Abstract
The human CHRNA5 D398N polymorphism (rs16969968) causes an aspartic acid to asparagine change in the nicotinic acetylcholine receptor (nAChR) α5 subunit gene. The N398 variant of CHRNA5 is linked to increased risk for nicotine dependence. In this study, we explored the effect of the CHRNA5 D398N polymorphism on the properties of human α3β4* nicotinic acetylcholine receptors in human embryonic kidney (HEK) cells.
Addition of either D398 or N398 variant of α5 subunit in the α3β4* receptor did not affect total [125I]-epibatidine binding or surface expression of the receptor. However, addition of α5D398 into α3β4* receptor decreased the maximal response to agonist without significantly affecting EC50 in aequorin intracellular calcium assay. α3β4α5N398 nAChRs showed further decreased maximal response. The differences in agonist efficacy between the receptor subtypes were found to be dependent upon the concentration of external calcium but independent of external sodium. Moreover, activation of α3β4α5 nAChRs led to significantly greater intracellular calcium release from IP3 stores relative to α3β4 nAChRs although no effect of the α5 polymorphism was observed. Finally, inclusion of the α5 variant caused a small shift to the left in IC50 for some of the antagonists tested, depending upon α5 variant but did not affect sensitivity of α3β4* receptors to desensitization in response to incubation with nicotine.
In conclusion, addition of either variant of a5 into an α3β4α5 receptor similarly effects receptor pharmacology and function. However, the N398 variant exhibits a reduced response to agonists when extracellular calcium is high and it may lead to distinct downstream cellular signaling.
Keywords: Nicotinic acetylcholine receptors, Polymorphism, rs16969968, Alpha5 subunit, CHRNA5, Intracellular calcium
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels of the cysteine loop superfamily, formed by five membrane-spanning subunits lining the central water-filled pore (for a recent review, see Barik and Wonnacott, 2009). When in open state, nAChRs are permeable to mono- and divalent cations, primarily sodium, Potassium and calcium ions. Mammalian neuronal nAChRs either consist of a combination of α2-α6 and β2-β4 subunits or are homomers of α7 or α9 subunits (Gotti et al., 2007). α10 forms heteromers with α9 subunit. At least two essential α subunits (α2, α3, α4 or α6) and two essential β subunits (β2 or β4) are needed to form functional heteromeric nAChRs and ACh binding sites. α5 and β3 cannot form functional receptors without the essential subunits and they do not participate in forming the ACh binding sites. But they can be incorporated in the pentamer as accessory subunits (Wang et al., 1996; Ramirez-Latorre et al., 1996; Groot-Kormelink et al., 1998), which can have dramatic effects on the conductance and desensitization of the receptor (Wang et al.,1996; Ramirez-Latorre etal.,1996; Gerzanich etal.,1998; Broadbent et al., 2006). The accessory subunits may also be involved in forming binding sites for positive allosteric modulators (Kuryatov et al., 2008).
In the brain, the most abundant nAChR subtype is α4β2* (the asterisk indicates that other subunits such as α5 can contribute to the receptor) but there are numerous other heteromeric nAChR subtypes. An example of the diversity of nAChRs are the five different subtypes – α4α6β2β3, α6β2βb3, α6β2, α4β2, α4α5β2 – that are expressed on dopaminergic nerve terminals (Zoli et al., 2002; for a review, see Grady et al., 2007). The α3β4* subtype is abundant in autonomic and sensory ganglia and is often dubbed the ganglionic subtype (Conroy and Berg, 1995; Xu et al., 1999a, 1999b; Mao et al., 2006). α3β4* receptors may contain α5, β2 or β3 as additional subunits (Conroy and Berg, 1995; Mao et al., 2006; Vernallis et al., 1993; Sheffield et al., 2000; Grady et al., 2009). In rodent superior cervical ganglia 25–30% of nAChRs are of α3β4α5 subtype (Mao et al., 2006; David et al., 2010). The distribution of α3β4* subtype is very limited in the brain, mainly confined to structures of habenulo-interpeduncular pathway – medial habenula, fasciculus retroflexus and interpeduncular nucleus (IPN) – (Sheffield et al., 2000; Grady et al., 2009; Duvoisin et al., 1989; Baddick and Marks, 2011) as well as the inferior colliculus (Baddick and Marks, 2011) and pineal gland (Marks et al., 1998; Hernandez et al., 2004). The habenular complex is activated by negative reward or the absence of anticipated positive reward (Matsumoto and Hikosaka, 2007; Ji and Shepard, 2007; Salas et al., 2010) and, consequently, its activity is apparently increased during nicotine withdrawal (De Biasi and Salas, 2008). α3β4* nAChRs in the habenulo-interpeduncular pathway also appear to be involved in nicotine self-administration and aversion. Glick and colleagues recently reported that inhibition of α3β4* nAChRs in the habenula blocks self-administration of nicotine in rats while inhibition of α3β4* nAChRs in the IPN increases nicotine self-administration (Glick et al., 2011). In addition, Frahm et al. (2011) reported that overexpression of the β4 subunit in mice increased aversion to nicotine and this aversion was eliminated when α5 was overexpressed in the medial habenula. Based on available data, α3β4* nAChRs may contribute to several aspects related to nicotine intake including reinforcement, aversion and withdrawal.
In 2007, Bierut and colleagues reported that a D398N (Asp398Asn, rs16969968) polymorphism in the human CHRNA5 gene is associated with nicotine dependence (Saccone et al., 2007; Bierut et al., 2007). Subsequent studies have convincingly replicated this association (Bierut et al., 2008; Chen et al., 2009, 2011; Saccone et al., 2009a, 2009b; Wanget al., 2009; Johnson et al., 2010; Sherva et al., 2010; Hong et al., 2010; Smith et al., 2011) and refined it by showing a significant link between D398N and smoking quantity (Stevens et al., 2008; Sarginson et al., 2011; Falcone et al., 2011) or “pleasurable buzz” received from smoking (Sherva et al., 2008). A recent meta-analysis has also confirmed a highly significant link between D398N polymorphism and smoking quantity (Saccone et al., 2010). Furthermore, the D398N polymorphism also appears to be associated with cocaine dependence (Sherva et al., 2010; Grucza et al., 2008), alcohol abuse or dependence (Chen et al., 2009), opioid dependence (Sherva et al., 2010; Erlich et al., 2010), lung cancer (Wang et al., 2009; Young et al., 2008; Falvella et al., 2009; Lips et al., 2010; Truong et al., 2010; Sakoda et al., 2011), upper aerodigestive tract cancers (Chen et al., 2011; Lips et al., 2010) as well as chronic obstructive pulmonary disease (Young et al., 2008). These clinically relevant findings have spurred research to characterize α5N* nAChRs.
Recent studies have demonstrated that the D398N polymorphism affects the function of α4β2α5 nAChRs (Bierutetal.,2008; Kuryatov et al., 2011). α4β2α5 nAChRs containing the variant of α5 associated with increased risk for nicotine dependence (Asn at position 398) exhibited diminished agonist-evoked intracellular calcium response, reduced calcium permeability as well as enhanced short-term desensitization compared to α4β2α5 nAChRs possessing the major variant of α5. However, conflicting results have been obtained regarding the effect of the polymorphism on the function of α3β4α5 nAChRs. One study found a similar effect of the D398N polymorphism on the function of α3β4α5 nAChRs (Frahm et al, 2011). However, two other reports indicated that the variant forms of the α5 subunit do not affect the electrophysiological properties of α3β4α5 nAChRs (Kuryatov et al, 2011; Li et al, 2011). The aim of the present study was to employ additional approaches to further evaluate whether the α5 D398N polymorphism can affect the function and pharmacology of α3β4α5 nAChRs.
2. Materials and methods
2.1. Materials
2.1.1. Chemicals
Coelenterazine was purchased from AnaSpec (San Jose, CA), amphotericin B, penicillin, streptomycin and G418 from Fisher Scientific (Waltham, MA), hygromycin B from Invitrogen (Carlsbad, CA), mecamylamine from Merck (Rahway, NJ), [125I]-epibatidine (specific activity 2200 Ci/mmol) from Perkin Elmer (Boston, MA) and FuGene transfection reagent from Roche Diagnostics (Indianapolis, IN). Acetylcholine (ACh), atropine, CdCl2, cytisine, dextrose, d-tubocurarine, HEPES, meglumine, nicotine hydrogen tartrate and Triton-X100 were from Sigma (St. Louis, MO). Bupropion, ryanodine, 2,2,6,6-tetramethylpiperidin-4-yl heptanoate (TMPH), varenicline and xestospongin C were purchased from Tocris Bioscience (Ellisville, MO).
