Abstract
Background/Aims
This study reports treatment outcomes after helical intensity-modulated radiotherapy (IMRT) in unresectable hepatocellular carcinoma (HCC) patients for whom transarterial chemoembolization (TACE) was considered ineffective or unsuitable.
Methods
From January 2008 to December 2011, 22 unresectable HCC patients received helical IMRT. A daily dose of 1.8 to 4 Gy was delivered at five fractions per week to deliver a total dose of 30 to 60 Gy. The most-prescribed dose fractionation was a total dose of 50 to 57.5 Gy, with a daily dose of 2.3 to 2.5 Gy.
Results
In the entire group, the objective response rate of the primary tumor was 72.7%. In the eight patients with portal vein thrombosis (PVT), the objective response rate of PVT was 50.0%. Median disease progression-free survival was 11.8 months, and the 1-year disease progression-free survival rate was 40.2%. The median overall survival was 14.4 months, and the 1- and 2-year overall survival rates were 86.4% and 69.1%, respectively. PVT and Child-Pugh classifications were significant prognostic factors for overall survival in multivariate analyses.
Conclusions
Helical IMRT in patients with unresectable HCC resulted in high treatment response and survival rates. This study suggests helical IMRT is a practical treatment option for HCC patients in whom TACE is unsuitable or ineffective.
Keywords: Helical intensity-modulated radiotherapy, Hepatocellular carcinoma, Objective response rate, Prognostic factor, Survival rate
INTRODUCTION
Treatments for unresectable hepatocellular carcinoma (HCC) include transarterial chemoembolization (TACE), percutaneous ethanol injection therapy, and radiofrequency ablation (RFA).1 TACE is one of the most widely used nonsurgical treatments for HCC, and two randomized trials showed improved survival using TACE compared with symptomatic therapy alone.2,3 In addition, sorafenib, a multikinase inhibitor, has recently been shown to improve survival in patients with advanced HCC.4,5
In the past, radiotherapy (RT) was used infrequently in HCC because the tumor was thought to be radioresistant and normal liver was radiosensitive. However, HCC was shown to be radiosensitive, and now, based on recent advances in three-dimensional conformal RT (3D-CRT), we may deliver clinically effective radiation dosage to HCC with minimal exposure to normal liver and acceptably low risk of liver toxicity.6 One of the newest conformal radiation treatment modalities employs helical intensity-modulated RT (IMRT), in which a gantry 6-MV linear accelerator is rotated continuously through 360° around the patient using tens of thousands of narrow beamlets.7 However, no randomized studies to date address the effectiveness of RT, and no consensus exists regarding the use of RT to treat unresectable HCC patients with or without portal vein thrombosis (PVT).
This study reports treatment outcomes and prognostic factors after helical IMRT of unresectable HCC patients for whom TACE was considered ineffective or unsuitable. The aim of this study is to promote consensus regarding the use of RT for unresectable HCC.
MATERIALS AND METHODS
1. Patient population
Eligibility criteria were: 1) HCC confirmed by histology or clinical examination; 2) medical inoperability due to underlying disease, or technical unresectability because of large tumor size; 3) TACE was considered ineffective or unsuitable because of collateral blood supplies, or arterioportal shunts, or PVT; 4) good general condition with performance status ≤2 in Eastern Cooperative Oncology Group (ECOG) classification; 5) Child-Pugh classification A or B; 6) no extrahepatic metastases; 7) documented helical IMRT for HCC; and 8) follow-up data available. From January 2008 to December 2011, 35 patients with unresectable HCC received helical IMRT at our hospital. Of those patients, 22 patients met eligibility criteria and were included in this study (Fig. 1).
Fig. 1.
Flow chart of patient selection.
HCC, hepatocellular carcinoma; IMRT, intensity-modulated radiotherapy; TACE, transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group.
A diagnosis of HCC was based on practice guidelines of the Korean Liver Cancer Study Group and the National Cancer Center.8 T classification was defined according to the American Joint Committee on Cancer TNM staging system (7th edition), and liver function was classified according to Child-Pugh classification. The Institutional Review Board approval was obtained for the review and analysis of patient data.
