Abstract
Background/Aims
Atrophic gastritis (AG) and intestinal metaplasia (IM) are premalignant gastric lesions. The aims of this study were to evaluate the prevalence of endoscopic AG and IM and to document the risk factors for these lesions.
Methods
In total, 4,023 subjects were enrolled at eight hospitals in Korea. AG and IM were diagnosed by endoscopy. Helicobacter pylori immunoglobulin G antibodies were measured.
Results
The prevalences of endoscopic AG and IM were 40.7% and 12.5%. In a multivariate analysis, the risk factors for AG were age groups of 40 to 59 years and >60 years, male sex, positive H. pylori serology, IM, and education below the college level (odds ratio [OR], 2.55, 5.00, 1.38, 1.41, 4.29, and 1.35, respectively). The risk factors for IM were age groups of 40 to 59 years and >60 years, male sex, positive H. pylori serology, AG, having relatives with gastric cancer, education below the college level and consumption of dairy products (OR, 3.16, 3.25, 1.88, 2.17, 3.68, 1.48, 1.47, and 1.40, respectively).
Conclusions
A nationwide survey regarding the prevalence of endoscopic AG and IM and their risk factors in Korea supports the hypothesis that endoscopic diagnosis of these premalignant lesions could be helpful to describe a group at high risk for gastric cancer.
Keywords: Atrophic gastritis, Intestinal metaplasia, Prevalence, Risk factors, Endoscopy
INTRODUCTION
Gastric cancer, the incidence of which became declining in many industrialized countries, is still one of the major causes of mortality from cancer death in the world.1 Whereas the postoperative 5-year survival rates are 90% to 95% for early gastric cancer (EGC), only 20% to 40% of patients with advanced gastric cancer are expected to survive for 5 years or more.2-4 Moreover, surgery is no more needed in a considerable fraction of patients who are diagnosed with EGC, owing to recent advances in endoscopic resection techniques and technologies. Therefore, early detection of EGC through the vigilant follow-up in the high risk groups is probably the effective strategy for improving survival and quality of life.
The pathogenesis of gastric cancer, particularly the intestinal type, can be explained by a cascade from chronic gastritis through atrophic gastritis (AG), intestinal metaplasia (IM), and dysplasia to cancer. AG is characterized by chronic inflammatory processes of gastric mucosa that leads to the loss of glandular structure and a reduction of gastric secretory function. The presence of AG is known to be a risk of gastric cancer, which increases with the degree and extension of atrophy.5 IM is defined as replacement of gastric columnar epithelial cells by cells of intestinal morphology with the presence of goblet cells, Paneth cells and absorptive cells.5 Both AG and IM represent an obligatory transitional step in gastric carcinogenesis and are undisputed indicators of an increased risk for gastric cancer as compared with chronic gastritis in the absence of these lesions.5-7 Therefore, the recognition of these lesions by endoscopy in a general population indicates the necessity of follow-up endoscopy, which helps the early detection of gastric cancer leading to a better prognosis and treatment by endoscopic resection.
The prevalence of AG and IM vary among countries. That is, they are relatively high in countries with a higher prevalence of Helicobacter pylori infection and gastric cancer.8 H. pylori infection is the major cause of chronic gastritis, which leads to AG and IM over a long period. However, despite a high rate of H. pylori infection, there are some regions with a low prevalence of precancerous lesions and gastric cancer.9 Therefore, other factors such as host and environmental factors might play a role in these differences.
In Western countries, AG and IM are generally observed in histological examination of random biopsies obtained during endoscopy, whereas in Asian countries including Korea, the presence and extension of AG and IM are frequently observed by endoscopy. A health check-up program designed to detect gastric cancer was implemented by the South Korean government in 2001 for biannual evaluation of Korean citizens over age 40. Thus, if we know the endoscopic prevalence of AG and IM in the general population and their role in the localization of high risk group of gastric cancer, it would be very useful for prevention of gastric cancer.
From this background, the aims of this study were to evaluate the prevalence of endoscopic AG and IM, and to document the risk factors for the development of these precancerous lesions with the special reference to H. pylori infection, host and environmental factors in a Korean general population.