2.1.2. Cell culture
The HEK293 cell line stably expressing the human α3β4 nAChR was developed as previously described (Li et al., 2011). The α3β4α5D and α3β4α5N stable lines were generated by transfecting the stable α3β4 line with constructs containing the α5D or α5N subunits in pCDNA3 with a hygromycin-selectable element. Cells were doubly selected with G418 (1000 μg/ml) and hygromycin B (200 μg/ml), Surviving populations were then immunoselected three times with monoclonal antibody 35 (Sigma-Aldrich, St. Louis MO), which binds to an epitope on both the α3 and α5 subunits (see Li et al., 2011). The human α5 subunit cDNA was obtained from J. Lindstrom (University of Pennsylvania), originally provided by F. Clementi (Università Degli Studi di Milano, Milano, Italy). The lines were maintained in DMEM (Fisher Scientific) supplemented with 4.5 g/l of glucose, l-glutamine, 10% of fetal bovine serum, 15 mM of HEPES and 1% each of penicillin, streptomycin and amphotericin B, 400 μg/ml of G418 and, for α3β4α5D/N lines, 100 μg/ml of hygromycin B.
2.2. Elisa
To measure cell surface nAChRs in stably expressing HEK293 cell lines, an ELISA was carried out. Cells seeded in 12-well dishes were washed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3). The cells were blocked with 4% (w/v) powdered milk in PBS and then incubated with an antibody against the α3 and α5 nAChR subunits (mAb35; Sigma-Aldrich, St. Louis MO). After three additional washes with milk-PBS, the cells were incubated with horseradish peroxidase conjugated goat anti-rat secondary antibody, washed again with milk-PBS, and finally incubated with the horseradish peroxidase substrate 3, 3′, 5, 5′-tetramethylbenzidine. The absorbance of the supernatant was measured with a plate reader (iMark, Biorad, Hercules CA). The nonspecific background binding of antibodies to cells was determined using non-transfected cells. The ELISA signal was normalized to the amount of protein in parallel wells and ratios between the α3β4-expressing cell line and cell lines expressing α3β4α5D or α3β4α5N were calculated.
2.3. Nicotinic acetylcholine receptor binding
2.3.1. Sample preparation
For binding samples of stably expressing HEK293 cell lines, the cells were seeded in 6-well plates as described above. For binding studies in nicotine-treated cells, the cells were incubated in 10 μM nicotine for 24 h before preparing the membrane samples. Control cells received equal amount of HBSS. Four days after plating, the cells were rinsed once with HBSS, scraped from wells and homogenized using a motorized tissue grinder with a Teflon pestle in 50 μl of 0.1× buffer (14 mM NaCl, 0.15 mM KCl, 0.1 mM MgSO4, 0.2 mM CaCl2). After homogenization, sample volumes were increased to 1 ml with 0.1× buffer and pelleted by centrifugation (5 min at 20,000 g). The homogenization – centrifugation wash cycle was repeated twice. Membrane homogenates were first incubated on ice for 1 h and then incubated in 0.01 mg/ml DNase I (Sigma, St Louis, MO) at 37 °C for 15 min. Incubated homogenates were pelleted by centrifugation at 15,000 g and resuspended three times in 1 ml of 0.1 × buffer. Pellets were stored at −80 °C under 0.1× buffer until binding assay.
2.3.2. Binding assay
Membrane pellets were homogenized in distilled deionized water. Reaction volume was 30 μl, consisting of membrane sample, 1× buffer (140 mM NaCl, 1.5 mM KCl, 1 mM MgSO4, 2 mM CaCl2) and 200 pM [125I]-epibatidine. 300 μM cytisine was included in non-specific binding samples. Reaction mixes were allowed to incubate for 2 h at room temperature. After incubation, samples were vacuum filtered to collect the [125I]-epibatidine binding into polyethyleneimine-soaked filter paper. Radioactivity was recorded with Packard Cobra Auto-Gamma counter (Meriden, CT).
Protein concentration of the samples was assessed by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Samples were prepared in 96-well plates and they were analyzed at 562 nm wavelength with Epoch microplate spectrophotometer (BioTek, Winooski, VT).
2.4. Aequorin intracellular calcium assay
Functional nAChR activities were assessed in HEK293 cells stably expressing human α3, β4 and α5D/N subtypes by measuring intracellular calcium changes using aequorin, a calcium sensing luminescent protein, as previously described (Bierut et al., 2008; Karadsheh et al., 2004). Cells were seeded in six-well plates (1.5 × 106 cells per well) and 24 h later they were transfected with human codon optimized aequorin cDNA (Vernon and Printen, 2002) using FuGene transfection reagent (Roche Diagnostics, Indianapolis, IN) and Opti-MEM reduced serum media (Invitrogen, Carlsbad, CA) as recommended by manufacturers.
Approximately 40–48 h after transfection, culture medium was replaced with DMEM with 0.1% fetal bovine serum plus 2.5 μl coelenterazine per well and the cells were incubated for three hours at room temperature and minimal light exposure. After loading, the cells were aspirated from the wells, transferred into 1.5 ml microcentrifuge tubes and pelleted by centrifugation (5 min at 800 g, 4 °C). Thereafter, supernatant was discarded and the cells were resuspended into fresh assay buffer [Hank's Balanced Salt Solution (HBSS, Cellgro, Mediatech, Manassas, VA) supplemented to 10 mM CaCl2] then pelleted and resuspended again. After washes the cells were incubated for one hour at 4 °C in fresh assay buffer under minimal light exposure. The cells were then pelleted by centrifugation (5 min at 800 g, 4 °C), resuspended in 1 ml of fresh assay buffer and 50 μl of cell suspension was aliquoted on opaque white 96-well plates. Luminescence (lux) was recorded in a Victor V3 plate reader with chemiluminescence detector (PerkinElmer, Waltham, MA). Recordings included three steps: 1) one second pre-read, 2) 20-s stimulation read at 0.2 s intervals after addition of 50 μl of appropriate agonist solution, 3) five-second lysis read at 0.1 s intervals after addition of 100 μl of lysis solution (0.1% Triton X-100 and 100 mM CaCl2 in distilled deionized water).
2.4.1. Agonist experiments
For agonist experiments, chemiluminescence was recorded immediately after cells were applied to 96-well plate. Concentration ranges were 1 μM–1 mM for ACh, 3 μM–1 mM for nicotine and 300 nM–100 μM for varenicline. For experiments with SAP-102, the cells in half of the wells were transfected with SAP-102 plasmid and the cells were stimulated with 300 nM–1 mM ACh. 200 nM atropine was included in the assay mix to inhibit muscarinic receptors whenever ACh was used as the agonist.
2.4.2. Antagonist experiments
For antagonist experiments, the cells were pre-incubated with 10 nM–100 μM mecamylamine, 100 nM–100 μM d-tubocurarine, 10 nM–100 μM bupropion or 3 nM–100 μM TMPH for 10 min before chemiluminescence was recorded. Cells were stimulated with 1 mM ACh (200 nM atropine included in the assay mix).
2.4.3. Desensitization experiments
For desensitization experiments, the cells were incubated with nicotine (100 nM–300 μM) for 30 min before chemiluminescence was recorded. Stimulation was carried out with 1 mM ACh in the presence of 10 mM Ca2+. For experiments measuring stimulation of nicotinic receptors, 200 nM atropine was included in the assay mix. For experiments measuring stimulation of muscarinic receptors, 10 μM mecamylamine was included in the assay mix.
2.4.4. Source of agonist-induced increase in intracellular calcium
To determine the mechanism of the agonist-induced change in intracellular calcium, the cells were incubated with CdCl2 (100 μM) to block voltage-operated calcium channels, ryanodine (30 μM) to inhibit ryanodine receptor dependent calcium release from endoplasmic reticulum, xestospongin C (10 μM) to antagonize IP3 receptor-mediated calcium release, or a combination of ryanodine and xestospongin C for 30 min before chemiluminescence was recorded. 1 mM ACh was used as agonist in these experiments (200 nM atropine included in the assay mix). Since ryanodine and xestospongin C were dissolved in ethanol, control cells were incubated in HBSS with equal amount of ethanol.
For experiments with varying calcium concentrations, the calcium concentrations in assay mix ranged from 0 to 10 mM. 1 mM ACh was used as agonist (200 nM atropine included in the assay mix).