2. RT
All patients underwent computed tomography (CT) simulation in the supine position after immobilization with a posterior vacuum bag and an anterior vacuum-sealed cover sheet. To reduce movement of the liver by respiration, all patients were asked to take shallow breaths. Gross tumor volume (GTV), including primary hepatic mass and PVT was contoured manually in CT data sets. Since most tumors had microscopic extension of less than 2 to 5 mm beyond the tumor margin,9,10 a margin of 5 mm was added to the GTV to account for clinical target volume (CTV). Planning target volume (PTV) was set by adding a 1 cm radial margin and a 1.5-cm craniocaudal margin to the CTV. The entire liver was contoured and the normal liver volume was quantified as the total liver volume minus the CTV.
Until now, the most effective dose-fractionation schedule for hepatoma has not been determined, so the prescription dose was decided by the physician's own judgments according to the patients' general condition, hepatic functional capacity, and tumor size. A daily dose of 1.8 to 4 Gy was delivered at five fractions per week to deliver a total dose of 30 to 60 Gy. Mostly prescribed dose fractionation protocol was total dose of 50 to 57.5 Gy with daily dose of 2.3 to 2.5 Gy. The biologically effective dose (BED) was calculated using a linear quadratic model with respect to acute effects on tumor, as the α/β ratio=10.11
IMRT inverse planning was generated using the Hi·Art Planning Station (TomoTherapy Inc., Madison, WI, USA). Each treatment plan was evaluated with a cumulative dose-volume histogram. In the optimized plan, 1) the PTV was covered by 95% of isodose curves; 2) inhomogeneity of the PTV ranged from 95% to 107%; 3) doses to normal organs were limited in their tolerances. Avoiding overdose to normal liver is critical because radiation-induced liver disease (RILD) is usually fatal. The guideline followed in this study was that no more than 30% of normal liver may receive more than 27 Gy (V27 <30%), and no more than 50% of normal liver may receive more than 24 Gy (V24 <50%). Also, the mean normal liver dose should be less than 28 Gy. The BED was calculated with α/β ratio=3.
3. Clinical evaluation
Primary tumor and PVT responses were determined using magnetic resonance imaging (MRI) or CT scan 4 to 8 weeks after completion of RT, and the status of disease progression was determined every 2 to 3 months thereafter. The response to RT was evaluated according to modified Response Evaluation Criteria in Solid Tumors guidelines.12 Objective response rates were defined as the sum of the complete response (CR) and partial response (PR) rates. Disease progression was defined as tumor reappearance at another site in liver, or tumor enlargement on CT/MRI or clinical examination compared with previous status, or development of extrahepatic metastases.
Radiation-induced general and gastrointestinal toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Patients who had baseline symptoms were scored based on CTCAE toxicity grade both before and after RT, and the difference in grade was recorded. Liver toxicity was focused on RILD, defined as anicteric elevation of alkaline phosphatase level of at least 2-fold and nonmalignant ascites and hepatomegaly in the absence of documented progressive disease (PD), or elevation of transaminases levels of at least 5-fold the upper limit of normal.13 Radiation-induced hepatitis B virus (HBV) reactivation was defined as an increase in serum HBV DNA of >2 log10 copies/mL compared with baseline.14
4. Statistical analysis
Duration of overall survival was determined from the completion of RT to the date of death or, for survivors, to the date of last follow-up. Duration of disease progression-free survival was determined from the completion of RT to the date of disease progression or to the date of last follow-up.
Survival rates were estimated by the Kaplan-Meier method and compared using a log-rank test. Parameters evaluated as potential prognostic factors of survival were: primary tumor response, presence of PVT, ECOG performance status, gender, age, underlying hepatitis, Child-Pugh classification, tumor size, T stage, multiplicity, pre-RT α-fetoprotein (AFP) level, AFP level change, interval between RT and previous treatment, and RT dose. AFP level change was calculated by post-RT AFP level/pre-RT AFP level. Post-RT AFP levels were checked 4 to 8 weeks after completion of RT. All parameters were categorized in two groups according to patient distribution. Parameters with a p-value less than 0.10 in univariate analysis were further assessed in a multivariate analysis, using a Cox regression hazard model.
Using chi-square test, the following parameters were evaluated as potential predictive factor of primary tumor response: presence of PVT, underlying hepatitis, Child-Pugh classification, tumor size, T stage, multiplicity, pre-RT AFP level, AFP level change, and RT dose. Parameters with a p-value less than 0.10 in univariate analysis were further assessed in multivariate analysis, using a multivariate logistic regression model.
All analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A p<0.05 was considered statistically significant.
