Abstract
Sphingosine 1-phosphate (S1P) is an important bioactive sphingolipid metabolite that has been implicated in numerous physiological and cellular processes. Not only does S1P play a structural role in cells by defining the components of the plasma membrane, but in the last 20 years it has been implicated in various significant cell signaling pathways and physiological processes: for example, cell migration, survival and proliferation, cellular architecture, cell-cell contacts and adhesions, vascular development, atherosclerosis, acute pulmonary injury and respiratory distress, inflammation and immunity, and tumorogenesis and metastasis [1, 2]. Given the wide variety of cellular and physiological processes in which S1P is involved, it is immediately obvious why the mechanisms governing S1P synthesis and degradation, and the manner in which these processes are regulated, are necessary to understand. In gaining more knowledge about regulation of the Sphingosine Kinase (SK)/S1P pathway, many potential therapeutic targets may be revealed. This review explores the roles of the SK/S1P pathway in disease, summarizes available SK enzyme inhibitors and examines their potential as therapeutic agents.
Keywords: sphingolipids, sphingosine kinase (SK), sphingosine 1-phosphate (S1P), inhibitor, disease, biomarker
1. Sphingolipid Metabolism
Over the past 20 years, sphingolipids have emerged on the scene as pleiotropic signaling molecules implicated in the regulation of various cellular functions [3]. The first necessary step in the de novo pathway of ceramide generation involves Palmitoyl Co-A and the amino acid serine condensation, via the action of the enzyme serine palmitoyl transferase (SPT), to form dihydrosphingosine (DHS) (Fig. 1). Recently shown, SPT can undergo a change in substrate preference, from serine to alanine or glycine, leading to the production of 1-deoxysphinganine and 1-deoxymethylsphinganine, respectively [4]. Following its synthesis, serine-derived DHS then becomes acylated via action of the ceramide synthases to become dihydroceramide (Fig. 1) [5]. Dihydroceramide is then desaturated to form ceramide. Members of the large family of CerS are responsible for the addition of varying lengths of acyl chains, resulting in numerous dihydroceramide and ceramide species (Fig.1). Ceramide may also be generated by the breakdown of membrane sphingomyelins or via degradation of complex glycosphingolipids by the action of sphingomyelinases (SMase) and glucosyl ceramidases (GCase) respectively, as seen in Fig 1. Degradation of ceramide is carried out by the ceramidases (CDase), whereby the acyl chain is removed from ceramide and the 18 carbon amino-alcohol compound sphingosine is formed. Sphingosine then serves as the substrate for the sphingosine kinases (SKs) which are responsible for phosphorylating sphingosine at the primary hydroxyl group, resulting in the production of sphingosine 1-phosphate (Fig.1) [6]. In lieu of being phosphorylated by SK to S1P, sphingosine can be recycled back to ceramide via CerS-mediated reacylation [7]; this mechanism of ceramide generation is referred to as the salvage pathway. Of particular interest to this review are the SK enzymes as well as their product, the bioactive sphingolipid molecule sphingosine 1-phosphate (S1P) (Figure 1).
Figure 1. Sphingolipid Metabolic Pathway.
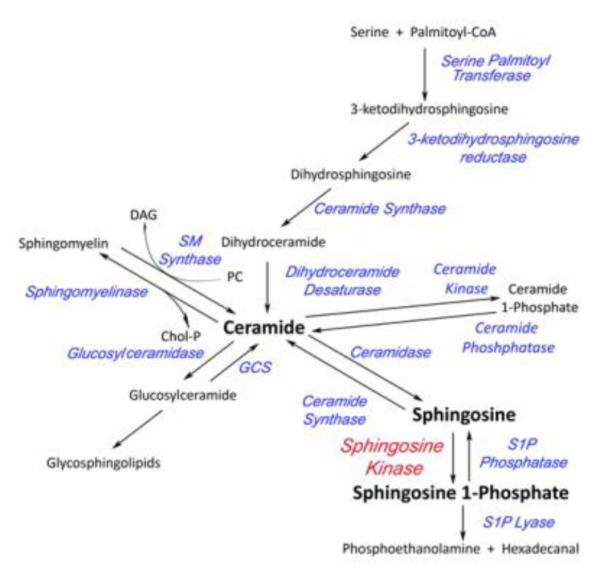
Phosphatidylcholine (PC), DAG (Diacylglycerol), SM Synthase (Sphingomyelin Synthase), Chol-P (phosphocholine), GCS (Glucoslyceramide Synthase). Besides Sphingosine Kinase in red, all enzyme names are in blue.
2. Sphingosine 1-Phoshpate (S1P)
2.1. Metabolism and Function
The bioactive signaling molecule sphingosine is phosphorylated via the action of the enzymes sphingosine kinase 1 (SK1) and sphingosine kinase 2 (SK2). A fine balance is maintained between the lipid signaling molecules ceramide, sphingosine and S1P and the SKs, along with other tightly regulated enzymes of sphingolipid metabolism, are attributed with preserving the aforementioned lipid equilibrium [8]. The phosphate can be removed from S1P by S1P phosphatases (SPPs) or other non-specific lipid phosphatases [9, 10]. Alternatively, S1P can be irreversibly broken down into phosphoethanolamine and hexadecenal by S1P lyase [1] (Figure 1). Sphingosine 1-phosphate has been shown to be involved in many normal physiological processes, as well as in disease processes [11]. Given the numerous important processes that rely on the SK/S1P pathway it is vital that we have a solid understanding of the mechanisms by which it is regulated.
2.2. S1P Signaling
S1P is implicated in both extracellular and intracellular-mediated signaling; however, to date, the majority of S1P effects have been attributed to its function as an extracellular signaling molecule [12]. The lack of S1P receptors in yeast and presence of a putative S1P receptor in the plant Arabadopsis thaliana provide significant evidence for intracellular function of S1P [13]. Despite the evidence for S1P as an intracellular signaling molecule, only recently have direct, intracellular molecular targets of S1P begun to be characterized. For example, intracellular S1P generated specifically by SK1 was shown to be necessary for TRAF2 E3 ubiquitin ligase activity, which is necessary for TNF-mediated events [13]. Moreover, nuclear S1P, derived from SK2, was reported to regulate epigenetic-mediated gene expression via inhibition of histone deacetylaces [13] . As mentioned above, many S1P functions are found to be receptor-mediated. The S1P family of G protein-coupled receptors, of which there are five (S1P1R-S1P5R), couple to different alpha subunits of heterotrimeric G proteins: for example Gαi, Gαq and Gα12/13. S1P receptor expression patterns, along with the Gα subunits to which each receptor couples dictates the activation of different downstream targets that occur upon receptor activation, including activation of Rac, ERK, PI3K, adenylyl cyclase, phospholipase C, Rho and JNK, resulting in the aforementioned cellular responses [14].
S1P is also capable of “inside-out” signaling whereby S1P is released, via the ABC family of transporters and the more recently described spinster 2 (spns2) transporter [15, 16], from the cell and is able to act in an autocrine or paracrine fashion, activating S1P receptors on the cell from which it was exported or on nearby cells [17-19]. “Inside-out” signaling is typically initiated by ligand-induced activation of SK which occurs in response to many signaling molecules, including growth factors, cytokines and even S1P itself [20] (for information regarding S1PRs as therapeutic targets, please see the comprehensive review by Aarthi, et. al [21]). Given the wide variety of significant processes in which the SK/S1P pathway is involved, it is of great importance to determine signals that are directly elicited by SK and S1P, as well as those that are indirectly mediated by the pathway.
3. Sphingosine Kinase (SK)
3.1. Sphingosine Kinase 1 (SK1)
Sphingosine kinase 1 and 2 (SK1 and SK2) are the enzymes responsible for the production of S1P from sphingosine. Surprisingly, very little is known concerning the structural features of the SKs, as little to no overall sequence similarity is shared between these enzymes and other known proteins, including lack of significant similarity with any well-characterized or recognizable regulatory or catalytic domains [22]. Sequence analysis in the forms of protein threading and motif searching have revealed weak structural similarities to other lipid kinases including diacylglycerol kinases (DAGK) and ceramide kinase (CK), as well as NAD Kinases and even 6-phosphofructokinases (PFKs) [23]. The crystal structure of PFK has been solved and displays close sequence homology with the ATP-binding domain of SK and therefore may share similar folding properties [23]. Moreover, the structure determination of a bacterial lipid kinase, YegS, possessing phosphatidylglycerol kinase activity, has provided a structural foundation that may aid in determining the structure-function relationship of other eukaryotic lipid kinases [24, 25]. Additionally, a SK1 homology model generated based on the known structure of DGKβ was used in the recent development of amidine-based SK inhibitors [26]. While the rational design of SK inhibitors is complicated by the lack of a defined structure, perhaps we can glean information from proteins with sequence similarity in hopes of identifying a potent inhibitor of SK which can then ultimately aid in the determination of the crystal structure.
