is not the source of sphingosine for S1P in circulation either.
Figure 4. Loss of nCDase in DSS-induced colitis leads to increases in circulating S1P.
Following 5 days of 5% DSS administration or regular drinking water, whole blood was collected from WT and nCDase−/− mice. Lipid analysis was performed using HPLC-ESI-MS by the Lipidomics Core Facility at MUSC for A) Ceramide and B) S1P. Data represent mean ± SD; n=5 mice per treatment group, *p<0.05, ***p<0.001, as compared to strain untreated; #p<0.05 as compared to WT DSS.
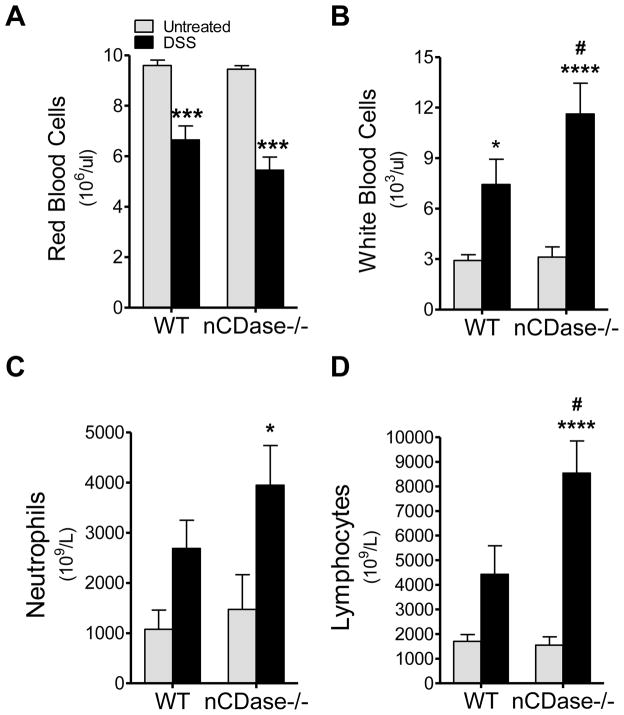
Since increases in S1P in colon epithelium may contribute to increased production of inflammatory mediators, and we had previously observed increases in S1P concomitant with increases in systemic inflammation we set out to determine the systemic inflammatory response in WT and nCDase−/− mice following DSS-induced colitis. To this end, we examined complete blood counts (Antech, Inc), to assess blood loss due to disease as well as markers of systemic inflammation. WT and nCDase−/− mice both demonstrated significant blood loss with DSS-induced colitis, as evidenced by significant decreases in circulating red blood cells (RBCs) (Figure 5A). Mirroring this loss in RBCs, WT and nCDase−/− mice also had significant decreases in hematocrit and hemoglobin (data not shown). It is interesting to note here that despite the loss of RBCs by both strains of mice, circulating S1P levels were increased in both strains. These data suggest an additional source for circulating S1P in DSS-induced colitis.
Figure 5. Systemic inflammation and circulating lymphocytes are increased in mice lacking nCDase.
Following 5 days of 5% DSS administration or regular drinking water, whole blood was collected from WT and nCDase−/− mice and complete blood counts were performed. A) Red blood cells, B) white blood cells, C) neutrophils, and D) lymphocytes were counted. Data represent mean ± SD; n=9 mice per treatment group, *p<0.05, ***p<0.001, ***0<0.0001 as compared to strain untreated; #p<0.05 as compared to WT DSS.
Circulating white blood cells (WBCs) increased in WT and nCDase−/− mice following DSS-induced colitis; however, nCDase−/− mice demonstrated a significant increase in these cells over that observed in WT (Figure 5B). This increase in WBCs included significant increases in circulating lymphocytes and neutrophils (Figure 5C & D) only in nCDase−/− mice. The increased systemic inflammation in nCDase−/− mice suggests that loss of nCDase may be detrimental not only to the local but systemic inflammatory responses as well.
