Abstract
Background/Aims
To investigate the beneficial effect of N-Acetylcysteine (NAC) on pancreatic microvascular perfusion in acute necrotizing pancreatitis (ANP).
Methods
Fifty-four rats were divided into a control group, an ANP group and an NAC-treated group. The ANP model was established by a retrograde injection of 3% sodium taurocholate into the pancreatic duct. The NAC-treated group received an intravenous infusion of NAC just 2 hours before and 30 minutes after the induction of ANP. The pancreatic microvascular perfusion was measured with laser Doppler flowmetry and pancreatic samples were collected for histological examination.
Results
The microvascular perfusion in the NAC-treated group decreased slightly and exhibited a significant increase compared to the ANP group (p<0.01). A pathological examination revealed that edema and inflammatory infiltration decreased, and the hemorrhaging and necrosis of the pancreas were significantly reduced.
Conclusions
NAC could improve pancreatic microvascular perfusion and alleviate the severity of sodium taurocholate-induced ANP, possibly representing a new therapeutic approach to prevent the progression of ANP.
Keywords: Acetylcysteine, Acute necrotizing pancreatitis, Pancreatic microvascular perfusion, Laser-Doppler flowmetry, Taurocholic acid
INTRODUCTION
Acute necrotizing pancreatitis (ANP) is a complex disease associated with significant complications and a high rate of mortality. The major cause of death due to ANP is organ failure.1,2 The pathophysiology of ANP has not yet been entirely elucidated, but is known to involve an alteration in the pancreatic microcirculation. Recent studies have focused on early microcirculatory changes in the ANP process. The disturbance of the microcirculation is a major etiological factor in the development of ANP and plays a key role in the development of multiple organ failure.3-5 Oxidative stress also plays a central role in the pathogenesis of ANP, contributing to the inflammatory response and the formation of a microcirculatory disturbance in early ANP.6,7
N-acetylcysteine (NAC) is a mucolytic with antioxidant properties that is used worldwide for the treatment of various acute and chronic pathologies of the respiratory system. Basic research suggests that NAC could inhibit tumor necrosis factor-α (TNF-α), exerting a powerful anti-inflammatory effect by decreasing the plasma level of interleukin (IL)-6 and blocking nuclear factor-kappaB (NF-κB) activation.8-12
There are a few clinical reports regarding the use of NAC to treat ANP. Virlos et al.13 recently completed a case-control analysis, showing no apparent benefits from the multiple antioxidant combination of selenium, NAC and ascorbic acid in severe acute pancreatitis. However, Narasimhan et al.14 reported that NAC administration was successful in resolving peripancreatic collections in a patient with severe pancreatitis. Therefore, we wish to evaluate the beneficial effect of the strong antioxidant NAC in the enhancement of pancreatic microvascular perfusion and its protective effect in a rat model of sodium taurocholate-induced ANP to provide a scientific basis for broad clinical applications.
MATERIALS AND METHODS
1. Chemicals and animals
The NAC and sodium taurocholate were purchased from Sigma Co. (St. Louis, MO, USA). The serum amylase (AMS) test kit and myeloperoxidase (MPO) test kit were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Adult male Sprague-Dawley (SD) rats weighing 250 to 350 g were provided by the Experimental Animal Center of the Huaxi Medical School of Sichuan University. The animals were bred and housed in standard cages and maintained in climate-controlled rooms with normal rat chow and water ad libitum. All of the studies were performed with the approval of the experimental animal committee at our university.
2. Experimental design
Fifty-four SD rats were randomly divided into three groups: a control group (n=6), an ANP group (n=24), and an NAC-treated group (n=24). The rat ANP model was established by a retrograde injection of 3% sodium taurocholate (0.1 mg/100 g) into the pancreatic duct with a micropump15 after laparotomy. The rats in the NAC-treated group received an intravenous infusion of NAC at 100 mg/kg 2 hours before and 30 minutes after the induction of ANP. The other groups were given the same amount of saline in place of the NAC. The ANP and NAC-treated groups were redivided into 3, 6, 12, and 24 hours time points, respectively. The pancreatic microvascular perfusion was measured and blood samples were collected at every time point.
3. Real-time pancreatic microvascular perfusion
The pancreatic microvascular perfusion was monitored with a laser Doppler flowmeter (Periflux System 5000; Perimed, Stockholm, Sweden). The Doppler probe (model P457; Perimed) was fixed with a special articulated holder, which connected with a fiber cable that was countersunk into the platform on the surface of pancreatic head. The laser light from the probe could be directed into the tissue. After 5 to 10 minute stabilization period, the pancreatic microvascular perfusion was measured at each time point and recorded in the form of wave on a microcomputer.
4. Histological examination and scoring
The rats were sacrificed with heart-blood letting at the indicated time point after laparotomy. The pancreatic samples were rapidly collected, fixed in formalin, embedded in paraffin, and cut into 5-µm thick sections. After staining with hematoxylin and eosin, 10 fields in each section were examined at high power (×200) by experienced morphologists who were blind to the experimental protocol. The sections were then scored after the criteria of Grewal.16 The interstitial edema, hemorrhage, hyperemia, necrosis, leukocyte infiltration, and adherence and basophil lamellation of the acinar cell cytoplasm were graded in 10 consecutive high power fields on a scale of 0 to 4. The score for each graded parameter was averaged, and the total pancreatic damage was calculated by adding all of the averages together.