MATERIALS AND METHODS
1. Study population
A total of 4,023 subjects who underwent screening endoscopy during a routine general check-up from January to December in 2011, were prospectively enrolled at eight nationwide healthcare centers in Korea. Subjects with a history of gastrointestinal surgery or with systemic disease requiring chronic medication except hypertension and diabetes mellitus were excluded. This study was reviewed and approved by the Institutional Review Board of the eight participating hospitals and written informed consent was obtained from all participating subjects.
2. Questionnaire
All subjects, who provided informed consents, underwent a clinical interview based on a structured questionnaire to assess personal and clinical data under the supervision of a well-trained interviewer before the endoscopy at the eight participating healthcare centers. The questionnaire included questions regarding demographic data, the presence of upper gastrointestinal symptoms, such as epigastric pain or discomfort, dyspepsia, epigastric soreness, and abdominal pain during the previous year, comorbid disease, history of H. pylori eradication, drug history including nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics, alcohol consumption, smoking history, consumption of dairy products, and relatives of gastric cancer.
3. Endoscopic examination
All of the endoscopic examinations were carried out and assessed by endoscopy experts of the eight participating healthcare centers. The endoscopic findings were examined, in a standardized manner, for typical macroscopic changes including erythema (diffuse, spotty, or linear), erosions (small superficial defects in the mucosa with flat edge and white/yellow color or small bleeding spots [petechiae]), absence of rugae, and visible blood vessels. Investigators did agree with simplification of endoscopic definition about AG and IM. Endoscopic AG was defined as thinning, whitish mucosal change or visible submucosal vascular patterns and endoscopic IM as white plaque-like elevation in antrum and corpus. Any overlapping findings were described.
4. Determination of H. pylori status
Blood samples were obtained from each participant immediately after endoscopy. Isolated serum samples were neatly arranged in storage boxes and stored at -70℃. H. pylori infection was diagnosed by enzyme-linked immunosorbent assay (ELISA) for anti-H. pylori immunoglobulin G (IgG) using a Genedia kit (Genedia H. pylori ELISA; Green Cross Medical Science Corp., Eumseong, Korea), with duplicate determinations according to the manufacturer's guidelines. The Genedia kit used H. pylori antigen obtained from Korean H. pylori strains, with a sensitivity and specificity in Korean adults of 97.8% and 92.0%, respectively. The cutoff optical density (OD, 450 nm) of H. pylori IgG was 0.406.
5. Statistical analysis
All statistical analyses were performed using the Stat View software package (SAS Institute, Cary, NC, USA). Continuous variables were analyzed by Student's t-test. Categorical variables were analyzed using chi-squared test or Fisher's exact test. Multivariate logistic regression was used for the analysis of risk factors, which were expressed as the odds ratio (OR) and 95% confidence intervals (CI). The p-values of less than 0.05 were considered statistically significant.
RESULTS
1. Baseline characteristics of subjects
A total of 4,023 subjects were included in the study. This study group comprised 2,358 males (58.6%) and 1,665 females (41.4%). The mean age was 48.7±11.3 (mean±standard deviation [SD]) years with a range from 15 to 98 years. H. pylori IgG positivity was demonstrated in 2,407 subjects (59.8%). Baseline characteristics of subjects including height, weight, body mass index (BMI), cholesterol, triglyceride, fasting glucose, smoking, alcohol, education, income level, NSAID use, high salt diet, history of H. pylori eradication, gastrointestinal symptoms, consumption of dairy product, and relatives of gastric cancer are described in Table 1.
Table 1.
Baseline Characteristics of the 4,023 Subjects

Data are presented as mean±SD or number (%).
NSAID, nonsteroidal anti-inflammatory drug; H. pylori, Helicobacter pylori; IgG, immunoglobulin G.