DISCUSSION
Neutral CDase is expressed primarily in the brush border of the intestinal epithelium and loss of this enzyme has been shown to decrease levels of sphingosine in the colon [30]. Thus, we hypothesized that nCDase would be a primary source for sphingosine and downstream S1P generation in IBD. Interestingly, our data demonstrate that under inflammatory conditions nCDase is not the CDase responsible for generation of sphingosine and downstream S1P in colon tissue or in circulation. Indeed we observed the opposite, as loss of nCDase in the animal model of IBD resulted in increases in both local and circulating levels of S1P. Colon tissue samples from patients with UC demonstrated expression of nCDase in the colonic epithelium, likewise this had been demonstrated previously with the knockout mouse [30]. Therefore, we harvested colon epithelium and sub-mucosal layers separately. While the method used was crude, data in Figure 1B and C, demonstrate that with this separation method we were able to distinguish epithelial and stromal layers based on the observation of increased nCDase activity. This increase nCDase activity in colon tissue of WT mice, specifically in the epithelium, was significantly increased following DSS administration. However, as mentioned previously, this increase in activity did not alter sphingolipid levels as expected. Ceramide levels were actually elevated in colon epithelium from WT and nCDase−/− mice following DSS, while S1P was significantly elevated only in nCDase−/− mice. In addition to the unexpected sphingolipid results, nCDase−/− mice demonstrated only a modest increase in TNFα in colon epithelium; however, these mice exhibited a significant increase in Cox-2. The exaggerated Cox-2 induction in nCDase−/− mice could explain why these mice were not protected from DSS-induced colitis, as they exhibited weight loss, splenomegaly and colon shortening equal to, or slightly greater than, WT mice (Supp. Figure 1). Similar to the increases in local S1P and inflammation, circulating S1P levels and systemic inflammation were also significantly increased in nCDase−/− mice, suggesting that loss of nCDase leads to increased inflammatory responses in a mouse model of inflammatory bowel disease.
We began investigating the role of nCDase in inflammation based on previous studies demonstrating a role of sphingolipid metabolizing enzymes and their lipid products in inflammation and inflammatory diseases. Specifically, S1P, and its synthetic enzyme SK1, have been previously been shown to play a role in the inflammatory processes involved in chronic inflammatory diseases such as IBD [16], and rheumatoid arthritis [17]. Sphingosine, the substrate for SK1, and precursor for S1P, can only be generated by the activity of a CDase. However, the role of CDases and other upstream sphingolipid metabolizing enzymes in inflammation is not well known.
There have been few studies on the role of CDases in inflammation, and many of those examine the role of aCDase while very little is known about the role of nCDase in inflammation. There are a few cellular studies implicating nCDase in a protective role against apoptosis and specifically against inflammation-induced apoptosis. Protection from apoptosis in beta cells treated with a cytokine “cocktail,” was attributed to overexpression of nCDase [37]. Conversely, knockdown of nCDase with siRNA increased cytokine-induced apoptosis. Increased expression and activity of nCDase in mesangial cells following treatment with interleukin-1β was also suggested to by cytoprotective [38]. These data support our own conclusion that nCDase may play a protective role in colon epithelium against cytokine induced toxicities and that loss of this enzyme may be detrimental, leading to inflammation and eventual cell death.
Ceramidase has also been shown to be stimulated by adiponectin or palmitate in beta cells [39], although the activity was described as in the range of pH 5.0 and 7.0, which could include both neutral and acid. This protection from apoptosis conferred by CDase activity was attributed to downstream generation of S1P. Interestingly in the DSS-induced colitis model, loss of nCDase did not prevent generation of S1P; in fact S1P levels were significantly elevated in colon epithelium of nCDase−/− mice following DSS. The differences in these studies and ours could be attributed to the distinct roles of S1P in protection from apoptotic stimuli and in promoting inflammation.