A result of alternative splicing, three different forms of SK1 have been identified in humans (SK1a, SK1b and SK1c) [27]. SK1 has many important evolutionarily conserved regions including glycine 82 which is required for catalysis, aspartate 278 which is required for substrate binding and a diacylglycerol kinase domain [1, 28, 29]. Other regions of the SK1 enzyme that are homologous include a Ca2+/Calmodulin binding site, along with a TNF receptor associated factor 2 (TRAF2) and ATP binding site [22]. It has been shown that SK1 contains residues that bind phosphatidylserine and other acidic phospholipids, thus contributing to the subcellular localization of the enzyme [22, 30-33]. As with other sphingolipid metabolizing enzymes, SK1 subcellular localization determines the outcome of S1P signaling. While SK1 resides predominantly in the cytosol, it has been shown to undergo translocation to the plasma membrane following phosphorylation by ERK [30]. Functionally, membrane targeting is thought to position SK1 into close proximity with its sphingosine substrate [31]. When S1P is formed at the plasma membrane, it can then be easily exported from the cell and act locally in an autocrine or paracrine fashion [34, 35]. S1P produced by endoplasmic reticulum-, nuclear- or mitochondrial-localized SK2 [34, 35] is likely quickly degraded due to its closeness to ER-bound S1P Lyase and S1P Phosphatase which degrade and dephosphorylate S1P, respectively [36]. There have been reports of extracellular SK1, suggesting that export of SK1a from endothelial cells may influence vascular S1P gradient [37]. Also in support of extracellular SK1, oxidized LDL immune complexes have been shown to mediate the release of SK1 in a monocytic cell line [38, 39]. Despite those that were briefly mentioned, there exists no general, widely-accepted mechanism of SK1 regulation as there does for many well characterized enzymes. Determining the specific signaling events in which the SK/S1P pathway is involved, as well as determining the mechanisms by which the pathway is regulated during these signaling events can help expose the pathway as a potential therapeutic target.
3.2 Sphingosine Kinase 2 (SK2)
Located on a separate gene from SK1 is the less well-characterized isoform of the SKs, Sphingosine Kinase 2 (SK2). SK2 was originally thought to function in apoptosis and other functions contradictory to those of SK1, including functioning as a putative BH3-only protein capable of stimulating apoptotic signaling [40-42]. With the advent of the putative SK2-specific enzyme inhibitor, ABC294640, many novel functions of SK2 have recently been uncovered. First of all, inhibition of SK2 has been shown to sensitize cells to apoptotic stimuli suggesting a positive role for SK2 in cancer [43]. Also implicating SK2 in cancer are reports that SK2 ablation via siRNA prevents tumor cell proliferation and migration [44]. Moreover, SK2 has recently been implicated in ischemic preconditioning such that its pharmacological inhibition abolished preconditioning-induced tolerance in ischemic injury [45-47]. Compared to SK1, SK2 displays different sub-cellular localization patterns and is thought to be in the ER, nucleus and mitochondria [48]. Other than its subcellular localization and ERK2-mediated phosphorylation and activation, it remains unknown the direct mechanisms by which SK2 is regulated [49]. Although SK1-deficient or SK2-deficient mice are viable, knocking out both isoforms of SK renders blood vessel formation inadequate for development, affects neurogenesis and neural tube closure and embryos die by ED 13.5 [50]. This provides evidence for yet another significant physiological process, embryonic development, which requires proper function of the SK/S1P pathway. Determining the manner in which the SK/S1P pathway contributes to development will only magnify the therapeutic potential of the SK/S1P pathway, especially in diseases displaying activation of developmental programs such as cancer.
4. SK/S1P Pathway as a Therapeutic Target
4.1. Biomarker of Disease
Use of SK/S1P pathway-components as prognostic indicators and biomarkers of disease highlights the therapeutic potential of targeting the SK/S1P pathway. Sphingosine Kinase 1 is well known to be overexpressed in numerous cancers including thyroid cancer [51], Head and Neck Squamous Cell Carcinoma [52], glioblastoma [53], breast cancer [54, 55], gastric cancer [56] and lung cancer [57] (Table 1). Furthermore, in clinical studies sphingosine kinase expression and S1P levels have been correlated with cancer grade and patient survival and therefore have the potential to be used as biomarkers of various malignancies: for example gastric [56], breast [54, 55] and prostate cancer [58] (Table 1). Not only does the SK/S1P pathway lend itself to cancer detection and prognosis, but it is also emerging as a descriptive biomarker of cardiovascular disease [59, 60]. S1P has been implicated as a potential marker of cardiovascular disease in Fabry disease and obstructive coronary artery disease (CAD), for example. Left ventricular hypertrophy (LVH) and increased intima-media thickening (IMT) are hallmarks of Fabry disease. Interestingly, when plasma from diseased patients was analyzed, S1P was the most abundant component [61] (Table 1). Also, it has been reported that serum S1P levels correlate with CAD; such that, S1P levels were able to predict occurrence and severity significantly better than traditional predictors [62]. It should be noted, however, that S1P is involved in many important cellular functions, including those that regulate normal cellular behavior; therefore, when manipulating the SK/S1P pathway with the intent of therapeutic development, it is important to understand all facets of physiology that may be affected.
Table 1.
| Disease/Pathology | Role of the SK/S1P Pathway | Refs |
|---|---|---|
| Cancer | ||
| Thyroid, Head & Neck, Glioblastoma, Breast and Lung |
Increased SK1 expression | [44-47, 50] |
| Gastric, Prostate, Breast and Colon | SK expression and SIP levels correlate with cancer grade and survival; Potential biomarker |
[48, 49, 51] |
| Cardiovascular Disease | ||
| Fabry Disease | S1P most abundant component in plasma from patients | [54] |
| Coronary Artery Disease | Serum S1P better predicted occurrence and severity of disease than traditional predictors |
[55] |
| Atherosclerosis | SK/S1P/S1P3R-mediated upregulation of adhesion molecule expression and macrophage recruitment |
[115, 116] |
| Inflammatory Disease | ||
| Multiple Sclerosis | Sphingosine analog, Fingolimod, clinically used to prevent MS relapse |
[56, 57] |
| Ulcerative Colitis/Irritable Bowel Disease | Inhibition of knockout of SK decreases parameters of disease by abrogating underlying inflammatory components |
[58, 59] |
| Arthritis | SK knockout decreased disease parameters in murine models of arthritis |
[58,117, 118] |
| Asthma/Anaphylaxis | S1P activation of mast cells promotes large scale allergic reaction via recruitment and activation of many types of immune cells; inhibition of SK abrogates parameters of inflammation in mouse model of allergic asthma |
[119-122] |
| Other | ||
| Obesity & Diabetes | SK is an intermediate in in vitro & in vivo models of diabetic nephropathy via regulation of matrix deposition |
[64,123] |
| Ischemia/Reperfusion Injury | ||
| -Myocardial | Role for SK2 in myocardial ischemic preconditioning; SK2 knockout prevents precondition-mediated protection |
[38, 66-69, 124] |
| Cerebral | SK2 inhibition or knockout prevents isofluorane-induced cerebral preconditioning |
|
| Renal | SK1 is upregulated following I/R injury; SK1 knockout mice exhibit greater damage following I/R injury |
|
| Fibrosis | ||
| Scleroderma | TGF-β, a major player in scleroderma, mediates myoblast transdifferentiation any myofibroblast accumulation via an SK/S1P- dependent manner |
[125] [71, 126] |
| -Idiopathic Pulmonary Fibrosis | Increased S1P levels lead to EMT, accumulation of myofibroblasts and correlates with lung function in patients |
[72] |
| -Cardiac Remodeling | TGF-β induces cardiac fibroblast activation and myocardial fibrotic remodeling in an SK1-dependent manner |
4.2. Inflammatory Diseases
More evidence for targeting the SK/S1P pathway therapeutically emerges in inflammatory diseases (Table 1). Fingolimod is a sphingosine analog, which acts as a substrate for SK2, and is currently used in the clinic to prevent relapse in relapse-remitting multiple sclerosis [63, 64]. FTY720, once phosphorylated to FTY720-P functions as an agonist to four out of five S1P receptors, resulting in the ubiquitin-mediated degradation of the S1P1R and inability of lymphocytes to egress from lymphoid tissues, thus dampening the inflammatory response [65, 66]. Besides MS, the SK/S1P pathway is known to play a critical role in many other pathological processes that possess important inflammatory components. For example, increased activity of the SK/S1P pathway correlates with the initiation and perpetuation of ulcerative colitis (UC) and inflammatory bowel disease (IBD) in patient samples and in mouse models of disease [67]. SK and S1P have been shown to modulate these diseases such that inhibition of either SK1 [68] or SK2 [43] has been shown to abrogate underlying inflammatory components thus decreasing parameters of disease. Moreover, a recently developed S1P1R-specific antagonist, KRP-203, was found to inhibit parameters of disease in an IL-10 knockout model of chronic colitis by promoting lymphocyte sequestration to secondary lymphoid tissues, reducing CD4+ T cell and B220+ B cell colon infiltrate and inhibiting IF-γ, IL-12 and TNFα production by colonic lymphocytes [69]. Other inflammatory pathologies that rely, at least in part, on SK/S1P signaling include arthritis, asthma, anaphylaxis and atherosclerosis [70-72] (Table 1). While S1P, via S1P1R signaling, is directly involved in recruiting lymphocytes to local areas of inflammation, it is also involved in perpetuation of inflammatory signaling. For example, inhibition of SK in a murine collagen-induced arthritis reduced disease severity and decreased plasma levels of TNF-α, IL-6, IF-γ, and S1P [73]. Pharmacological inhibition of SK can function on two levels to inhibit inflammatory diseases such as arthritis and atherosclerosis by 1) inhibition of lymphocyte egress as well as 2) inhibition of secondary cytokine signaling. Discussed later, obesity and diabetes are SK-mediated diseases with inflammatory components and treatment options for these diseases could be expanded to include SK-targeted anti-inflammatory therapies. Given the major role that SK/S1P has been shown to play in the aforementioned inflammatory pathologies, it is important that the field persists in the development of pathway modulators for the generation of novel anti-inflammatory treatment modalities.
