Introduction
Papillary renal cell carcinoma (RCC) is the most prevalent nonclear cell histologic subtype of renal carcinoma and constitutes approximately 10% of renal cancers, affecting 5,400 patients per year in the United States.1–3 Activating MET mutations are present in the majority of hereditary papillary RCCs and up to 13% of sporadic papillary RCCs.
Here we describe a patient with MET-mutated papillary RCC that responded to MET inhibition with a small molecule kinase inhibitor, PF-04217903, for 26 months. At the time of acquired resistance to treatment, we identified a genomic duplication that encompassed the mutated kinase domain of MET.
Case Report
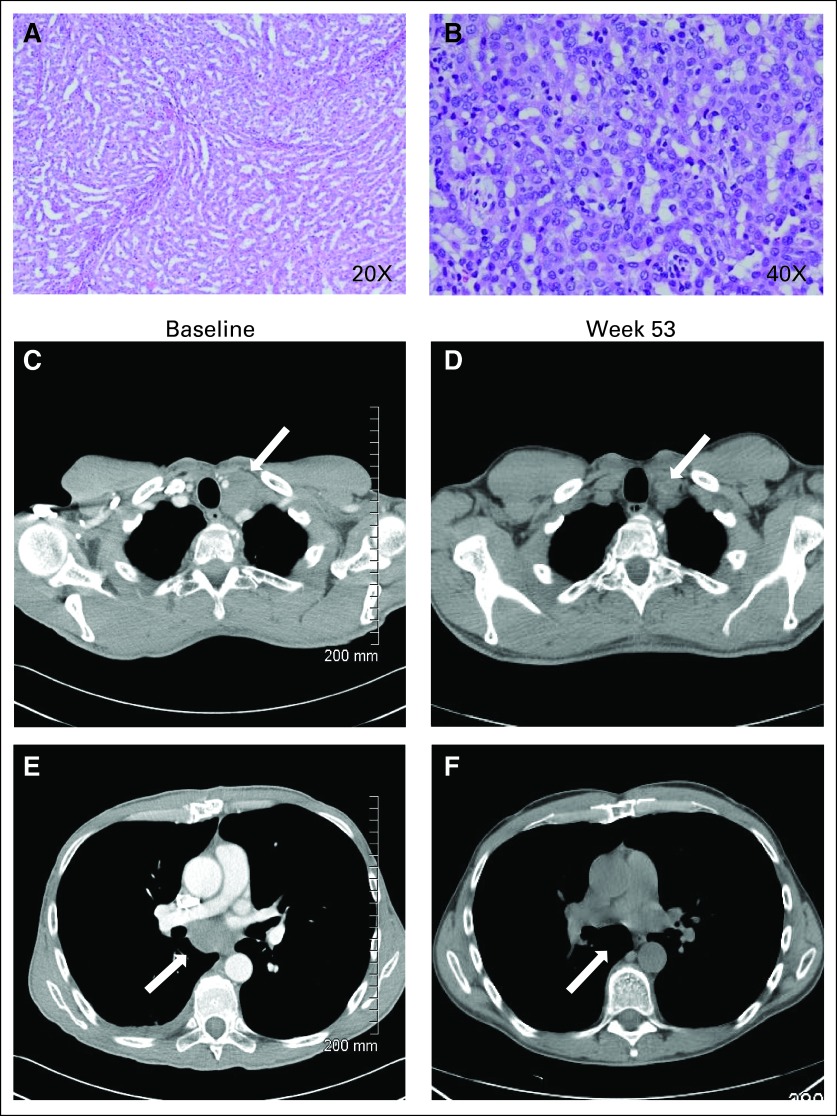
A 58-year-old white man with no significant medical comorbidities presented with back and flank pain and was found to have a 3.8-cm right-sided kidney mass with tumor thrombus that extended from the right renal vein into the inferior vena cava and was associated with retroperitoneal lymphadenopathy and pulmonary nodules. Biopsy of the kidney mass revealed compact papillary structures forming solid islands that were negative by immunohistochemistry for thyroid transcription factor 1 and positive for cytokeratin 7 and pancytokeratin (Figs 1A and 1B). These findings were consistent with papillary RCC of the solid variant.
Fig 1.
The patient was initially treated with a series of sequential systemic agents including sunitinib, temsirolimus, and ENMD-2076, an Aurora and angiogenic kinase inhibitor administered as part of a phase I clinical trial (An Open-Label, Dose-Escalation, Safety, and Pharmacokinetic Study of ENMD-2076 Administered Orally to Patients With Advanced Cancer). His best response to each of these therapies was disease progression after 2 months of treatment. Because of a marked resistance to systemic therapy and a good performance status, the patient underwent a palliative debulking surgery that included right-sided nephrectomy with cavotomy, removal of caval thrombus, and lymph node dissection. The pathology revealed multifocal papillary RCC.