The question still remains as to the source of sphingosine for SK1 and S1P. Colon tissue from nCDase−/− mice has been shown to exhibit lower levels of sphingosine [30]. Interestingly in colon epithelium, sphingosine and S1P levels were not basally different (data not shown); suggesting that in colon epithelium loss of nCDase doesn’t significantly alter sphingosine or S1P basally. Therefore, this makes the exaggerated increase in S1P in the colon epithelium, as well as the circulation, following DSS administration in nCDase−/− mice unexpected. One possible explanation is that sphingosine, which is toxic to cells, is metabolized to S1P in addition to being recycled back into ceramide. It has been previously demonstrated that in the presence of excess sphingosine, CDase can function in reverse to generate ceramide from sphingosine and free fatty acid [40, 41]. We did examine reverse CDase activity from colon tissue and the in vitro activity is high and present in colon tissue from WT, but not nCDase−/− mice (data not shown). However, it is difficult to translate this to in vivo for two reasons; one being that the activity is substrate driven, and the other is that the reverse activity does not increase with DSS-treatment. If the reverse activity is absent, as in the nCDase−/− mice, perhaps sphingosine could be more readily available for conversion to S1P, which could explain the dramatic increase in S1P in the colon tissue of nCDase−/− mice treated with DSS.
Systemically, S1P has been demonstrated to be stored in and released from platelets and red blood cells [42], which leads to the question of the source of S1P in circulation during IBD. S1P could still be generated by the vascular endothelium itself [43] or by activated platelets, in response to damage or prostaglandins, such as thromboxane [44]. Independent of the source, S1P has been shown to cause egress of immune cells into circulation [15], which suggests that the significant increase in S1P exhibited by nCDase−/− mice elicits the increased systemic inflammatory response.
This study began with the initial hypothesis that nCDase expression and activity in the colon epithelium is necessary to generate S1P and induce inflammation in DSS-induced colitis. However, we determined that loss of nCDase resulted in increased local and systemic inflammatory responses and S1P levels. This suggests that nCDase is not the CDase that couples to SK, and that the increase in activity of this enzyme in DSS-induced colitis may actually play a protective role in regulating inflammation and S1P levels. Future studies will be geared towards investigating the cellular sources of S1P and the role of nCDase in the generation of S1P in inflammation.
Supplementary Material
Highlights.
Neutral CDase activity is increased in colon epithelium in a mouse model of colitis.
Loss of nCDase increases Cox-2 expression in a mouse model of colitis.
Loss of nCDase increases tissue and circulating S1P levels in a model of colitis.
Systemic inflammation is increased with loss of nCDase in a model of colitis.
Acknowledgments
This work was supported by a Veterans Affairs Merit Award (LMO) and Career Development Award (AJS), as well as NIH Grant GM062887 (LMO). Lipid analyses provided by the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313) and the Lipidomics Core in the SC Lipidomics and Pathobiology COBRE, Department Biochemistry, MUSC (P20 RR017677). We thank Dr. Richard Proia (NIDDK, Bethesda, MD, USA) for providing the nCDase−/− mice, George Washington and the COBRE Animal Pathobiology Core, as well as Margaret H. Romano and the Core Histology Laboratory at MUSC for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781(9):424–34. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlinz K, Kopal G, Bernardo K, Linke T, Bar J, Breiden B, Neumann U, Lang F, Schuchman EH, Sandhoff K. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J Biol Chem. 2001;276(38):35352–60. doi: 10.1074/jbc.M103066200. [DOI] [PubMed] [Google Scholar]
- 3.Li CM, Hong SB, Kopal G, He X, Linke T, Hou WS, Koch J, Gatt S, Sandhoff K, Schuchman EH. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics. 1998;50(2):267–74. doi: 10.1006/geno.1998.5334. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, Xu R, Hu W, Jin J, Crellin HA, Bielawski J, Szulc ZM, Thiers BH, Obeid LM, Mao C. Upregulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J Invest Dermatol. 2008;128(2):389–97. doi: 10.1038/sj.jid.5701025. [DOI] [PubMed] [Google Scholar]