2. Prevalence rates of AG and IM
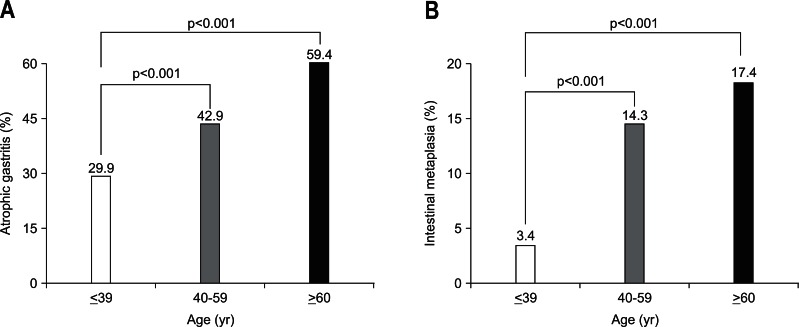
The prevalence rates of AG and IM, diagnosed by endoscopic findings, were 40.7% (1,638 of 4,023) and 12.5% (502 of 4,023), respectively. The prevalence rates of AG and IM in males were significantly higher than those in females (43.3% vs 37.1% and 15.4% vs 8.3%, respectively; p<0.001) (Tables 2 and 3). The prevalence rate of AG increased with age (from 29.9% in those aged less than 40 years to 59.4% in those aged 60 years or older) (Fig. 1A). The prevalence rate of IM also increased with age (from 3.4% in those less than 40 years of age to 17.4% in those 60 years or older; p<0.001) (Fig. 1B).
Table 2.
Univariate Analysis of the Risk Factors for Atrophic Gastritis

Data are presented as number (%).
H. pylori, Helicobacter pylori; IgG, immunoglobulin G; BMI, body mass index; NSAID, nonsteroidal anti-inflammatory drug.
*Some data were missing. Missing values were not included.
Table 3.
Univariate Analysis of the Risk Factors for Intestinal Metaplasia

Data are presented as number (%).
IM, intestinal metaplasia; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; BMI, body mass index; NSAID, nonsteroidal anti-inflammatory drug.
*Some data were missing. Missing values were not included.
Fig. 1.
Prevalence of atrophic gastritis (A) and intestinal metaplasia (B) in each age group. The prevalence of atrophic gastritis and intestinal metaplasia increased significantly with age.
3. The identification of risk factors for AG by univariate and multivariate analysis
In a univariate analysis of risk factors for AG, older age group of above 40 to 59, older age group of above 60 years, male gender, H. pylori IgG positivity, IM, and low education below college were found to be associated with a high risk of AG (Table 2). Multivariate analysis showed that the significant independent risk factors for AG were older age group of 40 to 59 (OR, 2.55; 95% CI, 2.05 to 3.18), above 60 years old (OR, 5.00; 95% CI, 3.71 to 6.74) compared with below 40, male gender (OR, 1.38; 95% CI, 1.17 to 1.64), H. pylori IgG positivity (OR, 1.41; 95% CI, 1.19 to 1.66), IM (OR, 4.29; 95% CI, 3.55 to 5.50), and low education below college (OR, 1.35; 95% CI, 1.01 to 1.79) (Table 4).
Table 4.
Multivariate Analysis of the Risk Factors for Atrophic Gastritis

B, estimate; SE, standard error; Exp(β), odds ratio; CI, confidence interval; H. pylori, Helicobacter pylori; IgG, immunoglobulin G.
4. The identification of risk factors for IM by univariate and multivariate analysis
In a univariate analysis of risk factors for IM, older age group of above 40 to 59, older age group of above 60 years, male gender, H. pylori IgG positivity, AG, BMI, triglyceride level, H. pylori eradication, relatives of gastric cancer, smoking, alcohol, low education, and consumption of dairy product were found to be associated with a high risk of IM (Table 3). Multivariate analysis showed that the significant independent risk factors for IM were older age group of 40 to 59 (OR, 3.16; 95% CI, 2.11 to 4.72), above 60 years old (OR, 3.25; 95% CI, 2.05 to 5.15), male gender (OR, 1.88; 95% CI, 1.39 to 2.54), H. pylori IgG positivity (OR, 2.17; 95% CI, 1.72 to 2.74), AG (OR, 3.68; 95% CI, 2.95 to 4.60), relatives of gastric cancer (OR, 1.48; 95% CI, 1.12 to 1.96), low education below college (OR, 1.47; 95% CI, 1.06 to 2.00), and consumption of dairy product at least five times per week (OR, 1.40; 95% CI, 1.12 to 1.76) (Table 5).
Table 5.