Given the association between activating MET mutations and papillary RCC, the patient provided informed consent for MET mutational analysis, which was performed on archival tumor tissue that was obtained during his debulking surgery. Before these results were available, the patient began treatment with an experimental MET inhibitor, PF-04217903, as part of a phase I clinical study (Phase 1 Safety, Pharmacokinetic and Pharmacodynamic Study of PF-4217903 in Patients With Advanced Cancer). Soon after starting therapy, the patient was confirmed as having a heterozygous MET mutation at M1268T. The patient had a family history of kidney cancer, but no germline MET mutation was identified.
The patient had a reduction of 35% in the sum of one-dimensional measurements of target lesions after receiving treatment for 53 weeks, achieving a confirmed partial response by RECIST version 1.04 (Figs 1C and 1D, white arrows illustrating a decrease in bulky lymphoadenopathy). The patient continued to be treated as part of this study for 26 months, during which time he was asymptomatic from his cancer. Unfortunately, the patient subsequently had rapid disease progression with development of malignant ascites and carcinomatosis, which led to death as a result of his cancer.
Formalin-fixed, paraffin-embedded tumor tissue from the patient's debulking surgery was obtained. DNA isolation, polymerase chain reaction amplification, and sequencing of predefined regions of MET were performed as previously described.5 DNA sequencing was performed on tumor tissue that was obtained before treatment with PF-04217903 (pretreatment sample) and using a cytospin preparation containing malignant cells from ascitic fluid that was obtained at the time of disease progression while the patient was receiving PF-04217903 (time-of-progression sample).
Dual-color fluorescent in situ hybridization (FISH) assays were performed on the pretreatment and time-of-progression tumor samples to check for a possible MET amplification. FISH was performed using a commercial MET probe (Abbott Molecular, Des Plaines, IL) and fosmid G248P87518A11 encompassing exons 12 through 21 of MET originating from the WIBR-2 Human Fosmid Library (BACPAC Resources, Oakland, CA) combined with alpha satellite probe CEP7 (Abbott Molecular), as previously described.6
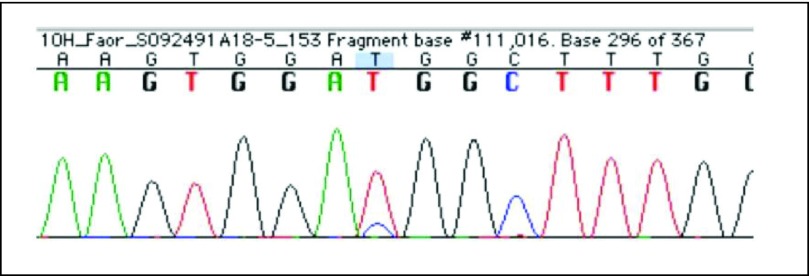
The initial screening MET-mutation testing of the patient's pretreatment tumor showed a heterozygous M1268T mutation that resulted in a methionine to threonine change (ATG>ACG) (Fig 2). We believe that this represents a de novo somatic mutation in the patient's tumor, given that germline MET mutation testing was negative.
Fig 2.
Our patient's clinical course was characterized by a prolonged period of response to therapy followed by rapid progression, which we suspected was a result of the tumor acquiring a secondary genetic defect that conferred resistance to PF-04217903. Massive parallel sequencing of the pretreatment sample and the time-of-progression sample revealed an increased representation of the M1268T mutated allele in the time-of-progression sample as compared with the pretreatment sample (Table 1). Additionally, other variant alleles in MET exon 21 were over-represented in the time-of-progression sample, which was consistent with a copy number gain. No additional therapy-driven MET mutations were identified.
Table 1.
Next-Generation Sequencing of Pretreatment and Time-of-Progression Samples
| Variant Allele |
Pretreatment Variant Allele |
Time-of-Progression Variant Allele |
|||
|---|---|---|---|---|---|
| Position on Chromosome 7 | Exon | Frequency (%) | Reads (No.) | Frequency (%) | Reads (No.) |
| *116423474 | 19 | 15.07 | 425 of 2,820 | 32.97 | 4,001 of 12,137 |
| 116436022 | 21 | 52.46 | 6,703 of 12,777 | 73.37 | 19,787 of 26,968 |
| 116436097 | 21 | 58.38 | 11,583 of 19,841 | 72.07 | 27,180 of 37,711 |
Increased representation of the M1268T mutated allele.