4.3. Other Diseases
Most recently identified, yet less well characterized are the roles of the SK/S1P pathway in obesity and diabetes, ischemia/reperfusion injury and fibrosis (Table 1). A recent report implicated the SK/S1P pathway as an intermediate in an in vitro model of diabetic nephropathy [74]. In glomerular mesangial cells, expression of dominant-negative SK1 or pharmacological inhibition impaired high glucose-stimulated fibronectin expression; meanwhile, kidneys from streptozotocin-induced diabetic rats exhibited hallmarks of diabetic nephropathy, such as matrix accumulation; this occurred concomitantly with increased SK1 message, protein and activity, as well as S1P levels [74]. Also, a recent study found correlations between S1P levels and circulating TNF-α, adiponectin, and free fatty acids in obese adolescents [75] which is in support of other reports implicating sphingolipids in the progression of obesity-related comorbidities in adults [76]. While obesity and diabetes possess many inflammatory components, it is likely that S1P-mediated lymphocyte egress and cytokine production can serve as a therapeutic target for some of the complications associated with these pathologies. In addition to obesity and diabetes, the SK/S1P pathway emerges as a potential player in ischemic preconditioning. Much of the evidence for SK in ischemic injury comes from studies carried out using knockout animals. SK2 has been shown to be involved in protection from ischemia in a number of studies. First, hearts from ischemic preconditioned SK2 null mice displayed greater area of damage and less recovery post-ischemia than wild-type mice, suggesting a role for SK2 in ischemic preconditioning of the myocardium [45]. Also, another study suggests that isofluorane-induced cerebral preconditioning occurs via SK2-mediated up regulation of Hif-1α because SK2-specific pharmacological inhibitor ABC294640-treated mice and SK2 knockout mice were not protected from injury following cerebral preconditioning [77]. On the other hand, some studies point to a role for SK1 in protection from I/R injury. Renal I/R injury in mice led to induction of kidney SK1 and mice lacking SK1 exhibited increased renal injury compared to wild-type mice [78-80]. This was rescued by overexpressing SK1, specifically in the kidney, through a mechanism involving S1P generation and S1P1R activation [78, 81]. Regardless of the specific isoform involved in I/R preconditioning and protection from injury, it is clear that the SK/S1P pathway has potential as a target in the development of I/R-related therapies. Lastly, targeting the SK/S1P pathway may be a potential arm of treatment for fibrotic diseases including scleroderma, idiopathic pulmonary fibrosis (IPF) and cardiac remodeling following myocardial infarction. A hallmark of IPF is the accumulation of myofibroblasts due to improper epithelial-mesenchymal transition (EMT). S1P is a well-known inducer of EMT and interestingly, levels are increased in IPF and correlate with lung function [82]. Also, transforming growth factor-β (TGF-β) activates cardiac fibroblasts following aortic banding in an SK1-dependent manner, suggesting that SK1 is involved in myocardial fibrotic remodeling and likely plays a role in cardiac fibrosis [83]. Given the role that TGF-β signaling plays in fibrotic diseases, as well as the role that TGF-β plays in activating the SK/S1P pathway, inhibition of TGF-β signaling and subsequent S1P signaling may function as a point of therapeutic intervention for pathologies possessing these components. The SK/S1P pathway has been shown to be involved in numerous pathologies ranging from inflammation and cancer to cardiovascular disease and diabetes; therefore, it is imperative that continued effort be put forth to develop pathway modulators, leading to novel and interesting therapeutic treatments.
5. Sphingosine Kinase Inhibitors
While overexpression studies using WT or kinase dead SK mutants have implicated the SK/S1P pathway in numerous cell biologies, gene silencing techniques have dramatically facilitated the study of SK in vitro and in vivo. Gene silencing in the form of small interfering siRNA, in vitro, has elucidated a role for SK in a plethora of signaling pathways associated with oncogenesis [84] and inflammation [85], for example, suggesting the SK/S1P pathway as a potential therapeutic target. More recently, however, siRNA has started to be employed in vivo in various models of disease. For example, Pushparaj, et. al. validated SK1 as a key player in C5a-mediated inflammation in vivo by i.v. injection of SK1 siRNA [86]. By optimizing in vivo siRNA administration, they avoided the previously employed “hydrodynamics” method whereby vascular damage occurred due to high speed and high volume i.v. injection [86]. Using longer administration times and lower volumes, the group achieved knockdown of SK1 in the liver, lung, spleen and peripheral blood mononuclear cells (PBMCs) [86]. In an additional study, the same group used tail vein injection of SK1 siRNA to establish a role for SK1 in mast cell-mediated anaphylaxis [87]. Interestingly, results from in vivo siRNA treatment revealed a compensatory mechanism in genetic knockout animals, as SK2 siRNA had no effect on mast cell function, while a role for SK2 was identified in mast cell-mediated anaphylaxis using a genetic knockout model [87]. In a novel mechanism of in vivo administration of siRNA, Masood, et. al. used nanotechnology to deliver SK1 siRNA in a mouse model of head and neck squamous cell carcinoma (HNSCC) [88]. SK1 siRNA carried by biocompatible gold nanorods lead to greater tumor regression and required lower radiation doses when injected intratumorally into subcutaneous tumors [88]. These studies lay the foundation for the use of SK siRNA for therapeutic interventions as well as further validate the need for the development of specific and potent mechanisms of SK inhibition.
It has become evident that the SK/S1P pathway is involved in multiple cellular processes that contribute to disease initiation, maintenance and progression; therefore, modulation of the pathway can prove useful in the development of important novel therapies for the treatment of various diseases including cancer, inflammatory diseases and vascular disorders. Two types of SK inhibitors will be discussed at length: sphingosine analog inhibitors and non-lipid small molecule inhibitors.