For experiments in the absence of sodium, one of these three solutions was used as the assay mix: 1) normal HBSS with 10 mM CaCl2, 2) sodium-free HBSS with 10 mM CaCl2, supplemented with dextrose to maintain osmolarity, 3) sodium-free HBSS with 10 mM CaCl2 supplemented with meglumine to maintain osmolarity. 1 mM ACh was used as agonist in these experiments (200 nM atropine included in the assay mix).
2.5. Determination of calcium permeability
The cells were grown overnight to two days after plating. Macroscopic currents were recorded using standard whole-cell voltage clamp. The pipette solution contained: 130 mM sodium gluconate, 2 mM MgCl2, 10 mM BAPTA, 10 mM HEPES, pH 7.4. The bath solution contained: 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4. In addition, the bath solution contained 2 mM CaCl2 (“low Ca2+”)or 20 mM CaCl2 (“high Ca2+”). The agonist (ACh) was applied through the bath using an SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT).
Calcium permeability estimates were based on the shift in the reversal potential of channel currents upon changes in the concentration of extracellular calcium. The cells were exposed to 1 s pulses of 100 μM ACh followed by a 9 s washout in bath. The cells were clamped at −25 to +30 mV with 5 mV step size and one current trace was recorded at each voltage. The currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 2 kHz, and acquired with a Digidata 1320 Series interface at 10 kHz. Data analysis was carried out using the pClamp 9.0 software package (Molecular Devices, Union City, CA). The peak current response at each voltage was determined following subtraction of leak currents measured in the absence of ACh at the same range of voltage steps For each cell, measurements were conducted in “low Ca2+” (i.e., 2 mM CaCl2-containing) and “high Ca2+” (20 mM CaCl2-containing) bath solutions to estimate the shift in the reversal potential upon changes in extracellular Ca2+. The reversal potentials were estimated using linear interpolation on the data points immediately below and above the reversal potential. The individual estimated reversal potentials ranged from 2.9 mV to 19.7 mV. The average shift in the reversal potentials was 4.9 ± 1.6 mV (mean ± SD; 6 cells) for α3β4 receptors, and 5.6 ± 2.0 mV (5 cells) and 5.6 ± 1.1 mV (5 cells) for α3β4α5D and α3β4α5N receptors, respectively.
The permeability ratio, PCa/PNa was calculated according to Uteshev (2010) (Equation (1))
| (1) |
where [Na+]out and [Ca2+]out stand for the concentrations of Na+ and Ca2+ in the extracellular medium, Vrev stands for reversal potential, γCa and γNa are the activity coefficients for calcium and sodium ions, and F, R, and T are the Faraday constant, universal gas constant, and absolute temperature, respectively. Parameters marked with asterisks stand for those in the presence of 20 mM external Ca2+. Parameters without asterisks mark those in the presence of 2 mM external Ca2+. The values of the individual activity coefficients were γCa = 0.27, γ*Ca = 0.29, γNa = 0.75, and γ*Na = 0.74.
2.6. Data analysis
The data are presented as means ± SEM. To control for differences in cell number per well and variation in coelenterazine loading, results of the aequorin assays were calculated as L/Lmax ratios, where L is the maximal peak value for agonist-stimulated luminescence and Lmax the maximal peak agonist-stimulated luminescence plus maximal peak luminescence resulting from cell lysis in the presence of high calcium concentration. To reduce between-day variation, results from each experiment day were normalized as follows. Generally, 0% was set to be 0% L/Lmax response and 100% the average of maximal response to agonist values in the α3β4 group. However, inhibition and desensitization data were normalized to the maximal response within each subtype. Further, data from the experiment exploring source of agonist-induced increase in intracellular calcium were normalized to the response of corresponding control cells within each subtype. For nAChR antagonist data, results were normalized within each receptor subtype. Further analysis and plotting of the data from aequorin assays was carried out using GraphPad Prism (GraphPad Software, San Diego, CA). Nonlinear regression for response on log agonist or antagonist concentration was used as the curve fitting model to calculate EC50 or half-maximal inhibitory concentration (IC50) values as well as minimum and maximum responses.
One-way and two-way ANOVAs were carried out in SPSS (IBM SPSS Statistics, Version 19). Because Rmax and EC50 data did not meet criteria for normal distribution, Kruskal–Wallis ANOVA was used to test these data. Bonferroni comparisons were used as the post hoc test for one-way and two-way ANOVAs. Dunn's test was used as the post hoc test for Kruskal–Wallis ANOVA. Furthermore, t-test was used to test two-group comparisons. Results where p < 0.05 were considered significant.
3. Results
3.1. Influence of the addition of the variant forms of the α5 subunit on the number and surface expression of nAChRs
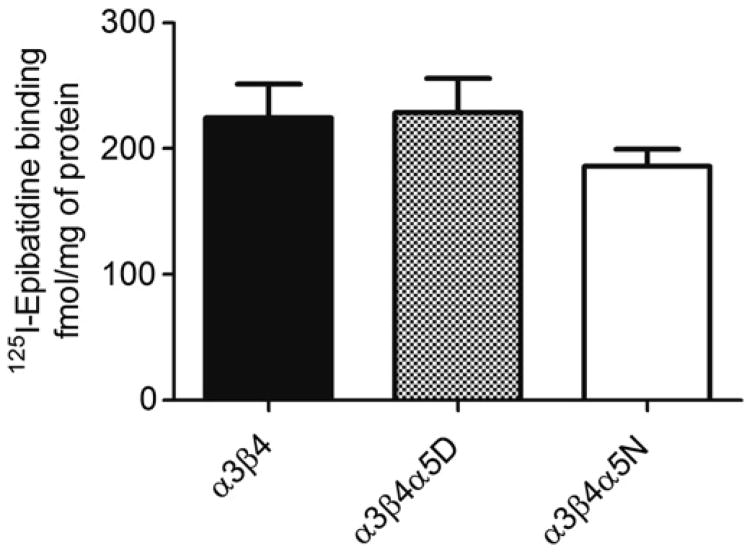
Fig. 1 shows [125I]-epibatidine binding in HEK293 cell lines stably expressing α3β4, α3β4α5D or α3β4α5N. No significant cell line differences were observed. ELISA data also indicated that the surface expression of nAChRs for the three cell types did not differ: the α3β4α5/α3β4 ratio was 0.81 ± 0.08 (t = 0.08, p > 0.05) for α3β4α5D and 1.03 ± 0.05 (t = 0.63, p > 0.05) for α3β4α5N.
Fig. 1.
[125I]-Epibatidine binding in HEK293 cells stably expressing human α3β4, α3β4α5D or α3β4α5N. n = 16. Results are given as means ± SEM of femtomoles of [125I]-epibatidine bound per milligram of protein. Two-way ANOVA did not show a significant difference between cell lines [F2, 47 = 1.018, p > 0.05].
