Abstract
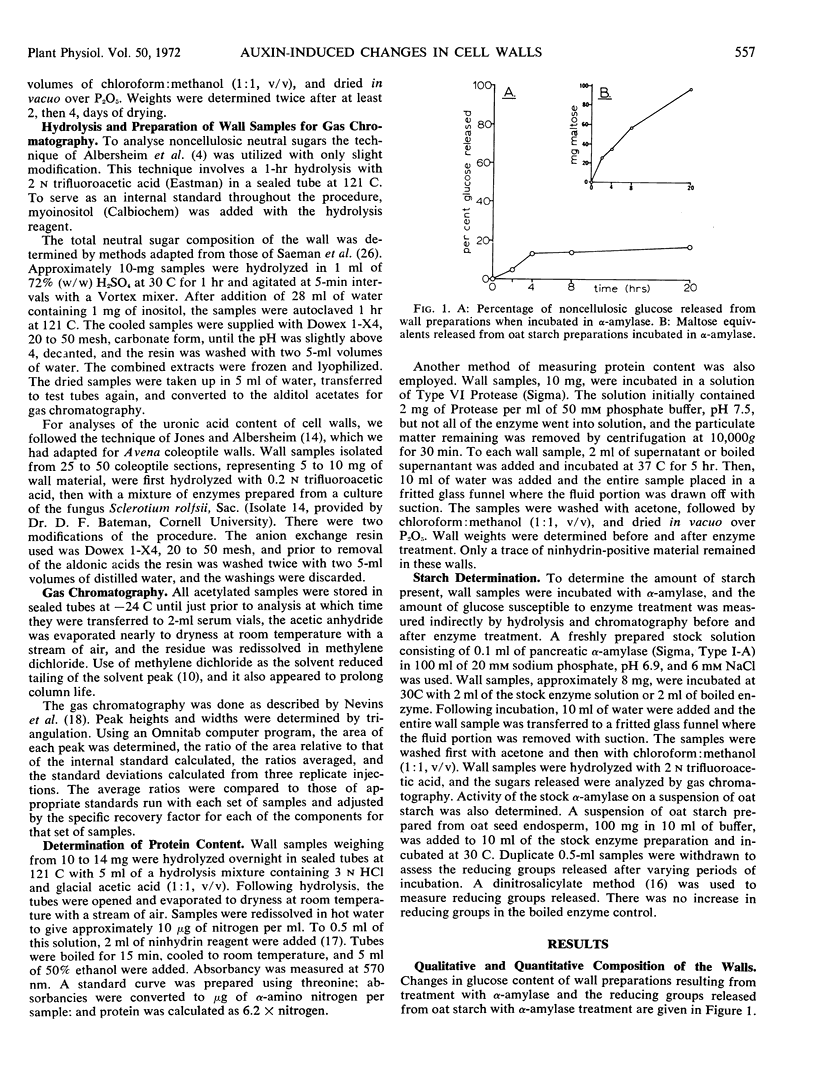
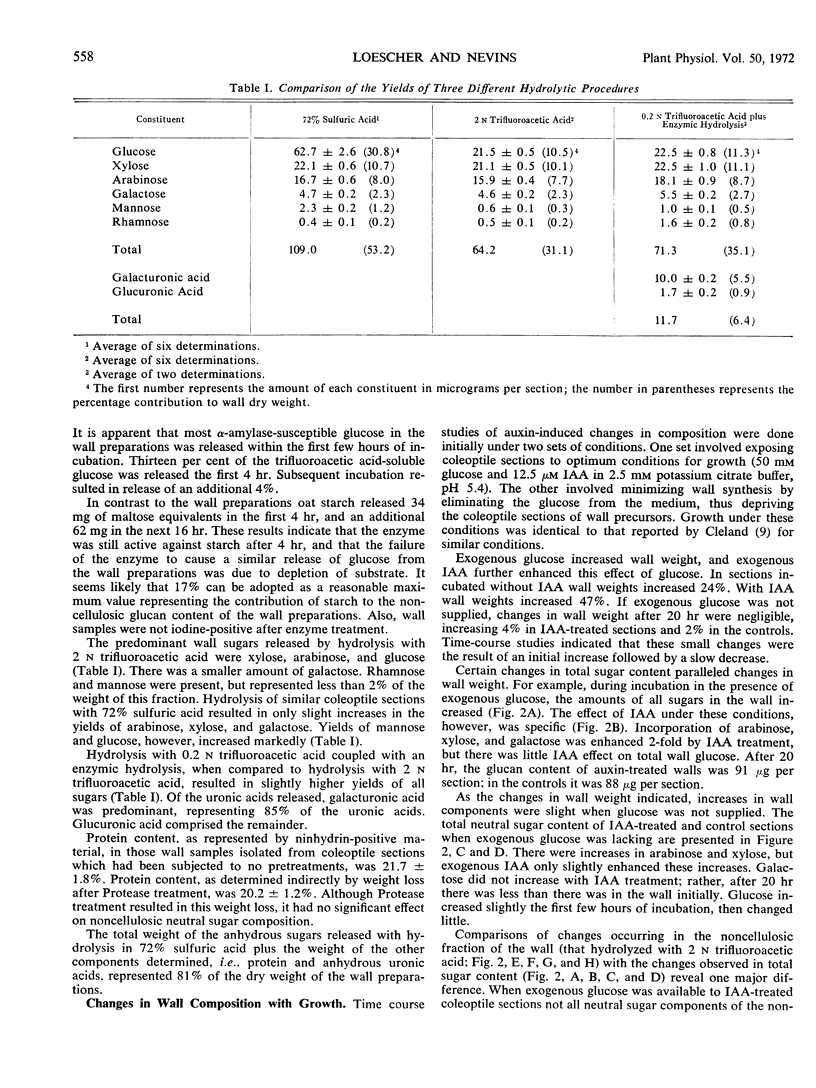
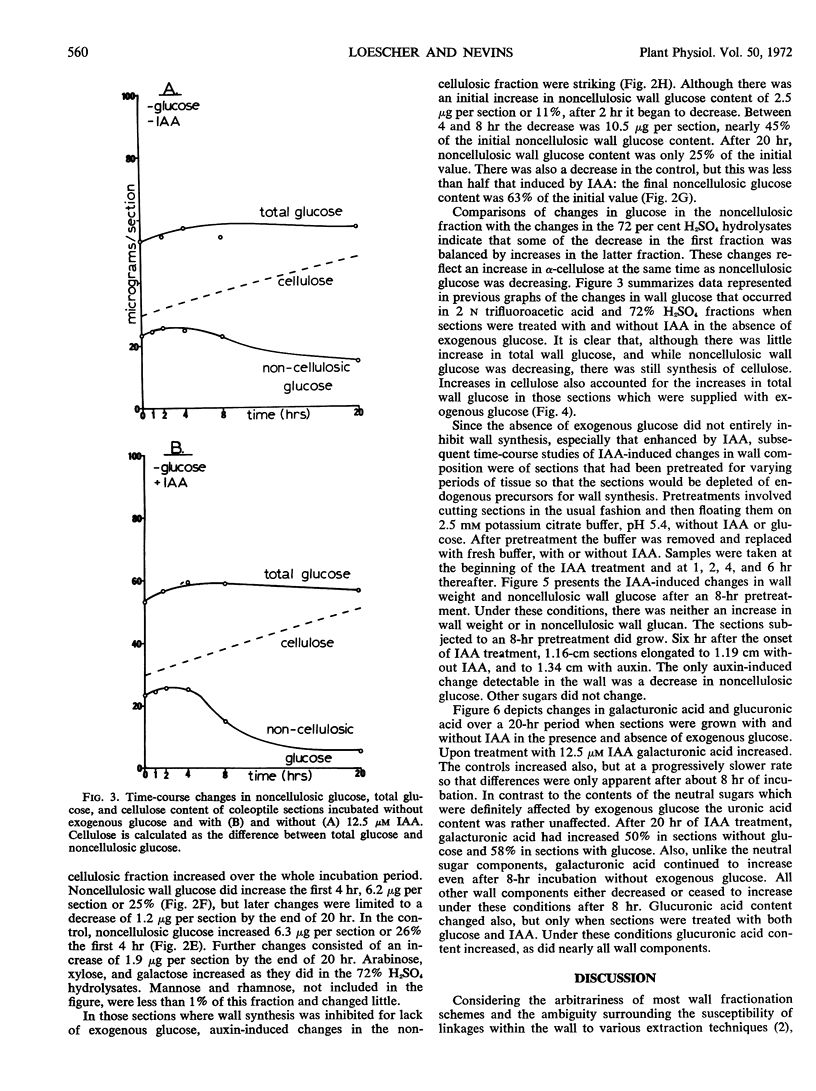
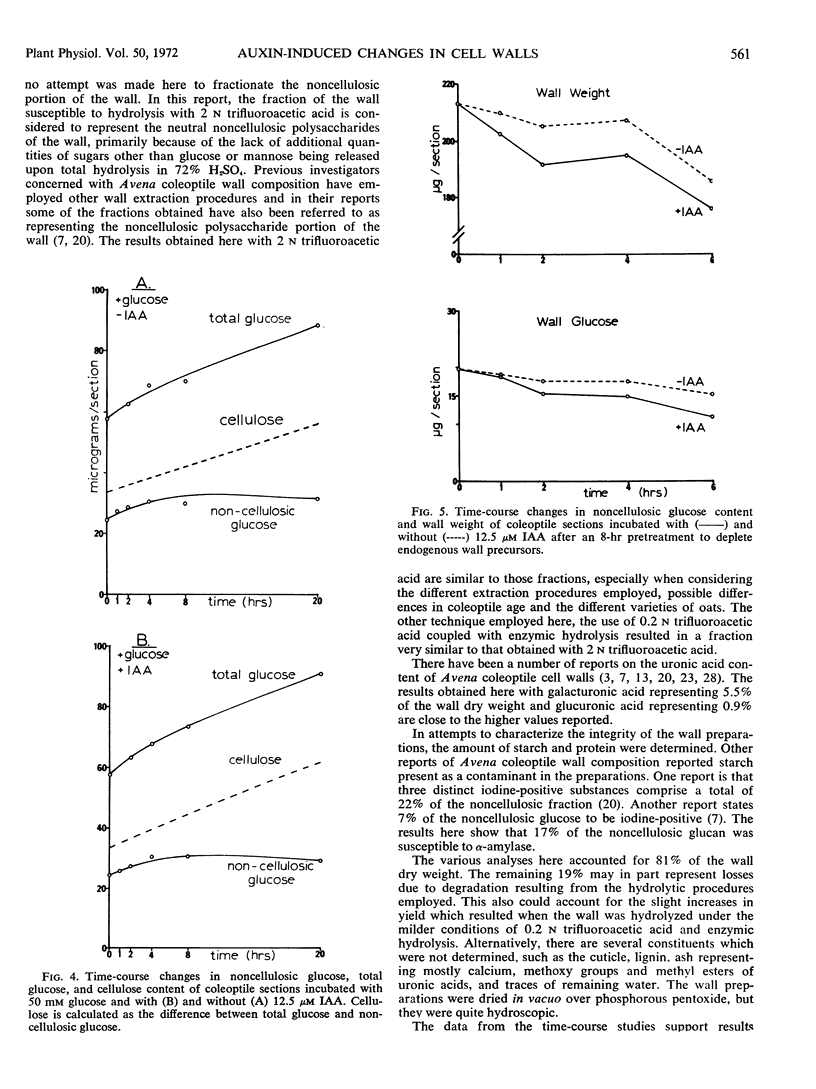
Sugar and uronic acid residues were derived from wall polysaccharides of oat (Avena sativa, var. Victory) coleoptiles by means of 2 N trifluoroacetic acid, 72% sulfuric acid, or enzymic hydrolysis. The products of hydrolysis were reduced and acetylated to form alditol acetates which were analyzed using gas chromatography. Time-course studies of auxin-promoted changes in various wall fractions indicate that when exogenous glucose was available, increases in certain wall constituents paralleled increases in length. However, under conditions where exogenous glucose was not available, and where wall synthesis was limited, such correlations with growth were not apparent. Under these latter conditions total wall weight initially increased slightly, then decreased. These changes in weight were the net of increases in cellulose and some noncellulosic constituents and a decrease