5.1. Sphingosine Analog Inhibitors
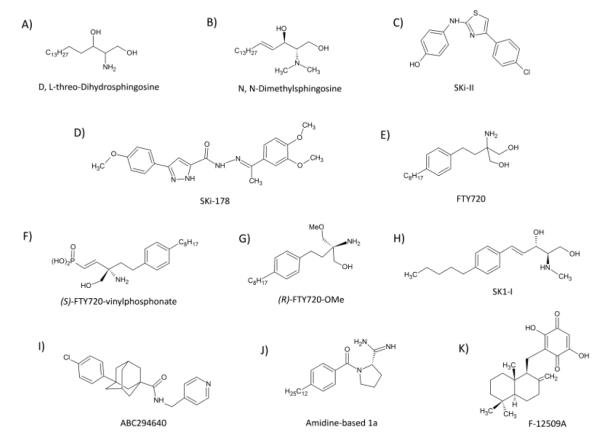
D,L-threo-dihydrosphingosine
D,L-threo-Dihydrosphingosine (DHS) (Fig..2), the synthetic threo stereoisomer of the naturally occurring D-erythro-dihydrosphingosine, is perhaps the earliest encountered inhibitor of SK [89]. Indicating a high degree of SK stereoselectivity, D,L-threo-DHS acts as a competitive inhibitor of SK1 with a Ki of approximately 3-6 μM (~0.2 mol%) (Fig. 2); alternatively, it acts as a substrate for SK2 and can be further metabolized, becoming incorporated into the sphingolipid metabolic pathway [89]. Although a fairly potent inhibitor of SK1, L-threo-DHS also inhibits other kinases and is, in fact, used as a PKC-α (PKCα)-specific inhibitor in both the laboratory and the clinic and is often referred to as safingol (Table 2) [90]. The off target effects and substrate properties of this compound make it a less-than-ideal SK inhibitor.
Figure 2.
Chemical Stucture of SK Inhibitors.
Table 2.
| Inhibitor | Kinase Inhibited |
Ki (μM) |
Type of Inhibition |
Comments | Refs |
|---|---|---|---|---|---|
| D,L-threo- DHS | SK1 | 3-6 | Competitive | Affects other kinases, i.e. PKC; substrate for SK2 |
[6, 20, 127- 131] |
| DMS | SK1 | 5 | Competitive | Lacks specificity: inhibits | [6, 127-130] |
| SK2 | 12 | Non-competitive | CK, PKC & MAPK; activates PI3K, SDK1, CKII & EGFR; activates SK at low concentrations; induces Ub-mediated SK1 proteolysis |
||
| SKi-II | SK1 | 17- | SK1: mixed | Lacks specificity; induces | [95-98] |
| SK2 | 50 | Kic=17; Kiu= 48 | Cathepsin-B-mediated proteolysis of SK1 |
||
| SK1-178 | SK1 | 1.3 | Competitive | Modification of SK1-l; First non-lipid, small molecule SK1-specific inhibitor |
[95, 96, 98] |
| FTY720 | SK1 | 2 | Competitive | Substrate for SK2; induces proteasomal degradation of SK1; used in the clinic as Gilenya® to treat MS |
[77, 90, 91, 132] |
| (S)-FTY720- vinyl-Pn |
SK1 | 15 | Uncompetitive | Induces SK1 proteasomal degradation; antagonist for all five S1P receptors |
[77, 90, 91, 132] |
| {R)-FTY720- OMe |
SK2 | 16.5 | Competitive | Induces Ub-mediated SK2 proteolysis |
[92] |
| SK1-I (BML- 241) |
SK1 | 10 | Competitive | NA | [93] |
| ABC294640 | SK2 | 9.8 | Competitive | NA | [99] |
| Amidine Based Inhibitors |
(la) SK1 (12aa)SK2 |
0.3 8 |
Competitive | Specificity affected by alkyl chain length |
[106, 109] |
| B-5354C | SK1/SK2 | 3 | Non-competitive | Natural product from marine bacterium |
[111, 113, 114] |
| F-12509A | SK1/SK2 | 5 | Competitive | Natural product from Trichopeziella barbata |
[111, 133] |
| S-1518S | ND | ~2 | ND | Natural product from Zopfiella inermis |
[114] |
Dimethylsphingosine
Originally established as an inhibitor of PKC [91], the N, N-Dimethyl derivative of sphingosine, (dimethylsphingosine, DMS, Fig. 2), is an inhibitor of both SK isoforms. DMS acts as a competitive inhibitor of SK1 (Ki=5μM) and a non-competitive inhibitor of SK2 (Ki=12μM) (Table 2) [92]. It was recently shown that DMS results in ubiquitin-mediated SK1 proteasomal degradation which may lead to more effective treatments for SK-reliant diseases [93, 94]. Unfortunately, DMS has been shown to have off target effects limiting its use as a specific inhibitor of SK in the laboratory, as well as in the clinic. DMS has been shown to have inhibitory effects on important cellular kinases including ceramide kinase (CK) [95] , PKC [91], SRC kinases [96], and MAPK [97], as well as stimulating effects on phosphatidylinositol kinase (PI3K) [98], sphingosine-dependent protein kinase 1(SDK1) [99], Casein Kinase II [100] and epidermal growth factor receptor (EGFR) (Table 2) [101] Concentration-dependent effects of DMS have also been observed, such that low concentrations have been shown to enhance SK activity [102]. While many studies have used DMS as a tool to identify functions of SK, it is important to consider its many off target effects when interpreting results.
FTY720
FTY720 (2-amino-2-[2-(4-octylphenyl) ethyl] propane-1, 3-diol) is structurally similar to sphingosine (Fig. 3) and the lead compound from which it was derived, ISP-1 (myriocin), was isolated from the fungus Isaria sinclairii [103]. Also known as Fingolimod, or by trade name GilenyaTM, this compound is currently used in the clinic to quell symptoms and slow the progression of multiple sclerosis (MS) (Table 2) [104, 105]. Fingolimod’s primary mode of action is through T-lymphocyte-specific immunosuppression [104]. Following phosphorylation by SK2, FTY720-P, acts as a receptor activator at four of the five S1PRs: S1P1R, S1P3R, S1P4R and S1P5R. Drug exposure leads to activation and subsequent ubiquitin-mediated degradation of S1P1R, the main chemotaxis-mediating receptor in lymphocytes, resulting in T-cell sequestration to the lymph nodes [104]. Not only does FTY720 act on the SK/S1P pathway by interacting with S1P cell-surface receptors, but it also acts as a competitive inhibitor of SK1 (with respect to sphingosine) with a Ki around 2μM (Table 2) [93]. Similar to other SK inhibitors (see DMS and FTY720-phosphonate discussions), FTY720 induces the proteasomal degradation of the SK1 splice variant, SK1a [93]. Due to its success in the clinic, FTY720 has served as the basis for chemical derivatives that have shown increased potency and specificity.
(S)-FTY720-vinylphosphonate
Rationally designed derivatives of the previously described FTY720, the unsaturated phosphonate enantiomers (R)- and (S)-FTY720-vinylphosphonate (R- or S-vinyl-Pn) (Fig. 2), also function to inhibit SK with the (S)-enantiomer being superior, inhibiting purified SK1 activity by 90% [93] and purified SK2 by 70%. (S)-vinyl-Pn is more effective at inhibiting SK1 than DMS, FTY720 and SKi-II (discussed below) and is an uncompetitive inhibitor with a Ki of approximately 15μM (Table 2) [93]. Like its parent compound, (S)-vinyl-Pn leads to the proteasomal degradation of SK1 and other than S1P, is the only antagonist known to act on all five S1PRs (Table 2) [106], with full antagonism at S1P1R, S1P3R and S1P4R and partial antagonism at S1P2R and S1P5R [107]. Also similar to its lead compound, both enantiomers of FTY720-vinyl-Pn induce transient peripheral lymphopenia when administered to mice [107]. Other non-specific effects include potent, dose-dependent inhibition of lysophospholipase D (autotaxin) [107]. Further in vivo characterization is necessary to determine the therapeutic potential of (S)-FTY720-vinyl-Pn.
(R)-FTY720-OMe
One of the most recently developed SK inhibitors is also a derivative of the clinically successful FTY720, (R)-FTY720 methyl ether (FTY720-OMe) (Fig. 2), and acts competitively with sphingosine to inhibit SK2 displaying a Ki of 27μM (Table 2) [108]. In order to block the FTY720 site of phosphorylation by SK2, Lim et. al. replaced the prochiral group with a methoxy group resulting in enantioselective, SK2-specific inhibition (Fig. 2) [108]. The methyl ether compound, similar to its parent compound, decreased the expression of SK2 and this was inhibited by the addition of ubiquitin-proteasomal pathway inhibitor, MG132 (Table 2) [108]. (R)-FTY720-OMe inhibited DNA synthesis and induced apoptosis, as well as stimulated focal adhesion assembly in HEK 293 cells [108]. Like other Fingolimod derivatives, future studies assessing the likelihood of (R)-FTY720-OMe’s use in the clinic are required.