3.2. Effect of nAChR subtype on agonist-induced increase in intracellular calcium
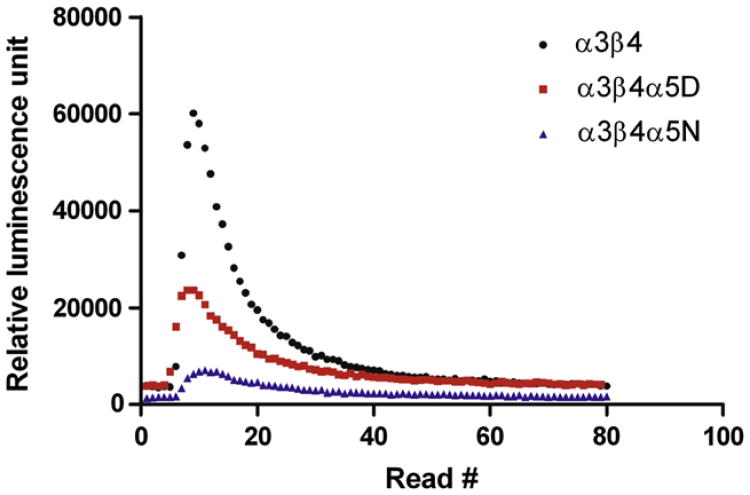
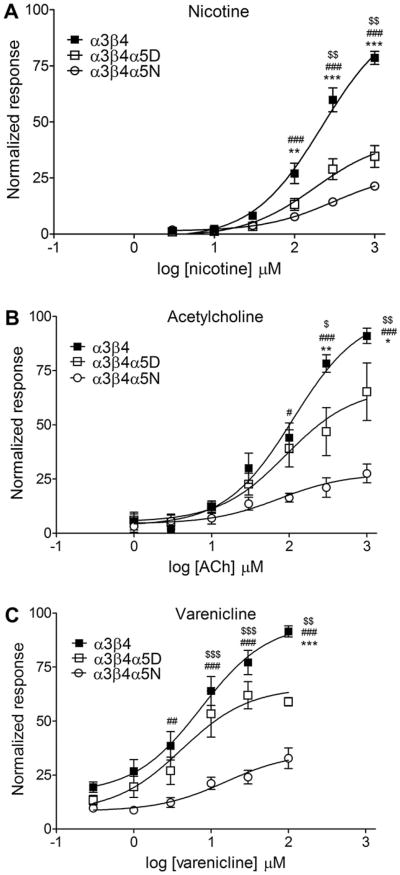
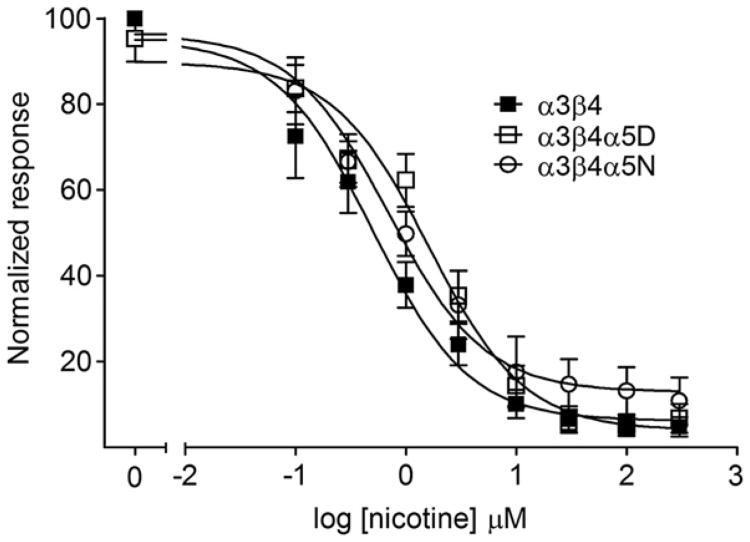
Representative calcium traces after stimulation with 1 mM nicotine are shown in Fig. 2. Nicotine, ACh and varenicline-induced increases in intracellular calcium in HEK293 cell lines stably expressing α3β4, α3β4α5D, or α3β4α5N are shown in Fig. 3. The calculated Rmax and EC50 values as well as statistics calculated for the data are shown in Table 1. Independent of the agonist, addition of the α5 subunit into α3β4 receptor decreases the response to agonist at high agonist concentrations without affecting the EC50 estimates. This effect is even more pronounced in α3β4α5N than in α3β4α5D receptors. Interestingly, α3β4α5N receptors showed higher EC50 for nicotine than α3β4α5D receptors. The α3β4α5N nAChRs exhibited reduced response relative to both the α3β4 and α3β4α5D nAChRs at the highest two or three agonist concentrations tested.
Fig. 2.
Representative calcium traces from aequorin assay in HEK293 cell lines stably expressing human nicotinic acetylcholine receptor subtypes α3β4, α3β4α5D or α3β4α5N. The cells were stimulated with 1 mM nicotine.
Fig. 3.
Nicotine, acetylcholine and varenicline-induced intracellular calcium response in HEK293 cell lines stably expressing human nicotinic acetylcholine receptor subtypes α3β4, α3β4α5D or α3b4α5N. Stimulation was performed with different concentrations of nicotine, acetylcholine or varenicline in the presence of 10 mM Ca2+. Atropine (200 nM) was included in the assay mix to block muscarinic receptors. Data is normalized to the average maximal response of α3β4 cells. α3β4α5 receptors showed decreased response to agonists. Two-way ANOVAs for the agonist curves: Nicotine, nAChR subtype effect F2,125 = 114.2, p < 0.001; Acetylcholine, nAChR subtype effect F2,104 = 32.46, p < 0.001; Varenicline, nAChR subtype effect F2, 161 = 74.32, p < 0.001. Post-hoc results for α3β4 vs. α3β4α5D: * = p < 0.05, ** = p < 0.01, *** = p < 0.001; for α3β4 vs. α3β4α5N: # = p < 0.05, ## = p < 0.01, ### = p < 0.001; and for α3β4α5D vs. α3β4α5N:$ = p < 0.05,$$ = p < 0.01,$$$ = p < 0.001. Results are given as normalized response values ± SEM.
Table 1.
EC50 values for agonist-induced stimulation of intracellular calcium response and IC50 values for antagonist-induced inhibition of acetylcholine-induced intracellular calcium response in HEK293 cells stably expressing human α3β4, α3β4α5D or α3β4α5N nicotinic acetylcholine receptor subtypes. Stimulation was performed in the presence of 10 mM Ca2+.
| Compound | n | α3β4 | α3β4α5D | α3β4α5N | |
|---|---|---|---|---|---|
| Agonists | |||||
| Nicotine | 8 | EC50 (μM) | 198 ± 26 | 149 ±11 | 531 ± 251# |
| Rmax % | 88 ±5 | 36 ± 5** | 29 ± 3*** | ||
| Acetylcholine | 6 | EC50 (μM) | 112 ± 28 | 89 ± 31 | 53 ± 21 |
| Rmax % | 100 ± 10 | 73 ± 23 | 24 ± 6* | ||
| Varenicline | 12 | EC50 (μM) | 24 ± 12 | 10 ± 2 | 36 ±26 |
| Rmax % | 110 ± 9 | 69 ±4* | 32 ± 5*** | ||
| Antagonists | |||||
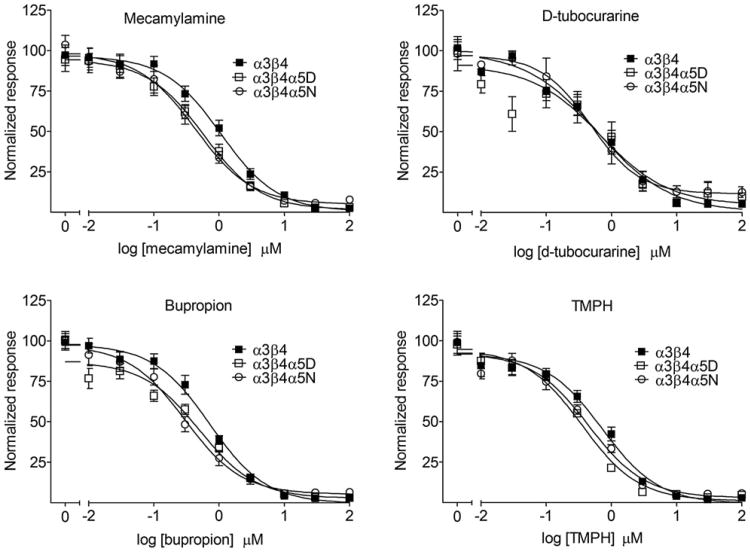
| Mecamylamine | 12 | IC50 (μM) | 1.18 ± 0.17 | 0.62 ± 0.09 | 0.44 ± 0.09** |
| D-Tubocurarine | 12 | IC50 (μM) | 1.01 ± 0.24 | 0.94 ± 0.21 | 0.64 ± 0.15 |
| Bupropion | 12 | IC50 (μM) | 0.67 ± 0.07 | 0.52 ± 0.07 | 0.38 ± 0.09* |
| TMPH | 12 | IC50 (μM) | 0.83 ± 0.10 | 0.36 ± 0.02*** | 0.45 ± 0.04* |
| Desensitization | |||||
| Nicotine | 10 | IC50 (μM) | 0.7 ± 0.2 | 1.9 ± 0.7 | 0.8 ± 0.3 |
Results of the statistical tests: Kruskal–Wallis tests for EC50, IC50 and R max values: nicotine EC50 H = 8.405; p < 0.05, Rmax H = 15.77; p < 0.001; acetylcholine EC50 H = 2.558, p > 0.05, Rmax H = 7.797, p < 0.05; varenicline EC50 H = 0.4230; p > 0.05, Rmax H = 23.42; p < 0.001; mecamylamine IC50 H = 12.53; p < 0.01; d-tubocurarine IC50 H = 1.891; p > 0.05; bupropion IC50 H = 6.444; p < 0.05; TMPH IC50 H = 14.77; p < 0.001; nicotine desensitization IC50 H = 4.025; p > 0.05.
p < 0.05,
p < 0.01,
p < 0.001 α3β4 vs. α3β4α5D or α3β4α5N;
p < 0.05 α3β4α5D vs. α3β4α5N. Data are given as means ± SEM.