of over 75% in noncellulosic glucose. When coleoptile sections were preincubated without exogenous glucose for 8 hours to deplete endogenous wall precursors and subsequently treated with auxin, there were no detectable increases in wall weight. There was instead an auxin-promoted decrease in wall weight, and this decrease paralleled a decrease in noncellulosic glucose. There were no significant changes in other wall components. The auxin-promoted decreases in noncellulosic glucose are interpreted as a possible step in the mechanism of growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERSHEIM P., BONNER J. Metabolism and hormonal control of pectic substances. J Biol Chem. 1959 Dec;234:3105–3108. [PubMed] [Google Scholar]
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. B., Ray P. M. Direct and Indirect Effects of Auxin on Cell Wall Synthesis in Oat Coleoptile Tissue. Plant Physiol. 1965 Mar;40(2):345–352. doi: 10.1104/pp.40.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. B., Ray P. M. Relation between Effects of Auxin on Cell Wall Synthesis and Cell Elongation. Plant Physiol. 1965 Mar;40(2):360–368. doi: 10.1104/pp.40.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C. T., Bayley S. T., Setterfield G. Chemical Constitution of the Primary Cell Walls of Avena Coleoptiles. Plant Physiol. 1958 Jul;33(4):283–289. doi: 10.1104/pp.33.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M., Reinhold L. Induction of coleoptile elongation by carbon dioxide. Plant Physiol. 1971 Mar;47(3):335–341. doi: 10.1104/pp.47.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E. F., Jang R. Pectic Metabolism of Growing Cell Walls. Plant Physiol. 1960 Jan;35(1):87–97. doi: 10.1104/pp.35.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. M., Albersheim P. A gas chromatographic method for the determination of aldose and uronic Acid constituents of plant cell wall polysaccharides. Plant Physiol. 1972 Jun;49(6):926–936. doi: 10.1104/pp.49.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. Metabolic turnover in cell wall constituents of Avena sativa L. coleoptile sections. Biochim Biophys Acta. 1967 Jun 13;141(1):118–125. doi: 10.1016/0304-4165(67)90250-4. [DOI] [PubMed] [Google Scholar]
- LUCHSINGER W. W., CORNESKY R. A. Reducing power by the dinitrosalicylic acid method. Anal Biochem. 1962 Oct;4:346–347. doi: 10.1016/0003-2697(62)90098-2. [DOI] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. The specific nature of plant cell wall polysaccharides. Plant Physiol. 1967 Jul;42(7):900–906. doi: 10.1104/pp.42.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Rottenberg D. A. Uronic acid constituents of oat-coleoptile cell walls. Biochem J. 1964 Mar;90(3):646–655. doi: 10.1042/bj0900646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Haughton P. M., Cleland R. An in vitro system that simulates plant cell extension growth. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1814–1817. doi: 10.1073/pnas.67.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. The growth of plant cell walls. Int Rev Cytol. 1964;17:1–49. doi: 10.1016/s0074-7696(08)60404-0. [DOI] [PubMed] [Google Scholar]