Multivariate Analysis of the Risk Factors for Intestinal Metaplasia

B, estimate; SE, standard error; Exp(β), odds ratio; CI, confidence interval; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; NS, not significant.
DISCUSSION
The development of gastric cancer is generally accepted to be a multistep progression from H. pylori-related chronic inflammation of the gastric mucosa, to AG, IM, dysplasia, and, finally, intestinal-type gastric cancer. The risk of gastric cancer is closely related to the degree and extension of AG and IM.5,10,11 Therefore, evaluating the prevalence and risk factors for these precancerous lesions such as AG and IM may be helpful to prevent the development of gastric cancer.
However, it is nearly impossible to take biopsy from antrum and body in the general population without definite lesion, especially in a huge population as our study. Thus, it is necessary to evaluate prevalence rates of endoscopic AG and IM, especially where the health check-up endoscopy is popular for gastric cancer screening. In the present study, the prevalence of AG and IM were found to be 40.7% and 12.5%, respectively. In Western Europe, the overall prevalence rates of AG and IM were approximately one-third and one-fourth of subjects, respectively.12-14 In contrast, the prevalence of AG and IM were 55.5% and 28.5%, respectively, in Japan.15 Previously, we have shown that the prevalence rates of AG and IM, diagnosed by histology, were 42.5% and 28.6%, respectively in the antrum.8 Considering histological diagnosis of IM is more accurate and sensitive than endoscopic diagnosis,13,16 the prevalence rate of endoscopic IM in the present study, 12.5%, is not very low. Another reason of lower prevalence of IM in this study could be explained by the population of the participating subjects: 21.5% was below 40 years old, and only 16.7% was ≥60.
In our study, the prevalence of AG and IM increased significantly with age and this phenomenon could be explained by H. pylori infection. H. pylori infection usually occurs in the childhood, but AG and IM progress in the elderly population due to long infectious periods with H. pylori.17 In addition, the prevalence of AG and IM in males were significantly higher than those in females. It is generally understood that the prevalence of H. pylori infection and gastric disorders in males is more common than that in females.16,17 Furthermore, seropositivity of H. pylori was found to be the risk factor of AG and IM in the present study. As it is well known, H. pylori infection is the most common risk factor of glandular atrophy and subsequent IM.8 Our consistent results support that endoscopic diagnosis of AG and IM could be a useful tool for localization of high risk group of gastric cancer without histology.
Interestingly, AG and IM were found to be a risk factor for each other in our study. Usually, these two lesions have been associated with chronic inflammatory processes such as H. pylori infection. Thus, these results might be originated from that atrophy and IM ensue sequentially over time after H. pylori infection.18,19 In addition, the degree of IM has been correlated with the degree of atrophy.20 However, IM can also occur without atrophy.5,21 Furthermore, the risk factors for AG and IM were rather different.8 Bacterial factors were found to be important risk factor for AG but host and environmental factors were more important for IM, suggesting that sometimes it does not go together.8
In our study, low education below college was a risk factor for the development of AG and IM. Previous reports showed that lower education was associated with H. pylori infection22 and the development of AG in H. pylori infected subjects.23 As lower education is usually associated with lower socioeconomic status and poorer hygiene, it is identified as risk factors for H. pylori infection.22,23 H. pylori infection is likely to contribute to this association between low education and the high prevalence of AG and IM.
The proportion of family history of gastric cancer was found to be 11% in this study, rather higher than that in the general population. This could be originated from more concern of the relatives of gastric cancer about their health they might frequently receive health check-up, compared general population. Interestingly, family history of gastric cancer was a risk factor for the development of IM, but not of AG in our study. First-degree relatives of gastric cancer were found to have a higher risk of developing gastric cancer.24,25 The possible causes of familial aggregation of gastric cancer are not only genetic factors but also environmental factors including H. pylori infection, excessive intake of salt and N-nitroso compound, and a deficiency of dietary antioxidants among gastric cancer patients and their family members.26 Relatives of gastric cancer have an increased prevalence of precancerous lesions including AG and IM, and H. pylori plays an instrumental role in determining the risk of precancerous lesions among relatives of gastric cancer.25,27,28 Consequently, prophylactic H. pylori eradication is advised to the relatives of gastric cancer in Korea for prevention of gastric cancer.29
Similar to the family history of gastric cancer, consumption of dairy product at least five times per week was a risk factor for the development of IM but not AG. Previously, milk or yogurt prevents the development of AG and IM through its defense mechanism against the attachment of H. pylori to the gastric mucosa30,31 and also, consumption of fermented dairy products confers an enhanced therapeutic benefit on H. pylori eradication.32 As our study showed different result further studies are necessary in the future.