Conroy et al. (2003) reported that the scaffolding protein SAP102 (Dlg3) specifically associates with α5 containing nAChRs. Therefore, SAP102 was tested for its effects on the function of the nAChR subtypes in the HEK293 stable cell lines. Transfection of the stably expressing HEK293 cell lines with SAP102 cDNA did not affect the response to ACh (data not shown) [F2, 18 = 18.801, p < 0.001].
3.3. Effect of nAChR subtype on noncompetitive antagonist inhibition of agonist-induced increase in intracellular calcium
The noncompetitive nicotinic receptor antagonists mecamylamine, bupropion, d-tubocurarine and TMPH were examined for their ability to inhibit ACh-induced calcium response in HEK293 cell lines stably expressing α3β4, α3β4α5D or α3β4α5N (Fig. 4 and Table 1). nAChR subtype affected IC50 values of mecamylamine, bupropion and TMPH but not d-tubocurarine. Both α5-containing nAChR subtypes differed from α3β4 nAChRs in IC50 for TMPH while only the α3β4α5N variant differed from α3β4 nAChRs for mecamylamine and bupropion. However, the magnitude of IC50 shift was moderate: in α3β4α5N expressing cells the IC50 values of mecamylamine, bupropion and TMPH were shifted only 1.6–2.5fold to lower concentration. The α3β4α5D and α3β4α5N subtypes did not differ in sensitivity to inhibition by any of the tested antagonists.
Fig. 4.
Inhibition of acetylcholine-induced intracellular calcium response by mecamylamine, d-tubocurarine, bupropion or TMPH in HEK293 cell lines stably expressing human nicotinic acetylcholine receptor subtypes α3β4, α3β4α5D or α3β4α5N. Cells were stimulated with 1 mM acetylcholine in the presence of 10 mM Ca2+. Atropine (200 nM) was added to block muscarinic receptors. TMPH = 2,2,6,6-tetramethylpiperidin-4-yl heptanoate. n = 10–12. Results are given as means of normalized response ± SEM. Curves are normalized to the response at 0 μM of antagonist within each cell line.
3.4. Desensitization of agonist-induced calcium influx in α3β4, α3β4α5D and α3β4α5N cell lines
Nicotine-induced desensitization of agonist-induced calcium response in HEK293 cell lines stably expressing α3β4, α3β4α5D or α3β4α5N is shown in Fig. 5 and IC50 values as well as results of statistical tests are shown in Table 1. The IC50 values for nicotine-induced desensitization were not statistically significant between the cell lines. The decrease in agonist-evoked changes in intracellular calcium following exposure to nicotine was due to desensitization and not loss of available aequorin due to extended exposure to agonist (Appendix A, Table 1). Nicotine pretreatment did not affect the function of endogenous muscarinic receptors (data not shown).
Fig. 5.
Nicotine-induced desensitization in HEK293 cell lines stably expressing human α3β4, α3β4α5D or α3β4α5N nicotinic acetylcholine receptor subtypes. Prior to stimulation, the cells were pretreated for 30 min with different concentrations of nicotine. Thereafter, the cells were stimulated with 1 mM acetylcholine in the presence of 10 mM Ca2+. Atropine (200 nM) was included in the assay mix to block muscarinic receptors. n = 9. Results are given as means of normalized response ± SEM. Data is normalized to the response at 0 μM nicotine within each cell line.
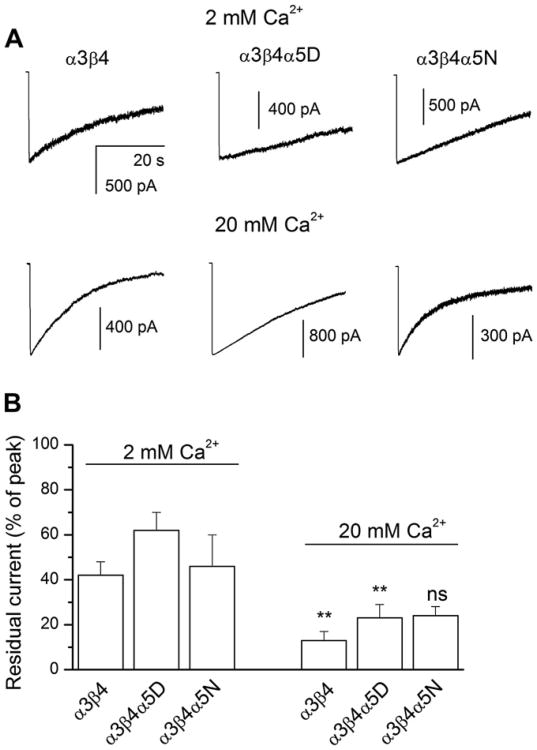
We probed the effect of change in external calcium concentration on receptor desensitization using whole-cell electrophysiology. Cells expressing α3β4, α3β4α5D or α3β4α5N receptors were exposed to a 40 s pulse of 100 μM ACh. Desensitization was quantified by calculating the ratio of the mean current response at the end of the 40 s drug application relative to the peak current. The data indicate that an increase in the concentration of external calcium significantly enhances the extent of desensitization for α3β4 and α3β4α5D receptors but not for α3β4α5N receptors (Fig. 6).
Fig. 6.
The effect of the α5 variant on receptor desensitization. (A) Macroscopic traces from HEK293 cells expressing α3β4, α3β4α5D or α3β4α5N receptors elicited by 40 s applications of 100 μM ACh. The bath solution contained 2 (“low calcium”) or 20 mM (“high calcium”) CaCl2. The membrane potential was −60 mV. The traces were analyzed with respect to the relative current amplitude at the end of the 40 s drug application. (B) Summary of the data. The plot shows mean ± SEM for the residual currents from 5 to 14 cells. Statistical analysis (t-test) was conducted with respect to the effect of change in external Ca2+ concentration. ** = p < 0.01; ns = not significant. We also examined the effect of the presence of the α5D or α5N subunit in the receptor-complex within the 2 mM and 20 mM Ca2+ groups. No significant differences (ANOVA with Bonferroni post hoc correction) were observed.
3.5. Effect of nAChR subtype on the source of agonist-induced increase of intracellular calcium
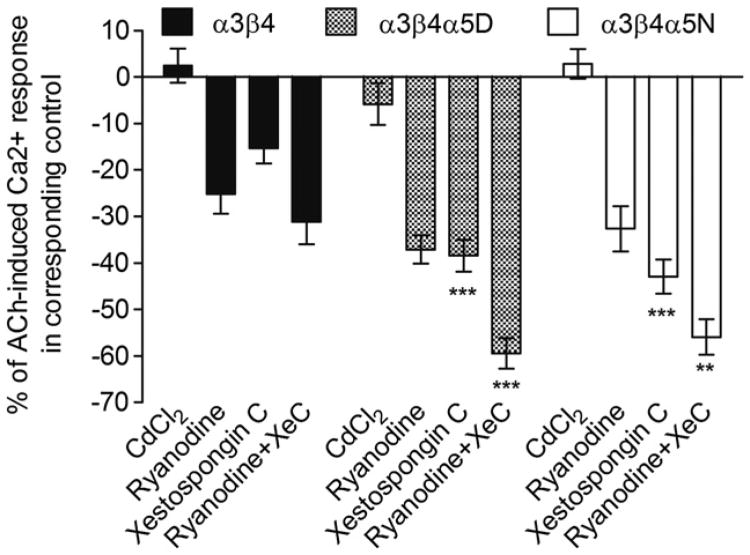
Agonist-evoked increases in intracellular calcium could arise though several sources including direct entry through the nAChR channel, activation of voltage operated calcium channels, or release from internal stores regulated by ryanodine or IP3 receptors. Each of these potential sources of calcium was assessed for the potential effect of the D398N polymorphism and/or their contribution to the agonist-evoked increase in intracellular calcium in the HEK293 cell lines expressing the α3β4, α3β4α5D and α3β4α5N nAChR subtypes. Electrophysiological experiments showed that the PCa/PNa ratio was 2.9 ± 0.4 for α3β4 (n = 6), 3.7 ± 0.7 for α3β4α5D (n = 5) and 3.7 ± 0.3 for α3β4α5N (n = 5). ANOVA with Bonferroni correction indicated that the differences between α3β4 and α3β4α5D/N are not significant (p > 0.5). These results indicate that calcium permeability was not significantly different between the nAChR subtypes. In addition, CdCl2, an inhibitor of voltage sensitive Ca2+ channels, did not affect the response induced by 1 mM ACh in any of the three cell lines (Appendix B, Fig. 1A). However, blockade of ryanodine receptors by ryanodine decreased the agonist-induced response and the effect was similar in all cell lines (Appendix B, Fig. 1B). Inhibition of the IP3 receptors by xestospongin C also decreased the agonist-induced response. However, the xestospongin C-induced inhibition of agonist-induced calcium response was significantly less in α3β4-expressing cells than in cells expressing α3β4α5D/N receptors (see Fig. 7 and Appendix B, Fig. 1C).