SK1-I
The first SK inhibitor displaying specificity for SK1 is the water soluble sphingosine analog, SK1-I (BML-258; (2R,3S,4E)-N-methyl-5-(4-pentylphenyl)-2-aminopent-4-ene-1,3-diol) (Figure 2) [109]. A competitive inhibitor of SK1 (Ki=10μM) (Table 2), SK1-I inhibited growth and survival of cultured leukemia cells as well as inhibited growth of leukemia xenograph tumors [109]. SK1-I resulted in down regulation of pro-survival ERK and Akt signals and did not show activity against numerous prominent cellular kinases [109]. SK1-mediated promotion of breast cancer was abolished via SK1-I-mediated inhibition of angio- and lymphangiogenesis [110]. With good bioavailability and low cytotoxicity towards non-cancerous cells, this compound has potential therapeutic value for the treatment of SK1-mediated diseases [109].
5.2. Non-lipid Small Molecule Inhibitors
SKi-II
Discovered through a screen, the SKi group of molecules were the first non-lipid, small molecule inhibitors of SK detected [111]. SKi-II (1-(p-hydroxyanilino)-4-(p-chlorophenyl)) thiazole (Fig. 2) , the most well-characterized SKi compound, is not specific for SK1 or SK2 and displays mixed inhibition of SK1 with a competitive inhibition constant of 17μM and an uncompetitive inhibition constant of 48μM (Table 2) [93] . SKi-II has been shown to inhibit S1P generation and proliferation, and induce apoptosis in numerous cancer cell lines [112]. It displays good oral bioavailability and has been shown to successfully inhibit parameters of disease in a DSS-induced mouse model of ulcerative colitis [68]. Mechanistically, SKi-II induces Cathepsin-B-mediated lysosomal degradation of SK1 such that the half-life is reduced from greater than 24h to less than one hour, suggesting potential off-target effects [113]. SKi-II has been shown to lack effects on PKC, ERK and PI3K [111]. A modification of SKi molecule, SKi-I (N’-[(2-hydroxy-1-napthyl) methylene]-3-(2-napthyl)-1H-pyrazole-5-carbohydrazide), led to development of SK1-specific inhibitor SKi-178 (N’-[(1E)-1-(3,4-dimethoxy) ethyldiene]-3-(4-methoxyphenyl-1H-pyrazole-5-carbohydrazide)) [111, 114]. One of the less well characterized SK1 inhibitors, SKi-178 is competitive with sphingosine and is more potent that its parent molecule, with a Ki of 1.3μM (Table 2). The addition of methyl and methoxy groups to the lead compound enhanced pharmacological properties, decreasing cytotoxicity and enhancing selectivity for SK1 and making it the first non-lipid small molecule SK1-specific inhibitor (Fig. 2) [114].
ABC294640
Perhaps one of the best characterized in vivo inhibitors of SK is the small molecule, non-lipid inhibitor ABC294640 (Fig. 2) [115]. An isoform-specific inhibitor of SK2, with a Ki=9.8μM (Table 2), ABC294640 has exposed novel roles for SK2 in a number of diseases, highlighting the importance of in vitro as well as and in vivo characterization of pharmacological inhibitors. In the two years since its introduction, ABC294640 has been shown to promote autophagy in tumor cells leading to non-apoptotic cell death [115], inhibit NFκB-mediated chemo resistance in breast cancer [116] and act synergistically with other chemotherapeutics to decrease pro-survival signals in hepatocellular cancer [117]. In addition to its antitumor activities, ABC294640 has shown promise as a potential therapy for inflammatory diseases. ABC294640 attenuated TNBS (2,4,6-trinitrobenzene sulfonic acid)-mediated gastric inflammation [118], inhibited colitis-driven colon cancer [43], prevented arthritis in two different rodent models [119] and improved mitochondria function following hepatic ischemia/reperfusion [47]. Development of SK2-specific inhibitor ABC294640 has revealed many previously unknown functions of SK2 and seems promising for future clinical use.
Amidine-based
Without a doubt, the most potent of the SK inhibitors are the recently developed amidine-based molecules. Moving away from potentially cytotoxic long-chain bases and adenosine analogs, potent and selective inhibitors possessing an amidine ‘warhead’ were developed (Fig. 2) [120]. The distinctive electrostatic properties of the basic amidine group and its direct interaction with ATP γ-phosphate are thought to be essential for its inhibitory properties [120] . As previously mentioned, the amidine-based SK1-specific inhibitor (referred to as 1a) is highly potent, exhibiting a Ki of 0.1uM, approximately 100 times more potent than other SK inhibitors (Table 2) [120]. Compared to the lead molecule, VPC94075 [121], the rigidity, direction of amidine functional group and the extended tail of 1a confers its specificity for SK1 [26]. Compound 1a is competitive with respect to sphingosine and does not affect other lipid kinases at 30-times the concentration of the Ki [120]. When administered in vitro, 1a decreases S1P levels as early as 10 minutes and keeps them down for approximately 24 hours [122]. Interestingly, 1a provokes less than a 2-fold increase in sphingosine and dihydrosphingosine and only significantly increases ceramide levels with 10μM treatment, 100 times the concentration needed for SK1 inhibition [123]. Also, 1a is shown to inhibit pro-survival ERK and Akt signals and induce PARP cleavage; however, this only occurs following 16 hours of treatment at 10μM [120]. In mice, S1P levels are decreased by 50% and the compound cleared from the bloodstream by one hour post-administration [120]. SK2-selective amidine-based compound, 12aa has been developed as well. Quaternary ammonium salts containing lipophilic phenyl backbones and cyclohexylammonium head groups have been described recently as low μM inhibitors of SK2 (Ki ~8μM) (Fig. 2) (Table 1) [124] and inhibit the phosphorylation of SK/S1P downstream effectors ERK and Akt [124]. Knott et. al. followed up on this SK2-specific inhibitor demonstrating that alkyl chain length greatly affects SK isoform selectivity [125]. Given the potency and specificity of the amidine-based SK inhibitors, there is great potential for these compounds to be developed into successful therapeutics.
Natural Products
Although less well characterized, there have been a few natural compounds isolated from bacteria and fungi that have inhibitory effects towards sphingosine kinase. B-5354 inhibits SK1 and SK2 and was isolated from a marine bacterium. With a Ki of approximately 3uM, it acts in a non-competitive manner with respect to sphingosine and has been shown to sensitize prostate cancer cells to chemotherapeutics [126-128]. Isolated from the culture broth of Trichopezizella barbata, F-12509A was shown to competitively inhibit both isoforms of SK, as well as induce apoptosis in cancer cells [126]. Lastly, S-15183a/b is a natural product isolated from the fungus Zopfiella inermis. Although the specificities of these compounds are not known, they have been shown to decrease S1P generated in platelets [129]. While the natural product SK inhibitors are less well-characterized, they provide potential lead compounds for the development of even more potent and specific SK inhibitors.
6. Conclusions
Sphingosine kinase is an important player in regulating the balance of bioactive sphingolipid signaling molecules ceramide, sphingosine and S1P. Pathological conditions resulting from the deregulation of sphingolipid metabolism are numerous, and a disproportionate amount of these conditions are known to be mediated by the SK/S1P pathway. The recent development of high throughput SK assays [130] will no doubt facilitate the identification of novel chemical structures and potential inhibitors by expediting the screening of chemical libraries. Previous and ongoing development of SK inhibitors will no doubt enhance treatment options for diseases possessing SK/S1P-dependent components.
Highlights.
The SK/S1P pathway is dysregulated in numerous pathologies and disease states.
Only recently have potent and specific inhibitors of SK begun to be developed.
Further development of SK inhibitors may provide novel disease treatments.
