Abstract
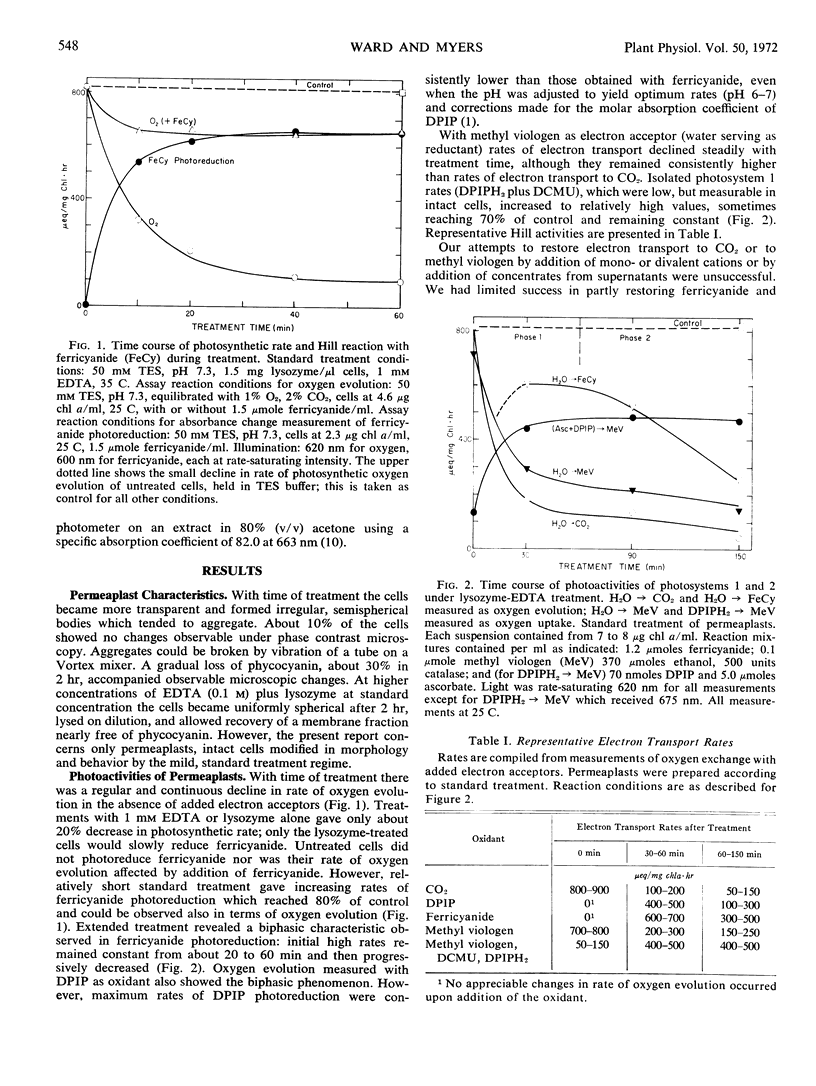
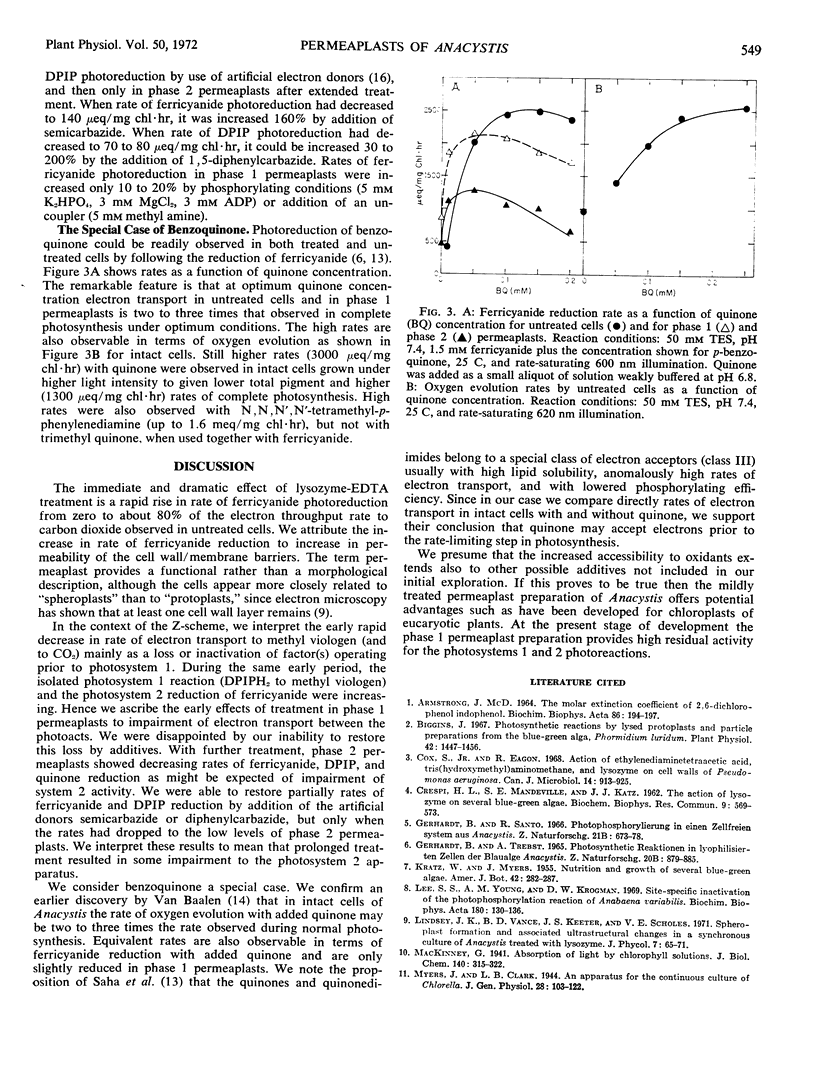
A treatment procedure using lysozyme and ethylenediaminetetracetic acid gave intact but permeable cells (permeaplasts) of Anacystis nidulans. Rates of electron transport from water to carbon dioxide, ferricyanide, 2,6-dichlorophenol indophenol, benzoquinone, and methyl viologen, and from reduced indophenol to methyl viologen were measured as a function of treatment time. Rates of oxygen evolution in complete photosynthesis and electron flow from water to methyl viologen showed rapid and parallel decline with treatment time. Electron flow from water to ferricyanide and from reduced indophenol to methyl viologen increased during the first half hour of treatment (phase 1) to 60 to 80% of the original photosynthetic rate. Longer treatment (phase 2) resulted in decreased rate of ferricyanide reduction but not in rate of methyl viologen reduction from indophenol. Electron flow from water to quinone was two to three times higher than for complete photosynthesis in intact cells. It remained high during phase 1 and declined during phase 2. Phase 1 permeaplasts apparently retain high activity for photosystems 1 and 2 photoreactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Biggins J. Photosynthetic Reactions by Lysed Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1447–1456. doi: 10.1104/pp.42.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESPI H. L., MANDEVILLE S. E., KATZ J. J. The action of lysozyme on several blue-green algae. Biochem Biophys Res Commun. 1962 Dec 19;9:569–573. doi: 10.1016/0006-291x(62)90127-4. [DOI] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- Gerhardt B., Trebst A. Photosynthetische Reaktionen in lyophilisierten Zellen der Blaualge Anacystis. Z Naturforsch B. 1965 Sep;20(9):879–889. [PubMed] [Google Scholar]
- Lee S. S., Young A. M., Krogmann D. W. Site-specific inactivation of the photophosphorylation reactions of Anabeana variabilis. Biochim Biophys Acta. 1969 May;180(1):130–136. doi: 10.1016/0005-2728(69)90200-x. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Vernon L. P., Shaw E. R. Photoreduction of 2,6-dichlorophenolindophenol by diphenylcarbazide: a photosystem 2 reaction catalyzed by tris-washed chloroplasts and subchloroplast fragments. Plant Physiol. 1969 Nov;44(11):1645–1649. doi: 10.1104/pp.44.11.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