RESULTS
1. Patient characteristics
The median age of patients was 61.1 years, and the ratio of men to women was 17:5. All patients had underlying liver cirrhosis and 16 patients had underlying hepatitis B. No patient had underlying hepatitis C. The median greatest tumor dimension before RT was 4.4 cm (range, 0.9 to 16.4 cm), and eight patients had PVT. The tumor growth patterns were nodular in 14 patients (massive exophytic nodular in one patient) and infiltrative in eight patients. Pre-RT AFP levels were <40 IU/mL in 54.5% of patients, and post-RT AFP levels were lower than pre-RT AFP levels in 50% of patients. Eighteen patients had received other treatments before the RT. Fourteen patients were treated with TACE (63.6%), one patient with TACE and RFA, one patient with RFA, and two patients with surgical resection and TACE. Median interval between previous last treatment and RT was 2.9 months (range, 0.4 to 14.7 months). No patient died during RT. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics

ECOG, Eastern Cooperative Oncology Group; AJCC, The American Joint Committee on Cancer; AFP, α-fetoprotein; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy; BED, biologically effective dose.
2. RT parameters and toxicity
For the entire study group, median normal liver volume was 982.09 cc (range, 249.56 to 1,252.3 cc), and median radiation dose to normal liver was 18.1 Gy (range, 3.85 to 28.0 Gy). The median normal liver V30 was 19.8% (range, 3.5% to 45.0%), and median normal liver V50 was 2.5% (range, 0% to 16.9%).
All patients received the complete course of scheduled RT. In general, radiation-induced toxicities were not severe. Fourteen patients (63.6%) experienced grade 1 general toxicity such as fatigue and malaise, and five patients (22.7%) experienced grade 2 general toxicity. No patient experienced general toxicity greater than grade 2. One patient experienced grade 2 gastrointestinal toxicity. This patient was diagnosed as grade 2 duodenal ulcer at 1.5 months after completion of RT and treated with antiulcer medication. Twelve patients experienced grade 1 gastrointestinal toxicity, and nine patients experienced no gastrointestinal toxicity. Only one patient experienced RILD. Normal liver volume for this patient was 794.8 cc and RT parameters were as follows: mean dose to normal liver was 28.0 Gy, V30 was 45.0%, and V50 was 16.9%. This patient also experienced radiation-induced HBV reactivation, and died 2.1 months after completion of RT. In the entire study group, three patients experienced radiation-induced HBV reactivation (Table 2).
Table 2.
Radiation-Induced Toxicity Following Helical Intensity-Modulated Radiotherapy for Unresectable Hepatocellular Carcinoma

3. Response to RT
In the entire group, primary tumor responses were CR in four patients (18.2%), PR in 12 patients (54.5%), stable disease (SD) in five patients (22.7%), and PD in one patient (4.6%). The objective response (CR+PR) rate of the primary tumor was 72.7%. In the eight patients with PVT, the PVT responses were PR in four patients (50.0%), SD in three patients (37.5%), and PD in one patient (12.5%). The objective response (CR+PR) rate of PVT was 50.0%.
Predictive factors for primary tumor response were analyzed. In univariate analysis, there was no significant predictive factor for primary tumor response. The patients with post-RT AFP levels lower than pre-RT AFP levels (AFP level change <1) showed higher objective primary tumor response rate, but this was not statistically significant (p=0.069). In multivariate analysis, however, the AFP level change was significantly associated with primary tumor response (p=0.043) (Table 3).
Table 3.
Analyses of Predictive Factors for Primary Tumor Response

AJCC, The American Joint Committee on Cancer; AFP, α-fetoprotein; BED, biologically effective dose.
4. Survival
At the time of last follow-up, 18 patients were alive. The median follow-up period for the whole group was 14.4 months (range, 1.9 to 38.2 months), and for living patients, 15.7 months (range, 7.2 to 38.2 months). Fourteen patients (63.6%) experienced disease progression. In-field progression developed in four patients (18.2%), out-field progression in 10 patients (45.5%), and extrahepatic metastases in two patients (9.1%). Two patients who developed extrahepatic metastases also showed in-field progression and out-field progression, respectively. The median period to infield progression and out-field progression were 4.1 months (range, 2.0 to 11.8 months) and 8.7 months (range, 1.2 to 22.4 months), respectively. Two extrahepatic metastases were developed at 1.2 and 2.8 months. In these patients who experienced disease progression, further treatments such as TACE, surgical resection, and systemic chemotherapy were implemented according to the patients' general condition and hepatic functional capacity. Median disease progression-free survival was 11.8 months, and the 1-year disease progression-free survival rate was 40.2% (Fig. 2). Univariate and multivariate analyses were performed to identify prognostic factors for disease progression-free survival. There was no significant prognostic factor for disease progression-free survival (Table 4). Median overall survival was 14.4 months, and the 1- and 2-year overall survival rates were 86.4% and 69.1%, respectively (Fig. 3). In univariate analysis, primary tumor response, PVT, and Child-Pugh classification were significantly associated with overall survival. In multivariate analysis, PVT and Child-Pugh classification showed significant association with overall survival (Table 5, Figs. 4 and 5).