Our study has limitations. The diagnosis of AG and IM was made only by endoscopic findings and not confirmed by histology. Although histological examination is considered as the gold standard for diagnoses of AG and IM, interobserver variation for diagnoses of AG on biopsy specimens has been shown to be high,33,34 and sampling errors exist, especially when multifocal AG is present.35,36 Also, there are few reports concerning the high agreement between the endoscopic and histological atrophy scores.37,38 The image quality of conventional endoscopes has improved dramatically over the last decade and typical endoscopic findings are interpreted as signs of AG and IM. In addition, to prevent the interobserver variation in the diagnosis of AG and IM, only endoscopic experts participated in this study. Even though the significance of typical endoscopic findings in relation to histology is still uncertain,16,39 similar results regarding the prevalence and risk factor of endoscopic AG and IM to those from the histological AG and IM support clinical significance of our study. Also, the severity and location of endoscopic AG and IM were not classified mainly because there is no standardized grading system of atrophy and IM. In addition, as the endoscopy of this study was performed as one of health check-up it was very difficult to ask the participating doctors to fill up all of those things in detail.
In conclusion, the prevalence of endoscopic AG and IM in the general population were not so high in Korea, especially in case of IM. The risk factors of AG and IM were similar but relatives of gastric cancer and consumption of dairy product were the risk factors of IM, but not of AG. Therefore, it is worthwhile to describe the degree and extent of AG and IM in detail during endoscopy, according to diagnostic criteria.
ACKNOWLEDGEMENTS
This work was supported by grant no. 06-2011-130 from the Seoul National University Bundang Hospital research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Katanoda K, Yako-Suketomo H. Comparison of time trends in stomach cancer incidence (1973-2002) in Asia, from cancer incidence in five continents, vols IV-IX. Jpn J Clin Oncol. 2009;39:71–72. doi: 10.1093/jjco/hyn150. [DOI] [PubMed] [Google Scholar]
- 2.Yuasa N, Nimura Y. Survival after surgical treatment of early gastric cancer, surgical techniques, and long-term survival. Langenbecks Arch Surg. 2005;390:286–293. doi: 10.1007/s00423-004-0482-y. [DOI] [PubMed] [Google Scholar]
- 3.Park JM, Ryu WS, Kim JH, et al. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat. 2006;38:13–18. doi: 10.4143/crt.2006.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 5.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Park RY, Cho SI, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol. 2008;42:448–454. doi: 10.1097/MCG.0b013e318046eac3. [DOI] [PubMed] [Google Scholar]
- 7.Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 8.Kim N, Park YS, Cho SI, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245–255. doi: 10.1111/j.1523-5378.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 9.You WC, Zhang L, Gail MH, et al. Precancerous lesions in two counties of China with contrasting gastric cancer risk. Int J Epidemiol. 1998;27:945–948. doi: 10.1093/ije/27.6.945. [DOI] [PubMed] [Google Scholar]
- 10.Tatsuta M, Iishi H, Nakaizumi A, et al. Fundal atrophic gastritis as a risk factor for gastric cancer. Int J Cancer. 1993;53:70–74. doi: 10.1002/ijc.2910530114. [DOI] [PubMed] [Google Scholar]
- 11.Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, et al. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol. 2004;57:177–182. doi: 10.1136/jcp.2003.11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuipers EJ, Uyterlinde AM, Peña AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 13.Petersson F, Borch K, Franzén LE. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol. 2002;37:262–266. doi: 10.1080/003655202317284156. [DOI] [PubMed] [Google Scholar]
- 14.Borch K, Jönsson KA, Petersson F, Redéen S, Mårdh S, Franzén LE. Prevalence of gastroduodenitis and Helicobacter pylori infection in a general population sample: relations to symptomatology and life-style. Dig Dis Sci. 2000;45:1322–1329. doi: 10.1023/a:1005547802121. [DOI] [PubMed] [Google Scholar]