Fig. 7.
Effect of CdCl2, ryanodine and xestospongin C on acetylcholine-induced calcium response in HEK293 cell lines stably expressing human α3β4, α3β4α5D or α3β4α5N nicotinic acetylcholine receptors. Cells were stimulated with 1 mM acetylcholine in the presence of 10 mM Ca2+ and 200 nM atropine. IP3 receptor blocker, xestospongin C, has a differential effect in cells expressing α3β4 receptors and α3β4α5D/N receptors. More precisely, the IP3-mediated intracellular calcium release is more pronounced in cells expressing α5* receptors. Two-way ANOVA results for the data presented in this figure: treatment effect: F3, 130 = 77.275, p < 0.001; subtype effect: F2,130 = 23.128, p < 0.001; treatment × subtype interaction: F6, 130 = 3.286, p < 0.01. XeC = xestospongin C. ** = p < 0.01, *** = p < 0.001 vs. corresponding treatment in α3β4. n = 8–12. Data is presented as means of percentages of the corresponding control ± SEM. Data is normalized to the response of corresponding control cells to 1 mM acetylcholine within each receptor subtype.
Combination of ryanodine and xestospongin C produced an inhibition of agonist-induced calcium response that was larger in α3β4α5D/N-expressing cell lines than in the cell line expressing α3β4 (see Fig. 7 and Appendix B, Fig. 1D). In the α3β4α5D/N-expressing cell lines the inhibition was also more pronounced than with ryanodine or xestospongin C alone. For α3β4α5 nAChRs, genotype of the α5 subunit did not appear to have an effect on any of these measures.
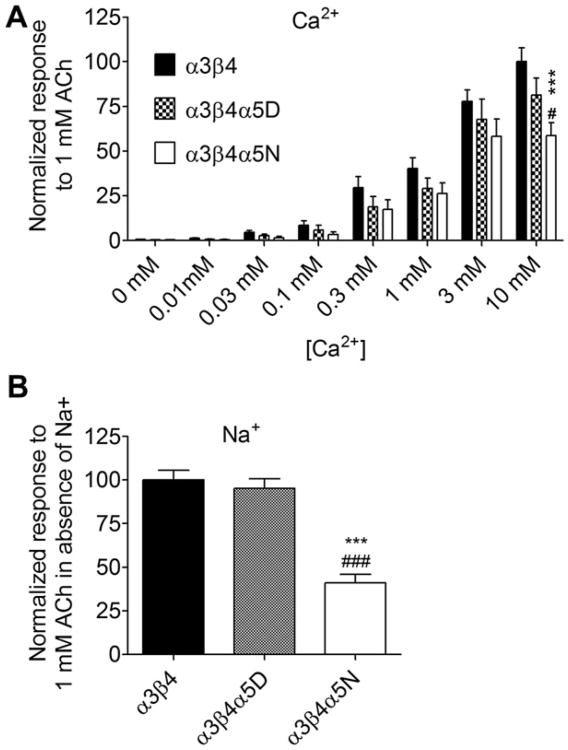
Previous studies have shown that increasing concentrations of extracellular calcium can potentiate the function of nAChRs (Mulle et al.,1992; Vernino et al.,1992). To assess whether the three nAChR subtypes are differentially responsive to the potentiation effect of external calcium and to determine the necessity of external sodium for agonist-elicited responses, nAChR function was assessed under conditions of altered extracellular calcium concentrations and in the presence or absence of extracellular sodium. Effects of varying extracellular calcium (Panel A) and sodium concentrations (Panel B) on ACh-induced calcium response in HEK293 cell lines stably expressing α3β4, α3β4α5D or α3β4α5N are shown in Fig. 8. Cell lines responded differently to varying extracellular calcium concentration. Differences in the ACh-stimulated intracellular calcium response between the cell lines were clearly visible only at 10 mM. At lower concentrations the differences were not observed. In addition, the effect of the D398N polymorphism on the response of α3β4α5 nAChRs to 1 mM ACh was found to be independent of extracellular sodium as the effect of the polymorphism was maintained when sodium was removed from the assay buffer (Fig. 8B).
Fig. 8.
Effects of varying extracellular calcium (Panel A) and absence of sodium (Panel B) on ACh-induced intracellular calcium response in HEK293 cell lines stably expressing human α3β4, α3β4α5D or α3β4α5N. The cells were stimulated with 1 mM acetylcholine in the presence of varying Ca2+ and Na+ concentrations. Atropine (200 nM) was included in the assay mix to block muscarinic receptors. Panel A: Ca2+ concentration effect: F7, 227 = 116.2, p < 0.001; subtype effect: F2, 227 = 11.08, p < 0.001; concentration × subtype interaction: F14, 227 = 2.095, p < 0.05; Bonferroni post hoc comparisons: α3β4 vs. α3β4α5N p < 0.001, α3β4α5D vs. α3β4α5N p < 0.05. Panel B: subtype effect: F2, 41 = 35.73, p < 0.001. ** = p < 0.01, *** = p < 0.001, α3β4 vs. α3β4α5N, # = p < 0.05, ### = p < 0.001 α3β4α5D vs. α3β4α5N. n = 12–14. Results are given as means of normalized response ± SEM. Data is normalized to the average maximal response of α3β4 subtype to 1 mM ACh in the presence of 10 mM Ca2+.
4. Discussion
In this study we found that the maximal agonist-induced calcium response is smaller in HEK293 cells expressing α3β4α5D nAChRs than α3β4 nAChRs. The response is further diminished in cells expressing the α3β4α5N nAChR variant suggesting that the D398N polymorphism in the α5 subunit affects the function of α3β4α5 nAChRs. The same general result was observed with all three agonists tested, acetylcholine, nicotine and varenicline. The cell line differences in nAChR subtype function could not be attributed to differences in receptor pharmacology, Ca2+ permeability, receptor number or surface expression of the nAChR subtypes. Therefore, we assessed whether inclusion of the α5 subunit affects downstream calcium signaling and if so, whether the α5D or α5N subunits differentially affect the release of calcium from intracellular stores. Ryanodine, an agent that inhibits ryanodine receptor-mediated calcium release from endoplasmic reticulum, diminished agonist-induced calcium response equally in all three cell lines. However, xestospongin C, which blocks IP3 receptor-mediated intracellular calcium release, induced a much more pronounced inhibition of agonist-induced calcium response in the α3β4α5D and α3β4α5N-expressing cell lines relative to the α3β4-expressing cell line. These data indicate that despite similar pharmacologies and calcium permeability, α3β4 and α3β4α5 nAChRs differ not only in maximal response to agonists but also lead to distinct downstream cellular signaling. Consequently, activation of α3β4 and α3β4α5 nAChRs may produce disparate effects on neuronal function. This finding is in accord with an earlier discovery that different nAChR subtypes are differently coupled to specific Ca2+ sources (Dajas-Bailador et al., 2002). Regardless, the sources of agonist-evoked increases in intracellular calcium were similar from α3β4α5 nAChRs containing either variant of the α5 subunit. Thus, the observed effect of the D398N variant on agonist-evoked responses cannot be attributed to a differential effect on the two intracellular calcium stores.