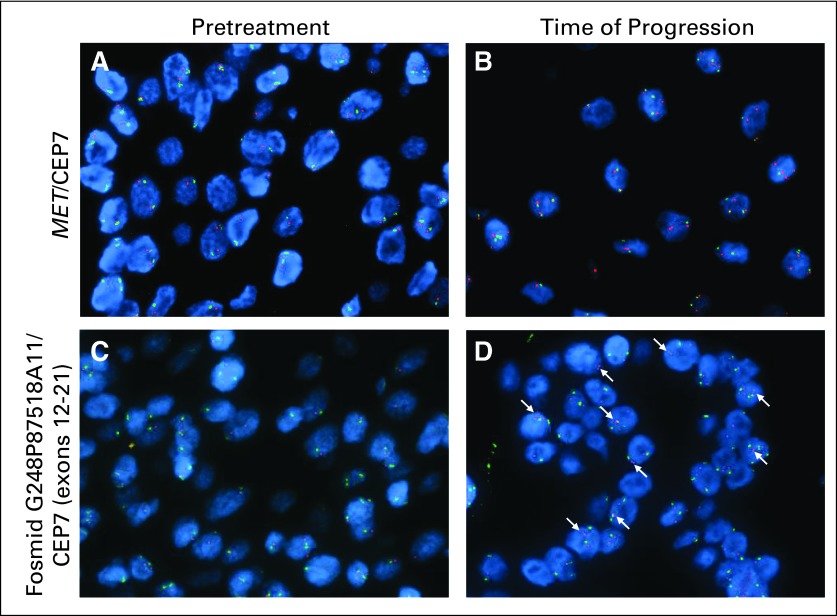
To validate our primary sequencing data, which was suggestive of genomic amplification of the mutated MET allele in the time-of-progression biopsy, we performed FISH using both a commercial MET probe and a genomic probe that was created from a fosmid that spanned exons 12 to 21, and included exon 19, where the M1268T mutation resides. Amplification of MET as defined as clustered signals or a ratio of MET/CEP7 greater than 2 was not observed (Figs 3A and 3B); however, duplication of chromosome 7 was evident in the time-of-progression sample (Table 2). In addition, using fosmid-mediated FISH directed at MET exons 12 to 21, we observed split signals or doublets in approximately 50% of tumor cells in the time-of-progression sample that were not present in the pretreatment sample (Fig 3; arrows indicate doublets).
Fig 3.
Table 2.
FISH Analysis With MET/CEP7 Probes
| Tumor Sample | Probes |
MET |
CEP7 |
MET/CEP7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | % Cells With |
Mean | SD | % Cells With |
|||||
| ≤ 2 Signals | ≥ 3 Signals | ≤ 2 Signals | ≥ 3 Signals | |||||||
| Pretreatment | MET/CEP7 | 1.64 | 0.56 | 96 | 4 | 1.58 | 0.54 | 98 | 2 | 1.04 |
| Fosmid G248P87518A11/CEP7 (exons 12 to 21) | 1.98 | 0.89 | 80 | 20 | 1.78 | 0.74 | 90 | 10 | 1.11 | |
| Fosmid G248P88585E3/CEP7 (exons 4 to 15) | 1.84 | 0.77 | 86 | 14 | 1.82 | 0.63 | 92 | 8 | 1.01 | |
| Time of Progression | MET/CEP7 | 2.56 | 0.79 | 42 | 58 | 2.22 | 0.91 | 72 | 28 | 1.15 |
| Fosmid G248P87518A11/CEP7 (exons 12 to 21) | 2.42 | 0.91 | 52 | 48 | 2.28 | 0.86 | 64 | 36 | 1.06 | |
| Fosmid G248P88585E3/CEP7 (exons 4 to 15) | 2.26 | 0.78 | 58 | 42 | 2.04 | 0.75 | 80 | 20 | 1.11 | |
Abbreviations: FISH, fluorescent in situ hybridization; SD, standard deviation.
Discussion
This patient with metastatic papillary RCC had an aggressive disease course with early disease progression in response to treatment with both sunitinib and temsirolimus. This is consistent with retrospective reviews of patients with nonclear cell tumors who have inferior survival outcomes compared with patients with clear cell tumors.1 Patients with nonclear cell RCC have traditionally been under-represented in clinical studies of new agents for the treatment of RCC; however, this is changing, and many trials now select for specific subtypes of RCC.