- 15.Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294–299. doi: 10.1046/j.1523-5378.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 16.Eshmuratov A, Nah JC, Kim N, et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci. 2010;55:1364–1375. doi: 10.1007/s10620-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 17.Gold BD. New approaches to Helicobacter pylori infection in children. Curr Gastroenterol Rep. 2001;3:235–247. doi: 10.1007/s11894-001-0028-1. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz N, Kato I, Peraza S, et al. Prevalence of precancerous lesions of the stomach in Venezuela. Cancer Epidemiol Biomarkers Prev. 1996;5:41–46. [PubMed] [Google Scholar]
- 19.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 20.Guarner J, Herrera-Goepfert R, Mohar A, et al. Gastric atrophy and extent of intestinal metaplasia in a cohort of Helicobacter pylori-infected patients. Hum Pathol. 2001;32:31–35. doi: 10.1053/hupa.2001.20889. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers EJ. Review article: Relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl 1):25–36. doi: 10.1111/j.1365-2036.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Hu F, Zhang L, et al. Prevalence of Helicobacter pylori infection and identification of risk factors in rural and urban Beijing, China. Helicobacter. 2009;14:128–133. doi: 10.1111/j.1523-5378.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 23.Ito LS, Oba-Shinjo SM, Marie SK, et al. Lifestyle factors associated with atrophic gastritis among Helicobacter pylori-seropositive Japanese-Brazilians in Sao Paulo. Int J Clin Oncol. 2003;8:362–368. doi: 10.1007/s10147-003-0355-3. [DOI] [PubMed] [Google Scholar]
- 24.Dhillon PK, Farrow DC, Vaughan TL, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93:148–152. doi: 10.1002/ijc.1294. [DOI] [PubMed] [Google Scholar]
- 25.Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34–e39. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]
- 26.Chang YW, Han YS, Lee DK, et al. Role of Helicobacter pylori infection among offspring or siblings of gastric cancer patients. Int J Cancer. 2002;101:469–474. doi: 10.1002/ijc.10637. [DOI] [PubMed] [Google Scholar]
- 27.El-Omar EM, Oien K, Murray LS, et al. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22–30. doi: 10.1016/s0016-5085(00)70410-0. [DOI] [PubMed] [Google Scholar]
- 28.Leung WK, Ng EK, Chan WY, et al. Risk factors associated with the development of intestinal metaplasia in first-degree relatives of gastric cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14:2982–2986. doi: 10.1158/1055-9965.EPI-05-0181. [DOI] [PubMed] [Google Scholar]
- 29.Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 30.Jarosz M, Rychlik E, Siuba M, et al. Dietary and socio-economic factors in relation to Helicobacter pylori re-infection. World J Gastroenterol. 2009;15:1119–1125. doi: 10.3748/wjg.15.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45–53. doi: 10.1097/MEG.0b013e32830d0eff. [DOI] [PubMed] [Google Scholar]
- 32.Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261–268. doi: 10.1111/j.1523-5378.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 33.Offerhaus GJ, Price AB, Haot J, et al. Observer agreement on the grading of gastric atrophy. Histopathology. 1999;34:320–325. doi: 10.1046/j.1365-2559.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- 34.el-Zimaity HM, Graham DY, al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 35.Satoh K, Kimura K, Taniguchi Y, et al. Biopsy sites suitable for the diagnosis of Helicobacter pylori infection and the assessment of the extent of atrophic gastritis. Am J Gastroenterol. 1998;93:569–573. doi: 10.1111/j.1572-0241.1998.166_b.x. [DOI] [PubMed] [Google Scholar]
- 36.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504–509. [PubMed] [Google Scholar]
- 37.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 38.Liu Y, Uemura N, Xiao SD, Tytgat GN, Kate FJ. Agreement between endoscopic and histological gastric atrophy scores. J Gastroenterol. 2005;40:123–127. doi: 10.1007/s00535-004-1511-x. [DOI] [PubMed] [Google Scholar]
- 39.Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946–950. doi: 10.1055/s-2003-43479. [DOI] [PubMed] [Google Scholar]