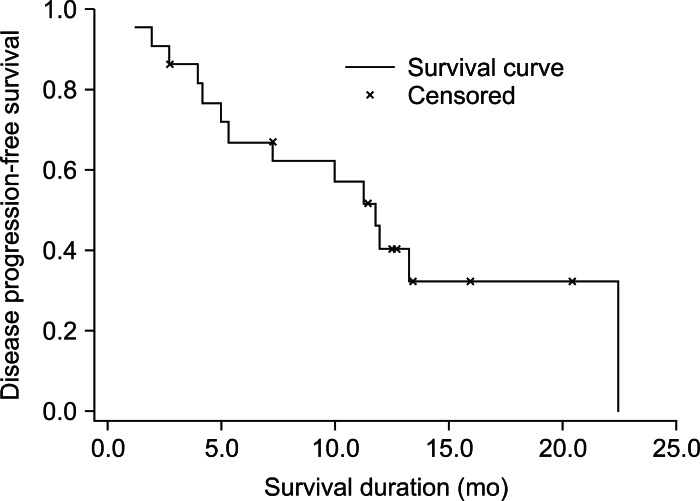
Fig. 2.
Disease progression-free survival in 22 patients with unresectable hepatocellular carcinoma treated with helical intensity-modulated radiotherapy. The median disease progression-free survival duration was 11.8 months, and the 1-year disease progression-free survival rate was 40.2%.
Table 4.
Analyses of Prognostic Factors for Disease Progression-Free Survival

ECOG, Eastern Cooperative Oncology Group; AJCC, The American Joint Committee on Cancer; AFP, α-fetoprotein; RT, radiotherapy; BED, biologically effective dose; CR, complete response; PR, partial response.
Fig. 3.
Overall survival in 22 patients with unresectable hepatocellular carcinoma treated with helical intensity-modulated radiotherapy. The median overall survival duration was 14.4 months, and the 1- and 2-year overall survival rates were 86.4% and 69.1%, respectively.
Table 5.
Analyses of Prognostic Factors for Overall Survival

ECOG, Eastern Cooperative Oncology Group; AJCC, The American Joint Committee on Cancer; AFP, α-fetoprotein; RT, radiotherapy; BED, biologically effective dose; CR, complete response; PR, partial response.
Fig. 4.
Overall survival of patients with Child-Pugh classifications A and B. The estimated 1- and 2-year overall survival rates were 100% and 75.0% for Child-Pugh classification A, and 57.1% and 57.1% for Child-Pugh classification B (p=0.026).
Fig. 5.
Overall survival of patients with and without portal vein thrombosis (PVT). The estimated 1- and 2-year overall survival rates for patients without PVT were 92.9% and 92.9%, respectively, while for patients with PVT, they were 75.0% and 0%, respectively (p=0.047).
DISCUSSION
Several studies reported the primary tumor response rates after 3D-CRT, with or without other local treatments in unresectable HCC, and the reported response rates range from 40% to 64%.15-19 Mornex et al.20 reported an objective response rate (CR+PR) of 92% to 3D-CRT in 27 HCC patients for whom surgical resection was not suitable. However, that study included only patients with small HCC (one nodule ≤5 cm, and two nodules ≤3 cm), and may therefore be biased toward a high response rate. With the advent of IMRT technique, a few studies also reported the primary tumor response rates after IMRT in unresectable HCC. From a study of 27 HCC patients treated with IMRT, Kang et al.21 reported an objective response rate of 44.4%. McIntosh et al.22 treated 20 HCC patients with helical tomotherapy and concurrent capecitabine, and reported an objective response rate of 56.25%. In our study, we used helical tomotherapy to treat 22 patients with unresectable HCC and a median greatest tumor dimension before RT of 4.4 cm (range, 0.9 to 16.4 cm). At 4 to 8 weeks after completion of RT, we found a CR rate of 18.2% (4/22), a PR rate of 54.5% (12/22), and an objective response rate of 72.7% for the primary tumor. The objective response rate of our study is relatively high compared with values previously reported.