The effect of the variant forms of the α5 subunit on receptor function is consistent with our previous finding that the N398 variant of the α5 subunit leads to a reduced maximal Ca2+ response to agonist in α3β4α5 nAChRs (Bierut et al., 2008). Recently, Frahm et al. (2011) reported that the N398 variant of α5 leads to reduced function of α3β4α5 nAChRs in frog oocytes. Kuryatov et al. (2011) also confirmed the effect of the D398N polymorphism in heterologously expressed α3β4α5 nAChRs and reported that the variant forms of the α5 subunit affected function by altering calcium permeability and short-term desensitization. In contrast, this group did not observe an effect of the polymorphism on either of these parameters in α3β4α5 nAChRs. Li et al. (2011) also did not find an effect of the α5 D398N polymorphism on a number of biophysical or pharmacological properties of α3β4α5 nAChRs. Although the results of Kuryatov et al. (2011) and Li et al. (2011) appear to be in conflict with the data reported here, we point out that at the external calcium concentrations used by Kuryatov et al. (2011) and Li et al. (2011) we also did not see an effect of the D398N polymorphism.
Prior studies have shown that high external calcium concentrations potentiate the function of nAChRs (Mulle et al., 1992; Amador and Dani, 1995; Booker et al., 1998; Liu and Berg, 1999; Zhang et al., 1999). The data presented in this report also demonstrate the potentiating effect of external calcium on α3β4, α3β4α5D and α3β4α5N nAChRs. As mentioned previously, this potentiating effect of external calcium is necessary to detect an effect of the D398N polymorphism on the function of α3β4α5 nAChRs. Although the mechanism by which high external calcium unmasks the functional effect of the polymorphism is not known, it is not likely due to nAChR subtype specific effects on sodium flux through the receptor since the effect of the polymorphism at high calcium concentrations is independent of external sodium. The variant forms of α5 also might differentially affect the ability of calcium to potentiate the function of α3β4α5 nAChRs. Further studies are needed to determine the mechanism by which increased external calcium unveils the functional effect of the α5 D398N polymorphism in α3β4α5 nAChRs.
The D398N polymorphism is located within the membrane-associated stretch (MA stretch) of large cytoplasmic loop of the α5 polypeptide between transmembrane domains three and four. In addition to having an impact on channel conductance, the large cytoplasmic loop interacts with chaperone proteins and other subunits, affecting stability of subunit complexes as well as assembly, clustering and trafficking of nAChRs (Williams et al., 1998; Jeanclos et al., 2001; Ortiz et al., 2005; Zhao et al., 2009). Within the large intracellular loop, the MA stretch is a key location determining channel conductance in α4β2* (Peters et al., 2006), α3β4α5 (Frahm et al., 2011) and 5-HT3 receptors (Kelleyet al., 2003; Peters et al., 2006). Adding an α5 subunit to a receptor complex does not seem to affect nAChR binding as seen in this study and in an earlier report of α4β2* receptors (Bierut et al., 2008). This is in accordance with the fact that the accessory subunits α5 and β3 do not contribute to the ACh binding site (Gotti et al., 2007). We also did not find changes in the surface expression of α3β4* receptors. These findings suggest that the D398N polymorphism probably does not have an impact on the interaction between the large intracellular loop and intracellular chaperone proteins that would affect the numbers of [125I]-epibatidine binding sites or surface receptors.
The EC50 value for activation of α3β4* nAChRs by nicotine is relatively high (approximately 4.6 μM) and, therefore, α3β4* nAChRs may not be activated by nicotine levels that smokers sustain in the plasma for most of their awake hours (0.2 μM; Benowitz et al., 1996). Despite the reduced probability of direct activation by tobacco-derived nicotine, α3β4* receptors are likely to be activated by endogenous ACh during the processes involved in the development of nicotine addiction. The medial habenula and the β4 and α5 nAChR subunits within it have been linked with nicotine self-administration and aversion to nicotine (Frahm et al., 2011; Fowler et al., 2011; Glick et al., 2011) as well as nicotine withdrawal symptoms (Salas et al., 2004, 2009; Jackson et al., 2008). In rats, α3β4 receptor antagonists have been shown to reduce the self-administration of cocaine (Glick et al., 1996), ethanol (Rezvani et al.,1997), methamphetamine (Glick et al., 2000, 2002b), morphine (Glick et al., 1996, 2002b), and nicotine (Glick et al., 2000, 2002a), as well as withdrawal symptoms of morphine (Rho and Glick, 1998). This effect is evidently mediated through the medial habenula (Glick et al., 2011, 2006, 2008) and, in the case of opioids and stimulants, IPN (Glick et al., 2006, 2008). Nicotine self-administration, instead, is increased if α3β4* receptors in IPN are blocked (Glick et al., 2011). Peripheral α3β4* receptors residing in autonomic ganglia also may be involved in somatic withdrawal symptoms. Therefore, alterations in the normal functioning of these receptors may affect proneness to addiction as well as severity of withdrawal symptoms, making it more easy to initiate smoking and more difficult to quit. Taken collectively, these findings suggest that α3β4*-mediated mechanisms are involved in drug self-administration as well as withdrawal symptoms. Since a large number of α3β4* receptors also contain an α5 subunit, the D398N polymorphism may affect self-administration as well as withdrawal symptoms of nicotine and possibly also other substances of abuse.
In conclusion, the D398N polymorphism affects the maximal agonist-induced calcium response from α3β4α5 nAChRs expressed in HEK293 cells under conditions of high external calcium. However, the mechanism through which the polymorphism affects receptor function remains unclear. Nonetheless, the data reported here do indicate that the CHRNA5 D398N polymorphism can affect the function of α3β4α5 nAChRs which could contribute to the role of this polymorphism in nicotine dependence.
Supplementary Material
Acknowledgments
This study was funded from the following grants: CA089392 (JS), DA026918 (JS), ES017484 (GA), NS22356 (J. H. Steinbach; Washington University), Academy of Finland grant # 135525 (AT), as well as Ella and Georg Ehrnrooth's Foundation (AT).
The expert help with cell lines of Ms. Vivian Nguyen is gratefully acknowledged. The authors thank Prof. J.H. Steinbach for his valuable comments on the manuscript.
Abbreviations
- ACh
acetylcholine
- cDNA
complementary DNA
- CHRNA5
human nicotinic acetylcholine receptor subunit α5 gene
- DMEM
Dulbecco's modification of Eagle's medium
- EC50
half-maximal effective concentration
- HBSS
Hank's balanced salt solution
- HEK293 cells
human embryonic kidney cells
- IC50
half-maximal inhibitory concentration
- IP3
inositoltrisphosphate
- IPN
interpeduncular nucleus
- nAChR
nicotinic acetylcholine receptor
- Rmax
maximal response
- TMPH
2,2,6,6-tetramethylpiperidin-4-yl heptanoate
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
Authorship credit: AT carried out aequorin and binding assays, analyzed data, prepared figures and wrote the first version of manuscript, PH carried out aequorin assays, PL prepared the stably expressing cell lines, CE carried out the surface ELISA assays, JRL maintained the stably expressing cell lines and seeded cells in plates, GA designed and conducted the calcium permeability experiments and contributed to manuscript preparation, JAS designed the experiments, analyzed data and prepared the manuscript.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neuropharm.2012. 07.022.