Acknowledgments
Funding This manuscript is based upon work supported in part by a MERIT Award, [BX000156-01A1] (L.M.O.) by the Office of Research and Development, Department of Veterans Affairs, Northport VA Medical Center, Northport, NY. The content of this material does not represent the views of the Department of Veterans Affairs or the United State Government; US Department of Education Graduate Assistance in Areas of National Need (GAANN) in Lipid Biology and New Technologies, [P200A070596] (K.A.O.G.); and National Cancer Institute [PO1-CA97132 (L.M.O.)] and National Institutes of Health National Institute of General Medical Sciences [R01-GM062887 (L.M.O.)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer D. Zu Heringdorf, Regulation and functional roles of sphingosine kinases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- [2].Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim.et Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [3].Hannun YA. Sphingolipid second messengers: tumor suppressor lipids. Adv. Exp Med. Bio. 1997;400A:305–312. doi: 10.1007/978-1-4615-5325-0_43. [DOI] [PubMed] [Google Scholar]
- [4].Penno A, Reilly MM, Houlden H, Laura M, Rentsch K, Niederkofler V, Stoeckli ET, Nicholson G, Eichler F, Brown RH, Jr., von Eckardstein A, Hornemann T. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- [6].Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- [7].Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baumruker T, Bornancin F, Billich A. The role of sphingosine and ceramide kinases in inflammatory responses. Immunol.Lett. 2005;96:175–185. doi: 10.1016/j.imlet.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [9].Pyne S, Long JS, Ktistakis NT, Pyne NJ. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem.Soc. Transact. 2005;33:1370–1374. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- [10].Alderton F, Darroch P, Sambi B, McKie A, Ahmed IS, Pyne N, Pyne S. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 2001;276:13452–13460. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- [11].Aarthi P, Harini R, Sowmiya M, Malathi J, Therese KL, Madhavan HN. Identification of bacteria in culture negative and polymerase chain reaction (PCR) positive intraocular specimen from patients with infectious endopthalmitis. J. Microbiol. Meth. 2011;85:47–52. doi: 10.1016/j.mimet.2011.01.010. [DOI] [PubMed] [Google Scholar]
- [12].Olivera A, Rosenfeldt HM, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. J. Biol. Chem. 2003;278:46452–46460. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- [13].Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews. Mol. Cell. Bio. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- [15].Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 Functions as a Sphingosine-1-Phosphate Transporter in Vascular Endothelial Cells. PLoS. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L, Lucas M, Estabel J, Ryder E, Adissu H, Adams NC, Ramirez-Solis R, White JK, Steel KP, Dougan G, Hancock RE. The role of sphingosine-1-phosphate transporter spns2 in immune system function. J. Immunol. 2012;189:102–111. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. & Therapeutics. 2000;88:115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- [18].Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. PNAS. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- [20].Alemany R, Kleuser B, Ruwisch L, Danneberg K, Lass H, Hashemi R, Spiegel S, Jakobs KH, Meyer zu Heringdorf D. Depolarisation induces rapid and transient formation of intracellular sphingosine-1-phosphate. FEBS Lett. 2001;509:239–244. doi: 10.1016/s0014-5793(01)03168-4. [DOI] [PubMed] [Google Scholar]
- [21].Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of the S1P/S1PR axis in health and disease. J. Dent. Res. 2011;90:841–854. doi: 10.1177/0022034510389178. [DOI] [PubMed] [Google Scholar]
- [22].Pitson SM, Moretti PA, Zebol JR, Zareie R, Derian CK, Darrow AL, Qi J, D’Andrea RJ, Bagley CJ, Vadas MA, Wattenberg BW. The nucleotide-binding site of human sphingosine kinase 1. J. Biol. Chem. 2002;277:49545–49553. doi: 10.1074/jbc.M206687200. [DOI] [PubMed] [Google Scholar]
- [23].Labesse G, Douguet D, Assairi L, Gilles AM. Diacylglyceride kinases, sphingosine kinases and NAD kinases: distant relatives of 6-phosphofructokinases. Trends Biochem. Sci. 2002;27:273–275. doi: 10.1016/s0968-0004(02)02093-5. [DOI] [PubMed] [Google Scholar]
- [24].Bakali HM, Herman MD, Johnson KA, Kelly AA, Wieslander A, Hallberg BM, Nordlund P. Crystal structure of YegS, a homologue to the mammalian diacylglycerol kinases, reveals a novel regulatory metal binding site. J. Biol. Chem. 2007;282:19644–19652. doi: 10.1074/jbc.M604852200. [DOI] [PubMed] [Google Scholar]
- [25].Bakali MA, Nordlund P, Hallberg BM. Expression, purification, crystallization and preliminary diffraction studies of the mammalian DAG kinase homologue YegS from Escherichia coli. Acta Crystallog. Struct. Biol. Cryst. Commun. 2006;62:295–297. doi: 10.1107/S1744309106004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer ML, East JE, Houck JD, Lynch KR, Macdonald TL. Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J. Med. Chem. 2011;54:3524–3548. doi: 10.1021/jm2001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh N, Khan F, Proia RL, Hla T. Extracellular export of Sphingosine Kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006 doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pitson SM, D’Andrea J, Vandeleur R,L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem. J. 2000;350(Pt 2):429–441. [PMC free article] [PubMed] [Google Scholar]
- [29].Yokota S, Taniguchi Y, Kihara A, Mitsutake S, Igarashi Y. Asp177 in C4 domain of mouse sphingosine kinase 1a is important for the sphingosine recognition. FEBS Lett. 2004;578:106–110. doi: 10.1016/j.febslet.2004.10.081. [DOI] [PubMed] [Google Scholar]
- [30].Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. J. Biol. Chem. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- [32].Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J. Biol. Chem. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- [33].Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D’Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- [34].Siow D, Wattenberg B. The compartmentalization and translocation of the sphingosine kinases: mechanisms and functions in cell signaling and sphingolipid metabolism. Crit. Rev. Biochem. Mol. Biol. 2011;46:365–375. doi: 10.3109/10409238.2011.580097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siow DL, Anderson CD, Berdyshev EV, Skobeleva A, Pitson SM, Wattenberg BW. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J. Lipid Res. 2010;51:2546–2559. doi: 10.1194/jlr.M004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim.Biophys. Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79:126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- [39].Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- [40].Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- [41].Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr., Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- [42].Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura SI. Involvement of N-terminally extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 2005 doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- [43].Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31:1787–1793. doi: 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gao P, Smith CD. Ablation of sphingosine kinase-2 inhibits tumor cell proliferation and migration. Molecular cancer research : Mol. Cell. Res. 2011;9:1509–1519. doi: 10.1158/1541-7786.MCR-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid. Med. Cell. Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huwiler A, Kotelevets N, Xin C, Pastukhov O, Pfeilschifter J, Zangemeister-Wittke U. Loss of sphingosine kinase-1 in carcinoma cells increases formation of reactive oxygen species and sensitivity to doxorubicin-induced DNA damage. Brit.J. Pharmacol. 2011;162:532–543. doi: 10.1111/j.1476-5381.2010.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shi Y, Rehman H, Ramshesh VK, Schwartz J, Liu Q, Krishnasamy Y, Zhang X, Lemasters JJ, Smith CD, Zhong Z. Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. J. Hepatol. 2012;56:137–145. doi: 10.1016/j.jhep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- [50].Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol, Cell. Bio. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Guan H, Liu L, Cai J, Liu J, Ye C, Li M, Li Y. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol. Endo. 2011;25:1858–1866. doi: 10.1210/me.2011-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Facchinetti MM, Gandini NA, Fermento ME, Sterin-Speziale NB, Ji Y, Patel V, Gutkind JS, Rivadulla MG, Curino AC. The expression of sphingosine kinase-1 in head and neck carcinoma. Cells Tissues Organs. 2010;192:314–324. doi: 10.1159/000318173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J. Neuropath. Exp. Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- [54].Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, Pyne S, Pyne NJ, Edwards J. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 2010;177:2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Karn T, Kaufmann M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Can. Res. Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- [56].Zhuge YH, Tao HQ, Wang YY. Relationship between sphingosine kinase 1 expression and tumor invasion, metastasis and prognosis in gastric cancer. Zhonghua Yi Xue Za Zhi. 2011;91:2765–2768. [PubMed] [Google Scholar]