The survival rate of our study is also high compared with values previously reported. From a study of 70 HCC patients with or without PTV, who were treated by 3D-CRT, Kim et al.19 reported 1- and 2-year overall survival rates of 43.1% and 17.6%, respectively. Liang et al.23 reported 1-, 2-, and 3-year overall survival rates of 65%, 43%, and 33%, respectively, for 128 HCC patients treated by hypofractionated 3D-CRT with or without TACE. McIntosh et al.22 treated 20 HCC patients with helical tomotherapy and reported 1-year overall survival rate of 73% and 11% for patients with Child-Pugh classification A and B disease, respectively. In our study, the 1- and 2-year overall survival rates were 86.4% and 69.1%, and 1-year disease progression-free survival rate was 40.2%. In addition, we found a 100% overall survival at 1 year for patients with Child-Pugh classification A or ECOG performance status 0. Possible reasons for these inconsistent results may stem from differences in tumor characteristics such as tumor size and multiplicity, patient characteristics such as performance status and underlying liver disease, PVT rate, treatment protocols such as combination of treatment modalities and RT dose, and the indications and regimens for post-RT treatment. We, nevertheless, believe that the results of our present study is very encouraging.
Several studies showed a dose-response relationship in RT for HCC. A higher radiation dose achieved a higher response rate,24,25 and a higher survival rate.18,19,26 In our study, BED was used to analyze the radiation dose affecting primary tumor response and survival because the fraction size ranged from 1.8 to 4 Gy. We found that in patients receiving doses of <65 Gy10 and ≥65 Gy10, the primary tumor response rates were 63.6% and 81.8%, the 1-year disease progression-free survival rates were 27.3% and 58.9%, and the 1-year overall survival rates were 81.8% and 90.0%. Thus, our study also showed a higher radiation dose achieved a higher response rate and higher survival rate. However, these differences were not statistically significant, possibly because of the small sample size, and/or the wide heterogeneity in tumor characteristics. Despite several studies showed this dose-response relationship, at this point in time, no consensus has been established on the optimal radiation dose-fractionation schedule for patients with HCC. Therefore, further investigations should be performed to establish the optimum fraction size and total dose.
Several studies have reported prognostic factors for survival of patients with HCC following RT. Zhou et al.27 found T stage, PVT, radiation dose, and Child-Pugh classification to be significantly associated with overall survival in univariate analysis, and in Cox regression analysis, T stage, radiation dose, and Child-Pugh classification were independent prognostic factor for overall survival. Kang et al.21 found significant associations of PVT, Child-Pugh classification, and primary tumor response with overall survival in univariate analysis, and in multivariate analysis, only primary tumor response remained significant. Kim et al.19 reported the Cancer of the Liver Italian Program score and primary tumor response were significant prognostic factors for overall survival in univariate analysis, and only primary tumor response in multivariate analysis. In our study, primary tumor response, PVT, and Child-Pugh classification were significantly associated with overall survival in univariate analysis. In multivariate analysis, PVT and Child-Pugh classification remained significant. Several studies, including our study, showed that primary tumor responders had better overall survival than non-responders. This finding indicates that RT may be considered as an alternative treatment for unresectable HCC patients for whom TACE is ineffective or unsuitable.
Most HCC patients referred for RT present with locally advanced disease and usually have underlying liver disease. Therefore, physicians must consider RT-induced toxicities as well as tumor control. Potential side effects of hepatic RT include general toxicity such as malaise and fatigue, gastrointestinal toxicity such as gastroduodenal ulcer and bleeding, and liver toxicity such as RILD. Especially, RILD may be the most serious complication of RT because it is almost always fatal. Previous studies reported a frequency of RILD between 0% and 15.4%.20,26,27 In our study, one patient (4.6%) experienced RILD, and this patient died 2.1 months after completion of RT. To avoid this serious complication, physicians have to pay much more attention to limit the radiation dose to normal liver. In addition to RILD, hepatic RT may induce HBV reactivation.14,28 Because treatment for RILD is supportive management, however, treatment for radiation-induced HBV reactivation requires antiviral therapy, radiation-induced HBV reactivation should be differentially diagnosed from RILD. In our study, three patients (13.6%) experienced radiation-induced HBV reactivation after hepatic RT.