Our patient with somatic papillary RCC was found to have a heterozygous M1268T mutation in MET that has previously been identified in both somatic and hereditary forms of this disease.7,8 This mutation results in an amino acid substitution in the P + 1 loop of the MET kinase domain, which is integral to substrate recognition. This mutation is one of the most effective in inducing MET phosphorylation, leading to downstream signal transduction.9,10 The patient case we report here serves as the first clinical proof of principle for the role of MET inhibition in a patient with papillary RCC harboring an activating MET mutation.
There are a number of MET inhibitors in various stages of preclinical and clinical development (Table 3). PF-04217903 is a highly selective MET inhibitor, whereas crizotinib (PF-02341066) is a potent inhibitor of both MET and ALK. On the basis of remarkable activity in ALK-translocated non–small-cell lung cancer, crizotinib received US Food and Drug Administration approval for use in the United States and represents the first commercially available MET inhibitor in the United States, even if it is technically licensed for its anti-ALK activity.11,12 The ongoing development of crizotinib includes an exploration of its activity in patients who are prescreened for evidence of MET mutations in papillary RCC (in the Phase 1 Safety, Pharmacokinetic and Pharmacodynamic Study of PF-02341066, a c-Met/HGFR Selective Tyrosine Kinase Inhibitor, Administered Orally to Patients With Advanced Cancer).
Table 3.
HGF/MET-Targeted Agents in Clinical Development
| Compound | Targets | Study Phase | Treatment Combinations and Disease Sites of Ongoing Studies | Reference |
|---|---|---|---|---|
| Crizotinib (PF-02341066) | MET, ALK TKI | Phase I/III | NSCLC with ALK translocation or inversion; ALK-positive NSCLC; crizotinib v pemetrexed and cisplatin; anaplastic large cell lymphoma; erlotinib for NSCLC; PF-00299804 for NSCLC; pharmacokinetic and bioavailability studies in advanced solid tumors | 11,12 |
| Cabozantinib (XL184) | MET, RET, VEGFR2 TKI | Phase I/III | Advanced solid tumors; temozolomide and radiation for malignant glioma; erlotinib for NSCLC; rosiglitazone for RCC, thyroid cancer; medullary thyroid cancer, lymphoma, malignant glioma | 13 |
| Foretinib (XL880) | MET, VEGFR2 TKI | Phase II | Erlotinib for NSCLC; lapatinib/breast cancer; papillary RCC, HCC, breast cancer | 14 |
| MetMAb (PRO143966) | Anti-MET Ab | Phase II | Paclitaxel, bevacizumab for breast cancer; erlotinib for NSCLC | 15 |
| Rilotumumab (AMG 102) | HGF Ab | Phase II | Platinum chemotherapy for SCLC; panitumumab for wild-type KRAS mCRC; erlotinib for advanced NSCLC; mitoxantrone and prednisone for CRPC; bevacizumab for malignant glioma; epirubicin, cisplatin, and capecitabine for gastric or esophagogastric junction cancer; pemetrexed and cisplatin/mesothelioma for RCC, ovarian cancer | 16–19 |
| AMG 208 | MET TKI | Phase I | Advanced solid tumors | 20 |
| ARQ 197 | MET TKI | Phase II | Irinotecan and cetuximab for mCRC; gemcitabine for advanced solid tumors; sorafenib for advanced solid tumors; erlotinib for NSCLC; RCC, alveolar soft tissue sarcoma, clear cell sarcoma, gastric cancer, HCC, GCT | 21 |
| AV-299 | HGF Ab | Phase I/II | Advanced solid tumors, lymphomas, myeloma; gefitinib for NSCLC | 20 |
| E7050 | Phase I/II | Sorafenib for HCC; advanced solid tumors | 20 | |
| MGCD265 | MET, VEGFR1-3, Ron, Tie2 TKI | Phase I/II | Erlotinib or docetaxel for advanced solid tumors or NSCLC; advanced solid tumors | 22 |
Abbreviations: ALK, anaplastic lymphoma kinase; CRPC, castration-resistant prostate cancer; GCT, germ cell tumor; HCC, hepatocellular carcinoma; HGF, hepatocyte growth factor; HGF Ab, neutralizing antibody against human HGF; mCRC, metastatic colorectal carcinoma; NSCLC, non–small-cell lung cancer; RCC, renal cell carcinoma; SCLC, small-cell lung cancer; SD, standard deviation; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
This report adds to an increasing body of evidence that supports MET as an attractive target for blockade in cancers that are dependent on its constitutive activation for growth and metastasis and proves that blockade of this single kinase has potential for antitumor activity.23 Because it is assumed that it is the molecular biology, rather than the histology per se, that is driving the benefit from the MET inhibitor, we propose that selection that is based solely on papillary renal histology will be insufficient. Instead, screening papillary renal cancers for MET mutations in advance to most appropriately direct them to MET inhibition within clinical trials should be considered.