- 5.Mao CG, Xu RJ, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase - A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 6.Hwang YH, Tani M, Nakagawa T, Okino N, Ito M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochem Biophys Res Commun. 2005;331(1):37–42. doi: 10.1016/j.bbrc.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 7.Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J Biol Chem. 286(28):25352–62. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259(5102):1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 9.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270(51):30701–8. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 10.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G- protein-coupled receptors. Biochim Biophys Acta. 2002;1582(1–3):72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 11.Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ Res. 2001;89(6):496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- 12.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–8. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonesu K, Nakamura T, Mizuno Y, Suzuki C, Nagayama T, Satoh S, Nara F. A novel sphingosine-1-phosphate receptor 1 antagonist prevents the proliferation and relaxation of vascular endothelial cells by sphingosine-1-phosphate. Biol Pharm Bull. 33(9):1500–5. doi: 10.1248/bpb.33.1500. [DOI] [PubMed] [Google Scholar]
- 14.Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol. 2006;3(1):11–9. [PubMed] [Google Scholar]
- 15.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 16.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. Faseb J. 2009;23(1):143–52. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 185(4):2570–9. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1- phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68(2):330–5. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 19.Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17(10):1203–17. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1085–93. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 21.Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116(11):2935–44. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 2008;74(10):1319–26. doi: 10.1038/ki.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai WQ, Irwan AW, Goh HH, Melendez AJ, McInnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183(3):2097–103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Tan W, Guo D, He S. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur J Pharmacol. 2007;573(1–3):230–40. doi: 10.1016/j.ejphar.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene- deficient mice. J Pharmacol Exp Ther. 2008;324(1):276–83. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- 26.Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53(4):997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel C, Sartory NA, Zahn N, Schmidt R, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44(13):3305–16. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178(4):2458–68. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 29.Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, Norris JS, Hannun YA. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J Biol Chem. 2006;281(34):24695–703. doi: 10.1074/jbc.M604713200. [DOI] [PubMed] [Google Scholar]
- 30.Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem. 2006;281(11):7324–31. doi: 10.1074/jbc.M508382200. [DOI] [PubMed] [Google Scholar]
- 31.Wu BX, Zeidan YH, Hannun YA. Downregulation of neutral ceramidase by gemcitabine: Implications for cell cycle regulation. Biochim Biophys Acta. 2009;1791(8):730–9. doi: 10.1016/j.bbalip.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu BX, Snook CF, Tani M, Bullesbach EE, Hannun YA. Large-scale purification and characterization of recombinant Pseudomonas ceramidase: regulation by calcium. J Lipid Res. 2007;48(3):600–8. doi: 10.1194/jlr.M600423-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39(2):82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32(6):1372–4. 1376, 1378–9. [PubMed] [Google Scholar]
- 35.Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem. 2006;281(11):7324–31. doi: 10.1074/jbc.M508382200. [DOI] [PubMed] [Google Scholar]
- 36.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. Faseb J. 2003;17(11):1411–21. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q, Jin JF, Shan XH, Liu CP, Mao XD, Xu KF, Liu C. Chronic activation of neutral ceramidase protects beta-cells against cytokine-induced apoptosis. Acta Pharmacol Sin. 2008;29(5):593–9. doi: 10.1111/j.1745-7254.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 38.Franzen R, Pautz A, Brautigam L, Geisslinger G, Pfeilschifter J, Huwiler A. Interleukin-1beta induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J Biol Chem. 2001;276(38):35382–9. doi: 10.1074/jbc.M102153200. [DOI] [PubMed] [Google Scholar]
- 39.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 17(1):55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J Biol Chem. 2001;276(20):16758–66. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- 41.Kita K, Okino N, Ito M. Reverse hydrolysis reaction of a recombinant alkaline ceramidase of Pseudomonas aeruginosa. Biochim Biophys Acta. 2000;1485(2–3):111–20. doi: 10.1016/s1388-1981(00)00029-9. [DOI] [PubMed] [Google Scholar]
- 42.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1- phosphate in blood. FASEB J. 2007;21(4):1202–9. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 43.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102(6):669–76. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulrych T, Bohm A, Polzin A, Daum G, Nusing RM, Geisslinger G, Hohlfeld T, Schror K, Rauch BH. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J Thromb Haemost. 9(4):790–8. doi: 10.1111/j.1538-7836.2011.04194.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.