Some studies reported dosimetric advantages in using IMRT as compared to 3D-CRT in hepatic RT. Eccles et al.29 compared IMRT with 3D-CRT for hypofractionated liver RT, and reported better target coverage and lower dose to normal liver and other normal organs. Cheng et al.30 compared the dose-volume data from IMRT and 3D-CRT for HCC patients who had developed RILD after 3D-CRT, they found that using IMRT significantly reduced risk of complications involving normal tissue. We included in our analysis the patients who received only helical IMRT for treatment of HCC, and excluded those who received other RT modalities such as 3D-CRT. Thus, we achieved very favorable treatment response and survival rates. However, our study was not randomized comparative study. To confirm the clinical benefit of IMRT over 3D-CRT in hepatic irradiation, randomized prospective trials with large sample sizes and long-term follow-up periods are required.
There are some limitations in this study. First, this study is retrospective, and therefore, may have inherent bias. Second, the sample size was small. Third, the follow-up period was not sufficiently long. Despite these limitations, however, we believe that our study contributes to resolving some controversial issues in the management of unresectable HCC, and establishing consensus regarding the use of RT for unresectable HCC.
In conclusion, hepatic RT with helical IMRT in patients with unresectable HCC resulted in high treatment response and survival rates (objective response rate, 72.7%; and 1-year overall survival rate, 86.4%) with an acceptable level of toxicity. These findings suggest helical IMRT is a practical treatment option in patients with HCC for whom TACE is unsuitable or ineffective.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan S, Dawson LA, Seong J, et al. Radiotherapy for hepatocellular carcinoma: an overview. Ann Surg Oncol. 2008;15:1015–1024. doi: 10.1245/s10434-007-9729-5. [DOI] [PubMed] [Google Scholar]
- 7.Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 9.Wang MH, Ji Y, Zeng ZC, et al. Impact factors for microinvasion in patients with hepatocellular carcinoma: possible application to the definition of clinical tumor volume. Int J Radiat Oncol Biol Phys. 2010;76:467–476. doi: 10.1016/j.ijrobp.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Feng X, Zhang T, et al. Prospective evaluation of microscopic extension using whole-mount preparation in patients with hepatocellular carcinoma: definition of clinical target volume for radiotherapy. Radiat Oncol. 2010;5:73. doi: 10.1186/1748-717X-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Cheng SH, Lin YM, Chuang VP, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 16.Seong J, Keum KC, Han KH, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393–397. doi: 10.1016/s0360-3016(98)00415-5. [DOI] [PubMed] [Google Scholar]
- 17.Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73:1091–1097. doi: 10.1259/bjr.73.874.11271902. [DOI] [PubMed] [Google Scholar]
- 18.Liu MT, Li SH, Chu TC, et al. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2004;34:532–539. doi: 10.1093/jjco/hyh089. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Kim DY, Park JW, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 20.Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies: mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Kang MK, Kim MS, Kim SK, et al. High-dose radiotherapy with intensity-modulated radiation therapy for advanced hepatocellular carcinoma. Tumori. 2011;97:724–731. doi: 10.1177/030089161109700608. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh A, Hagspiel KD, Al-Osaimi AM, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115:5117–5125. doi: 10.1002/cncr.24552. [DOI] [PubMed] [Google Scholar]
- 23.Liang SX, Zhu XD, Lu HJ, et al. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–2188. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 24.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 25.Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZH, Liu LM, Chen WW, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007;80:194–201. doi: 10.1259/bjr/33521596. [DOI] [PubMed] [Google Scholar]
- 28.Kong M, Hong SE, Kim BH, Choi J. Three cases of radiation-induced hepatitis B virus reactivation after hepatic tomotherapy: case report. J Korean Soc Ther Radiol Oncol. 2011;29:53–62. [Google Scholar]
- 29.Eccles CL, Bissonnette JP, Craig T, Taremi M, Wu X, Dawson LA. Treatment planning study to determine potential benefit of intensity-modulated radiotherapy versus conformal radiotherapy for unresectable hepatic malignancies. Int J Radiat Oncol Biol Phys. 2008;72:582–588. doi: 10.1016/j.ijrobp.2008.06.1496. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JC, Wu JK, Huang CM, et al. Dosimetric analysis and comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy for patients with hepatocellular carcinoma and radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2003;56:229–234. doi: 10.1016/s0360-3016(03)00091-9. [DOI] [PubMed] [Google Scholar]