At the time of disease progression, the M1268T mutation remained, with no evidence of additional mutations; however, the patient's tumor acquired tandem duplication of the mutated MET allele in approximately 50% of tumor cells. In our patient, we speculate that this cytogenetic change in mutated gene copy number was selected at the time of resistance to treatment because of the mutated gene's ability to overcome inhibition by PF-04217903.
Copy number gain has been observed as a mechanism of resistance to crizotinib in ALK-rearranged non–small-cell lung cancer, both in preclinical models and in patients with an initial response to crizotinib treatment that is followed by disease progression.24,25 The significance of tandem duplication of M1268T MET will need to be explored in additional models of MET-mutated papillary RCC and in additional acquired resistance specimens, but may be useful in the future as a marker of resistance to small-molecule MET inhibitors. This patient case illustrates the importance of performing tumor tissue sampling when it is safe and feasible at the time of acquired resistance to therapy to understand mechanisms of resistance, with a goal of developing new treatments or combinations of treatments to overcome resistance.
Acknowledgment
Supported in part by National Institutes of Health (NIH)/National Cancer Institute (NCI) Grants No. 5R01CA100750-06 (R.S.) and 5R01CA125541-05, and by NIH/NCI Grant No. 5K12CA086913-10 (J.R.D.). We thank Pamela Vizcarra for preparing the tumor slides for analysis.
Footnotes
Clinical trial information: NCT00706355.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Keith Wilner, Pfizer (C); Keith A. Ching, Pfizer (C); Maruja E. Lira, Pfizer (C); Eric F.P.M. Schoenmakers, Pfizer (C); James G. Christensen, Pfizer (C) Consultant or Advisory Role: Patricia M. LoRusso, Pfizer (C) Stock Ownership: Keith Wilner, Pfizer; Keith A. Ching, Pfizer; Maruja E. Lira, Pfizer; Eric F.P.M. Schoenmakers, Pfizer; James G. Christensen, Pfizer Honoraria: D. Ross Camidge, Pfizer Research Funding: Patricia M. LoRusso, Pfizer; Jeffrey W. Clark, Pfizer; S. Gail Eckhardt, Pfizer Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 2.Reuter VE, Presti JC., Jr Contemporary approach to the classification of renal epithelial tumors. Semin Oncol. 2000;27:124–137. [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14:288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res. 2009;15:5714–5723. doi: 10.1158/1078-0432.CCR-09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitts TM, Tan AC, Kulikowski GN, et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: Approach to individualized therapy in early development. Clin Cancer Res. 2010;16:3193–3204. doi: 10.1158/1078-0432.CCR-09-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubensky IA, Schmidt L, Zhuang Z, et al. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol. 1999;155:517–526. doi: 10.1016/S0002-9440(10)65147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 9.Maritano D, Accornero P, Bonifaci N, et al. Two mutations affecting conserved residues in the Met receptor operate via different mechanisms. Oncogene. 2000;19:1354–1361. doi: 10.1038/sj.onc.1203431. [DOI] [PubMed] [Google Scholar]
- 10.Jeffers M, Schmidt L, Nakaigawa N, et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci U S A. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodig SJ, Shapiro GI. Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Investig Drugs. 2010;11:1477–1490. [PubMed] [Google Scholar]
- 13.Durante C, Russo D, Verrienti A, et al. XL184 (cabozantinib) for medullary thyroid carcinoma. Expert Opin Investig Drugs. 2011;20:407–413. doi: 10.1517/13543784.2011.559163. [DOI] [PubMed] [Google Scholar]
- 14.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 16.Giordano S. Rilotumumab, a mAb against human hepatocyte growth factor for the treatment of cancer. Curr Opin Mol Ther. 2009;11:448–455. [PubMed] [Google Scholar]
- 17.Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13:437–446. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen PJ, Sweeney CJ, Park DJ, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2677–2687. doi: 10.1158/1078-0432.CCR-09-2862. [DOI] [PubMed] [Google Scholar]
- 19.Gordon MS, Sweeney CS, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 20.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 2010;46:1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–1279. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 22.Kollmannsberger CK, Hurwitz H, Vlahovic G. Phase I study of daily administration of MGCD265 to patients with advanced malignancies (Study 265-101) J Clin Oncol. 2011;27(suppl):68e. abstr e14525. [Google Scholar]
- 23.Eder JP, Vande Woude GF, Boerner SA, et al. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 24.Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–7540. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]