References
- Amador M, Dani JA. Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. J Neurosci. 1995;15:4525–4532. doi: 10.1523/JNEUROSCI.15-06-04525.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol. 2011;82:828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol. 2009;192:173–207. doi: 10.1007/978-3-540-69248-5_7. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Perez-Stable E. CYP2D6 phenotype and the metabolism of nicotine and cotinine. Pharmacogenetics. 1996;6:239–242. doi: 10.1097/00008571-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker TK, Smith KW, Dodrill C, Collins AC. Calcium modulation of activation and desensitization of nicotinic receptors from mouse brain. J Neurochem. 1998;71:1490–1500. doi: 10.1046/j.1471-4159.1998.71041490.x. [DOI] [PubMed] [Google Scholar]
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, Sivilotti LG. Incorporation of the beta3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol Pharmacol. 2006;70:1350–1357. doi: 10.1124/mol.106.026682. [DOI] [PubMed] [Google Scholar]
- Chen D, Truong T, Gaborieau V, Byrnes G, Chabrier A, Chuang SC, Olshan AF, Weissler MC, Luo J, Romkes M, Buch S, Nukui T, Franceschi S, Herrero R, Talamini R, Kelsey KT, Christensen B, McClean MD, Lacko M, Manni JJ, Peters WH, Lubinski J, Trubicka J, Lener M, Muscat JE, Lazarus P, Wei Q, Sturgis EM, Zhang ZF, Chang SC, Wang R, Schwartz SM, Chen C, Benhamou S, Lagiou P, Holcatova I, Richiardi L, Kjaerheim K, Agudo A, Castellsague X, Macfarlane TV, Barzan L, Canova C, Thakker NS, Conway DI, Znaor A, Healy CM, Ahrens W, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Fabianova E, Bucur A, Bencko V, Foretova L, Janout V, Curado MP, Koifman S, Menezes A, Wunsch-Filho V, Eluf-Neto J, Fernandez L, Boccia S, Hashibe M, Hayes RB, Boffetta P, Brennan P, McKay JD. A sex-specific association between a 15q25 variant and upper aerodigestive tract cancers. Cancer Epidemiol Biomarkers Prev. 2011;20:658–664. doi: 10.1158/1055-9965.EPI-10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron. 2003;38:759–771. doi: 10.1016/s0896-6273(03)00324-6. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Mogg AJ, Wonnacott S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci. 2010;31:978–993. doi: 10.1111/j.1460-9568.2010.07133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med. 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, Linda Kao WH, Gerhard GS, Stewart WF, Boscarino JA. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum Genet. 2010;128:491–499. doi: 10.1007/s00439-010-0876-6. [DOI] [PubMed] [Google Scholar]
- Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, Baldwin D, Tyndale RF, Lerman C, Ray R. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine Tob Res. 2011;13:498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvella FS, Galvan A, Frullanti E, Spinola M, Calabro E, Carbone A, Incarbone M, Santambrogio L, Pastorino U, Dragani TA. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res. 2009;15:1837–1842. doi: 10.1158/1078-0432.CCR-08-2107. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. Alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002a;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002b;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur J Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Sell EM, McCallum SE, Maisonneuve IM. Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol. 2011;669:71–75. doi: 10.1016/j.ejphar.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:110, 2–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit beta3 into a functional nicotinic receptor. J Biol Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Jr, Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SC, Vicini S, Xiao Y, Davila-Garcia MI, Yasuda RP, Wolfe BB, Kellar KJ. The nicotinic receptor in the rat pineal gland is an alpha3beta4 subtype. Mol Pharmacol. 2004;66:978–987. doi: 10.1124/mol.104.002345. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3eta interacts with the nicotinic acetylcholine receptor alpha 4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem. 2001;276:28281–28290. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, Saccone NL, Grucza RA, Bierut LJ. Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction. 2010;105:2014–2022. doi: 10.1111/j.1360-0443.2010.03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadsheh MS, Shah MS, Tang X, Macdonald RL, Stitzel JA. Functional characterization of mouse alpha4beta2 nicotinic acetylcholine receptors stably expressed in HEK293T cells. J Neurochem. 2004;91:1138–1150. doi: 10.1111/j.1471-4159.2004.02801.x. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Li P, McCollum M, Bracamontes J, Steinbach JH, Akk G. Functional characterization of the {alpha}5(N398) variant associated with risk for nicotine dependence in the {alpha}3{beta}4{alpha}5 nicotinic receptor. Mol Pharmacol. 2011;80:818–827. doi: 10.1124/mol.111.073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, Hashibe M, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Field JK, Liloglou T, Xinarianos G, McLaughlin J, Liu G, Skorpen F, Elvestad MB, Hveem K, Vatten L, Study E, Benhamou S, Lagiou P, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Castellsague X, Macfarlane TV, Barzan L, Canova C, Lowry R, Conway DI, Znaor A, Healy C, Curado MP, Koifman S, Eluf-Neto J, Matos E, Menezes A, Fernandez L, Metspalu A, Heath S, Lathrop M, Brennan P. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Berg DK. Extracellular calcium regulates responses of both alpha3-and alpha7-containing nicotinic receptors on chick ciliary ganglion neurons. J Neurophysiol. 1999;82:1124–1132. doi: 10.1152/jn.1999.82.3.1124. [DOI] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose ganglia. Mol Pharmacol. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther. 1998;285:377–386. [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Mulle C, Lena C, Changeux JP. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Ortiz JA, Castillo M, del Toro ED, Mulet J, Gerber S, Valor LM, Sala S, Sala F, Gutierrez LM, Criado M. The cysteine-rich with EGF-like domains 2 (CRELD2) protein interacts with the large cytoplasmic domain of human neuronal nicotinic acetylcholine receptor alpha4 and beta2 subunits. J Neurochem. 2005;95:1585–1596. doi: 10.1111/j.1471-4159.2005.03473.x. [DOI] [PubMed] [Google Scholar]
- Peters JA, Carland JE, Cooper MA, Livesey MR, Deeb TZ, Hales TG, Lambert JJ. Novel structural determinants of single-channel conductance in nicotinic acetylcholine and 5-hydroxytryptamine type-3 receptors. Biochem Soc Trans. 2006;34:882–886. doi: 10.1042/BST0340882. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rho B, Glick SD. Effects of 18-methoxycoronaridine on acute signs of morphine withdrawal in rats. Neuroreport. 1998;9:1283–1285. doi: 10.1097/00001756-199805110-00004. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009a;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, Johnson EO, Rice JP, Goate AM, Bierut LJ. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009b;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda LC, Loomis MM, Doherty JA, Neuhouser ML, Barnett MJ, Thornquist MD, Weiss NS, Goodman GE, Chen C. Chromosome 15q24-25.1 variants, diet, and lung cancer susceptibility in cigarette smokers. Cancer Causes Control. 2011;22:449–461. doi: 10.1007/s10552-010-9716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Baldwin P, de Biasi M, Montague PR. BOLD responses to negative reward prediction errors in human habenula. Front Hum Neurosci. 2010;4:36. doi: 10.3389/fnhum.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, Murphy GM., Jr Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–1931. doi: 10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Alachkar H, Papp AC, Wang D, Mash DC, Wang JC, Bierut LJ, Sadee W. Nicotinic alpha5 receptor subunit mRNA expression is associated with distant 5′ upstream polymorphisms. Eur J Hum Genet. 2011;19:76–83. doi: 10.1038/ejhg.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T, Hung RJ, Amos CI, Wu X, Bickeboller H, Rosenberger A, Sauter W, Illig T, Wichmann HE, Risch A, Dienemann H, Kaaks R, Yang P, Jiang R, Wiencke JK, Wrensch M, Hansen H, Kelsey KT, Matsuo K, Tajima K, Schwartz AG, Wenzlaff A, Seow A, Ying C, Staratschek-Jox A, Nurnberg P, Stoelbenm E, Wolf J, Lazarus P, Muscat JE, Gallagher CJ, Zienolddiny S, Haugen A, van der Heijden HF, Kiemeney LA, Isla D, Mayordomo JI, Rafnar T, Stefansson K, Zhang F, Chang SC, Kim JH, Hong YC, Duell EJ, Andrew AS, Lejbkowicz F, Rennert G, Muller H, Brenner H, Le Marchand L, Benhamou S, Bouchardy C, Teare MD, Xue X, McLaughlin J, Liu G, McKay JD, Brennan P, Spitz MR. Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J Natl Cancer Inst. 2010;102:959–971. doi: 10.1093/jnci/djq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. Evaluation of Ca2+ permeability of nicotinic acetylcholine receptors in hypothalamic histaminergic neurons. Acta Biochem Biophys Sin. 2010;42:8–20. doi: 10.1093/abbs/gmp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vernon WI, Printen JA. Assay for intracellular calcium using a codon-optimized aequorin. BioTechniques. 2002;33:730–734. doi: 10.2144/02334bm02. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J Biol Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, Bierut LJ, Goate AM COGEND collaborators and GELCC collaborators. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BM, Temburni MK, Levey MS, Bertrand S, Bertrand D, Jacob MH. The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat Neurosci. 1998;1:557–562. doi: 10.1038/2792. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999b;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RP, Hopkins RJ, Hay BA, Epton MJ, Black PN, Gamble GD. Lung cancer gene associated with COPD: triple whammy or possible confounding effect? Eur Respir J. 2008;32:1158–1164. doi: 10.1183/09031936.00093908. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xiao Y, Abdrakhmanova G, Wang W, Cleemann L, Kellar KJ, Morad M. Activation and Ca2+ permeation of stably transfected α3/β4 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1999;55:970–981. doi: 10.1124/mol.55.6.970. [DOI] [PubMed] [Google Scholar]
- Zhao CJ, Noack C, Brackmann M, Gloveli T, Maelicke A, Heinemann U, Anand R, Braunewell KH. Neuronal Ca2+ sensor VILIP-1 leads to the upregulation of functional alpha4beta2 nicotinic acetylcholine receptors in hippocampal neurons. Mol Cell Neurosci. 2009;40:280–292. doi: 10.1016/j.mcn.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.