- [57].Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J. Histochem. Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- [58].Nunes J, Naymark M, Sauer L, Muhammad A, Keun H, Sturge J, Stebbing J, Waxman J, Pchejetski D. Circulating sphingosine-1-phosphate and erythrocyte sphingosine kinase-1 activity as novel biomarkers for early prostate cancer detection. Brit. J. Can. 2012;106:909–915. doi: 10.1038/bjc.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Baranowski M, Blachnio-Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M, Zabielski P, Gorski J. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J. Lipid. Res. 2010;51:74–80. doi: 10.1194/jlr.M900002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Keul P, Sattler K, Levkau B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail. Rev. 2007;12:301–306. doi: 10.1007/s10741-007-9038-x. [DOI] [PubMed] [Google Scholar]
- [61].Brakch N, Dormond O, Bekri S, Golshayan D, Correvon M, Mazzolai L, Steinmann B, Barbey F. Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Euro. Heart J. 2010;31:67–76. doi: 10.1093/eurheartj/ehp387. [DOI] [PubMed] [Google Scholar]
- [62].Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- [63].Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yeh EA, Weinstock-Guttman B. Fingolimod: an oral disease-modifying therapy for relapsing multiple sclerosis. Adv. Ther. 2011;28:270–278. doi: 10.1007/s12325-011-0004-6. [DOI] [PubMed] [Google Scholar]
- [65].Zhi L, Kim P, Thompson BD, Pitsillides C, Bankovich AJ, Yun SH, Lin CP, Cyster JG, Wu MX. FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus. J. Immunol. 2011;187:2244–2251. doi: 10.4049/jimmunol.1100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- [67].Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Digest. Dis. Sci. 2008;53:997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J. Pharmacol. Exp. Therapeut. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- [70].Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92:707–715. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hammad SM. Blood sphingolipids in homeostasis and pathobiology. Adv. Exp. Med. Biol. 2011;721:57–66. doi: 10.1007/978-1-4614-0650-1_4. [DOI] [PubMed] [Google Scholar]
- [72].Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, McInnes IB, Melendez AJ, Leung BP. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol. 2008;181:8010–8017. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- [74].Lan T, Liu W, Xie X, Xu S, Huang K, Peng J, Shen X, Liu P, Wang L, Xia P, Huang H. Sphingosine kinase-1 pathway mediates high glucose-induced fibronectin expression in glomerular mesangial cells. Mol. Endo. 2011;25:2094–2105. doi: 10.1210/me.2011-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Majumdar I, Mastrandrea LD. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine. 2012 doi: 10.1007/s12020-011-9589-4. [DOI] [PubMed] [Google Scholar]
- [76].Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- [77].Yung LM, Wei Y, Qin T, Wang Y, Smith CD, Waeber C. Sphingosine kinase 2 mediates cerebral preconditioning and protects the mouse brain against ischemic injury. Stroke. 2012;43:199–204. doi: 10.1161/STROKEAHA.111.626911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2012;23:266–280. doi: 10.1681/ASN.2011050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Park SW, Kim M, D’Agati VD, Lee HT. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Inter. 2011;80:1315–1327. doi: 10.1038/ki.2011.281. [DOI] [PubMed] [Google Scholar]
- [80].Park SR, Cho HJ, Moon KJ, Chun KH, Kong SY, Yoon SS, Lee JS, Park S. Cytotoxic effects of novel phytosphingosine derivatives, including N,N-dimethylphytosphingosine and N-monomethylphytosphingosine, in human leukemia cell line HL60. Leuk. Lymphoma. 2010;51:132–145. doi: 10.3109/10428190903383419. [DOI] [PubMed] [Google Scholar]
- [81].Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab. Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Milara J, Navarro R, Juan G, Peiro T, Serrano A, Ramon M, Morcillo E, Cortijo J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- [83].Pchejetski D, Foussal C, Alfarano C, Lairez O, Calise D, Guilbeau-Frugier C, Schaak S, Seguelas MH, Wanecq E, Valet P, Parini A, Kunduzova O. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Euro. Heart J. 2011 doi: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- [84].Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Current Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- [85].Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- [86].Pushparaj PN, H’Ng S C, Melendez AJ. Refining siRNA in vivo transfection: silencing SPHK1 reveals its key role in C5a-induced inflammation in vivo. Int. J. Biochem. Cell Biol. 2008;40:1817–1825. doi: 10.1016/j.biocel.2008.01.015. [DOI] [PubMed] [Google Scholar]
- [87].Pushparaj PN, Manikandan J, Tay HK, H’Ng S C, Kumar SD, Pfeilschifter J, Huwiler A, Melendez AJ. Sphingosine kinase 1 is pivotal for Fc epsilon RI-mediated mast cell signaling and functional responses in vitro and in vivo. J. Immunol. 2009;183:221–227. doi: 10.4049/jimmunol.0803430. [DOI] [PubMed] [Google Scholar]
- [88].Masood R, Roy I, Zu S, Hochstim C, Yong KT, Law WC, Ding H, Sinha UK, Prasad PN. Gold nanorod-sphingosine kinase siRNA nanocomplexes: a novel therapeutic tool for potent radiosensitization of head and neck cancer. Integr. Biol. (Camb) 2012;4:132–141. doi: 10.1039/c1ib00060h. [DOI] [PubMed] [Google Scholar]
- [89].Buehrer BM, Bell RM. Inhibition of sphingosine kinase in vitro and in platelets. Implications for signal transduction pathways. J. Biol. Chem. 1992;267:3154–3159. [PubMed] [Google Scholar]
- [90].Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH, Jr., Schwartz GK. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–193. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- [91].Igarashi Y, Hakomori S. Enzymatic synthesis of N,N-dimethyl-sphingosine: demonstration of the sphingosine: N-methyltransferase in mouse brain. Biochem. Biophys. Res. Commun. 1989;164:1411–1416. doi: 10.1016/0006-291x(89)91827-5. [DOI] [PubMed] [Google Scholar]
- [92].Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- [93].Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, Pyne NJ. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J. Biol. Chem. 2011;286:18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Loveridge C, Tonelli F, Leclercq T, Lim KG, Long JS, Berdyshev E, Tate RJ, Natarajan V, Pitson SM, Pyne NJ, Pyne S. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol. Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J. Biol. Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- [96].Igarashi Y, Hakomori S, Toyokuni T, Dean B, Fujita S, Sugimoto M, Ogawa T, el-Ghendy K, Racker E. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochem. 1989;28:6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- [97].Sakakura C, Sweeney E, Shirahama T, Ruan F, Solca F, Kohno M, Hakomori S, Fischer E, Igarashi Y. Inhibition of MAP kinase by sphingosine and its methylated derivative, N,N-dimethylsphingosine. Int. J. Oncol. 1997;11:31–39. doi: 10.3892/ijo.11.1.31. [DOI] [PubMed] [Google Scholar]
- [98].King CC, Zenke FT, Dawson PE, Dutil EM, Newton AC, Hemmings BA, Bokoch GM. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 2000;275:18108–18113. doi: 10.1074/jbc.M909663199. [DOI] [PubMed] [Google Scholar]
- [99].Megidish T, Cooper J, Zhang L, Fu H, Hakomori S. A novel sphingosine-dependent protein kinase (SDK1) specifically phosphorylates certain isoforms of 14-3-3 protein. J. Biol. Chem. 1998;273:21834–21845. doi: 10.1074/jbc.273.34.21834. [DOI] [PubMed] [Google Scholar]
- [100].McDonald OB, Hannun YA, Reynolds CH, Sahyoun N. Activation of casein kinase II by sphingosine. J. Biol. Chem. 1991;266:21773–21776. [PubMed] [Google Scholar]
- [101].Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochem. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- [102].Jin YX, Yoo HS, Kihara A, Choi CH, Oh S, Moon DC, Igarashi Y, Lee YM. Sphingosine kinase assay system with fluorescent detection in high performance liquid chromatography. Arch. Pharmacol. Res. 2006;29:1049–1054. doi: 10.1007/BF02969290. [DOI] [PubMed] [Google Scholar]
- [103].Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect. Medicin. Chem. 2008;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- [104].Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fingolimod for multiple sclerosis. Drug. Ther. Bull. 2012;50:18–20. doi: 10.1136/dtb.2012.02.0086. [DOI] [PubMed] [Google Scholar]
- [106].Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell. Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Valentine WJ, Kiss GN, Liu J, Gotoh SE,M, Murakami-Murofushi K, Pham TC, Baker DL, Parrill AL, Lu X, Sun C, Bittman R, Pyne NJ, Tigyi G. (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. Cell. Signal. 2010;22:1543–1553. doi: 10.1016/j.cellsig.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S. (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: Effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell. Signal. 2011;23:1590–1595. doi: 10.1016/j.cellsig.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Can. Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Can. Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- [112].French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J. Pharmacol. Exp. Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- [113].Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell. Physiol. Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- [114].Hengst JA, Wang X, Sk UH, Sharma AK, Amin S, Yun JK. Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorg. Med. Chem. Lett. 2010;20:7498–7502. doi: 10.1016/j.bmcl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- [115].French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Can. Biol. & Therapy. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Can. Biol & Therapy. 2011;11:524–534. doi: 10.4161/cbt.11.5.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacol. 2010;18:73–85. doi: 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- [119].Fitzpatrick LR, Green C, Frauenhoffer EE, French KJ, Zhuang Y, Maines LW, Upson JJ, Paul E, Donahue H, Mosher TJ, Smith CD. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacol. 2011;19:75–87. doi: 10.1007/s10787-010-0060-6. [DOI] [PubMed] [Google Scholar]
- [120].Kharel Y, Mathews TP, Gellett AM, Tomsig JL, Kennedy PC, Moyer ML, Macdonald TL, Lynch KR. Sphingosine kinase type 1 inhibition reveals rapid turnover of circulating sphingosine 1-phosphate. Biochem. J. 2011;440:345–353. doi: 10.1042/BJ20110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mathews TP, Kennedy AJ, Kharel Y, Kennedy PC, Nicoara O, Sunkara M, Morris AJ, Wamhoff BR, Lynch KR, Macdonald TL. Discovery, biological evaluation, and structure-activity relationship of amidine based sphingosine kinase inhibitors. J. Med. Chem. 2010;53:2766–2778. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kharel MK, Pahari P, Shepherd MD, Tibrewal N, Nybo SE, Shaaban KA, Rohr J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012;29:264–325. doi: 10.1039/c1np00068c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kharel P, Sellmyer DJ. Anomalous Hall effect and electron transport in ferromagnetic MnBi films. J. Phys. Condens. Matter. 2011;23:426001. doi: 10.1088/0953-8984/23/42/426001. [DOI] [PubMed] [Google Scholar]
- [124].Raje MR, Knott K, Kharel Y, Bissel P, Lynch KR, Santos WL. Design, synthesis and biological activity of sphingosine kinase 2 selective inhibitors. Bioorg. & Med. Chem. 2012;20:183–194. doi: 10.1016/j.bmc.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Knott K, Kharel Y, Raje MR, Lynch KR, Santos WL. Effect of alkyl chain length on sphingosine kinase 2 selectivity. Bioorg Med. Chem. Lett. 2012 doi: 10.1016/j.bmcl.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kono K, Sugiura M, Kohama T. Inhibition of recombinant sphingosine kinases by novel inhibitors of microbial origin, F-12509A and B-5354c. J. Antibiot. 2002;55:99–103. doi: 10.7164/antibiotics.55.99. [DOI] [PubMed] [Google Scholar]
- [127].Kono K, Tanaka M, Ogita T, Kohama T. Characterization of B-5354c, a new sphingosine kinase inhibitor, produced by a marine bacterium. J. Antibiot. 2000;53:759–764. doi: 10.7164/antibiotics.53.759. [DOI] [PubMed] [Google Scholar]
- [128].Kono K, Tanaka M, Mizuno T, Kodama K, Ogita T, Kohama T. B-535a, b and c, new sphingosine kinase inhibitors, produced by a marine bacterium; taxonomy, fermentation, isolation, physico-chemical properties and structure determination. J. Antibiot. 2000;53:753–758. doi: 10.7164/antibiotics.53.753. [DOI] [PubMed] [Google Scholar]
- [129].Kono K, Tanaka M, Ono Y, Hosoya T, Ogita T, Kohama T. S-15183a and b, new sphingosine kinase inhibitors, produced by a fungus. J. Antibiot. 2001;54:415–420. doi: 10.7164/antibiotics.54.415. [DOI] [PubMed] [Google Scholar]
- [130].Kharel Y, Mathews TP, Kennedy AJ, Houck JD, Macdonald TL, Lynch KR. A rapid assay for assessment of sphingosine kinase inhibitors and substrates. Analyt. Biochem. 2011;411:230–235. doi: 10.1016/j.ab.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Keul P, Lucke S, von Wnuck K. Lipinski, C. Bode, M. Graler, G. Heusch, B. Levkau, Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- [132].Weis T, Volker W, Holtwick R, Al Chahaf M, Schmidt A. Sphingosine 1-phosphate (S1P) induces expression of E-selectin and adhesion of monocytes via intracellular signalling pathways in vascular endothelial cells. Eur. J. Cell. Biol. 2010;89:733–741. doi: 10.1016/j.ejcb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- [133].Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J. Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Lai WQ, Irwan AW, Goh HH, Melendez AJ, McInnes IB, Leung BP. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J. Immunol. 2009;183:2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- [135].Lai WQ, Wong WS, Leung BP. Sphingosine kinase and sphingosine 1-phosphate in asthma. Biosci. Rep. 2011;31:145–150. doi: 10.1042/BSR20100087. [DOI] [PubMed] [Google Scholar]
- [136].Lai WQ, Goh HH, Bao Z, Wong WS, Melendez AJ, Leung BP. The role of sphingosine kinase in a murine model of allergic asthma. J. Immunol. 2008;180:4323–4329. doi: 10.4049/jimmunol.180.6.4323. [DOI] [PubMed] [Google Scholar]
- [137].Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- [138].Oskeritzian CA, Milstien S, Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol. & Therapeutics. 2007;115:390–399. doi: 10.1016/j.pharmthera.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lan T, Shen X, Liu P, Liu W, Xu S, Xie X, Jiang Q, Li W, Huang H. Berberine ameliorates renal injury in diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P signaling pathway. Arch. Biochem. Biophys. 2010;502:112–120. doi: 10.1016/j.abb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- [140].Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am. J. Physiol. Heart. Circ. Physiol. 2009;297:H1429–1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol. Cell. 2010;21:1111–1124. doi: 10.1091/mbc.E09-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Milara J, Mata M, Mauricio MD, Donet E, Morcillo EJ, Cortijo J. Sphingosine-1-phosphate increases human alveolar epithelial IL-8 secretion, proliferation and neutrophil chemotaxis. Euro. J. Pharmacol. 2009;609:132–139. doi: 10.1016/j.ejphar.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [143].Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- [144].Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251:19–26. doi: 10.1016/s0378-1119(00)00205-5. [DOI] [PubMed] [Google Scholar]
- [145].Yang L, Yatomi Y, Satoh K, Igarashi Y, Ozaki Y. Sphingosine 1-phosphate formation and intracellular Ca2+ mobilization in human platelets: evaluation with sphingosine kinase inhibitors. J. Biochem. 1999;126:84–89. doi: 10.1093/oxfordjournals.jbchem.a022440. [DOI] [PubMed] [Google Scholar]
- [146].Sakakura C, Sweeney EA, Shirahama T, Hagiwara A, Yamaguchi T, Takahashi T, Hakomori S, Igarashi Y. Selectivity of sphingosine-induced apoptosis. Lack of activity of DL-erythyro-dihydrosphingosine. Biochem. Biophys. Res. Commun. 1998;246:827–830. doi: 10.1006/bbrc.1998.8719. [DOI] [PubMed] [Google Scholar]
- [147].Dragusin M, Gurgui C, Schwarzmann G, Hoernschemeyer J, van Echten-Deckert G. Metabolism of the unnatural anticancer lipid safingol, L-threo-dihydrosphingosine, in cultured cells. J. Lipid Res. 2003;44:1772–1779. doi: 10.1194/jlr.M300160-JLR200. [DOI] [PubMed] [Google Scholar]
- [148].Valentine WJ, Godwin VI, Osborne DA, Liu J, Fujiwara Y, Van Brocklyn J, Bittman R, Parrill AL, Tigyi G. FTY720 (Gilenya) phosphate selectivity of sphingosine 1-phosphate receptor subtype 1 (S1P1) G protein-coupled receptor requires motifs in intracellular loop 1 and transmembrane domain 2. J. Biol. Chem. 2011;286:30513–30525. doi: 10.1074/jbc.M111.263442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kono K, Tanaka M, Ogita T, Hosoya T, Kohama T. F-12509A, a new sphingosine kinase inhibitor, produced by a discomycete. J. Antibiot. 2000;53:459–466. doi: 10.7164/antibiotics.53.459. [DOI] [PubMed] [Google Scholar]