5. AMS assays
The collected blood was centrifuged at 3,000 g for 15 minutes to obtain the serum and stored at -20℃ for the AMS assay. The AMS activity was determined by the method of iodine colorimetry using a UV spectrophotometer (Eppendorf DU730; Eppendorf, Hamburg, Germany). The absorbance is read against distilled water at a wavelength of 660 nm, One unit of AMS is defined as the amount of enzyme that will catalyze the hydrolysis of 10 mg starch in 100 mL serum (plasma) in 30 minutes at 37℃. The test process followed the kit instructions strictly.
6. Pancreatic MPO assays
The pancreatic samples were weighed to within 1 g, dissected and perfused with a phosphate buffered saline solution, pH 7.4, containing 0.16 mg/mL heparin to remove any red blood cells and clots. Five percent tissue homogenate samples were then prepared and stored at -70℃. The MPO levels were tested with the enzyme histochemical method. One hundred microliters of reagent were added to 0.9 mL of the tissue homogenate, which was then vortexed and incubated at 37℃ for 15 minutes. Two hundred microliters of the mixture were removed for the assay. The reagent was added to the mixture in accordance with the kit, vortexed and incubated at 60℃ for 10 minutes. The absorbance of each tube at 460 nm was recorded using a UV spectrophotometer. The MPO activity was calculated using the following equation: MPO (U/g)=(ODassay-ODcontrol)/11.3×weight of the tissue used (grams). One unit is defined as the amount of enzyme in 1.0 g of tissue that will catalyze 1.0 µmoL of H2O2 for 30 minutes at 37℃.
7. Statistical analysis
The results were expressed as the mean±SD. The between-group comparisons were made with a one-way analysis of variance (ANOVA). A p<0.05 was considered statistically significant.
RESULTS
1. Pancreatic microvascular perfusion
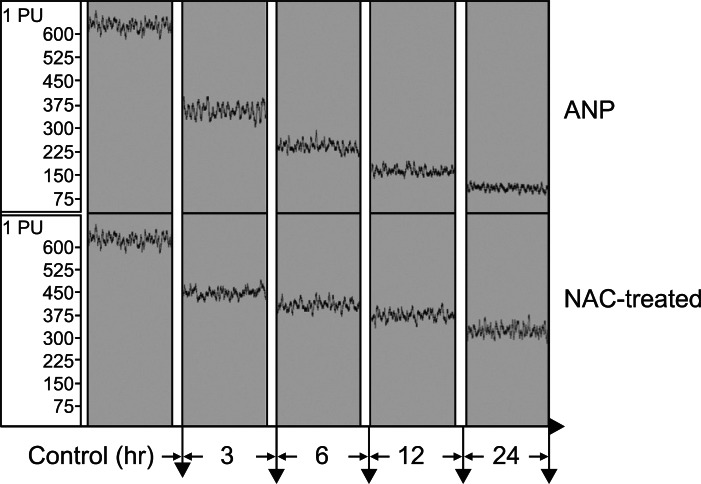
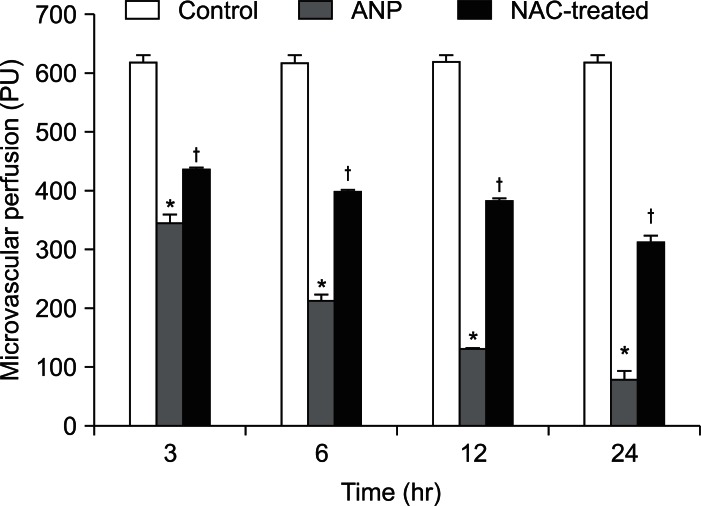
The pancreatic microvascular perfusion in the ANP group decreased sharply compared to the control group (p<0.01), falling from 619.94±9.03 to 82.24±11.83 perfusion within 24 hours. The microvascular perfusion of the NAC-treated group decreased slightly from 438.44±3.91 to 313.59±9.12, reaching more than 50% of the value for the control group and exhibiting a significant increase over the ANP group from 3 to 24 hours (p<0.01) (Figs. 1 and 2).
Fig. 1.
Record of pancreatic microvascular perfusion in different groups. The pancreatic microvascular perfusion in the acute necrotizing pancreatitis (ANP) group decreased sharply compared to the control group (p<0.01), falling from 619.94±9.03 perfusion (PU) to 82.24±11.83 PU within 24 hours. The microvascular perfusion of the N-acetylcysteine (NAC)-treated group decreased slightly from 438.44±3.91 to 313.59±9.12, reaching over 50% of the value for the control group and exhibiting a significant increase over the ANP group from 3 to 24 hours (p<0.01).
Fig. 2.
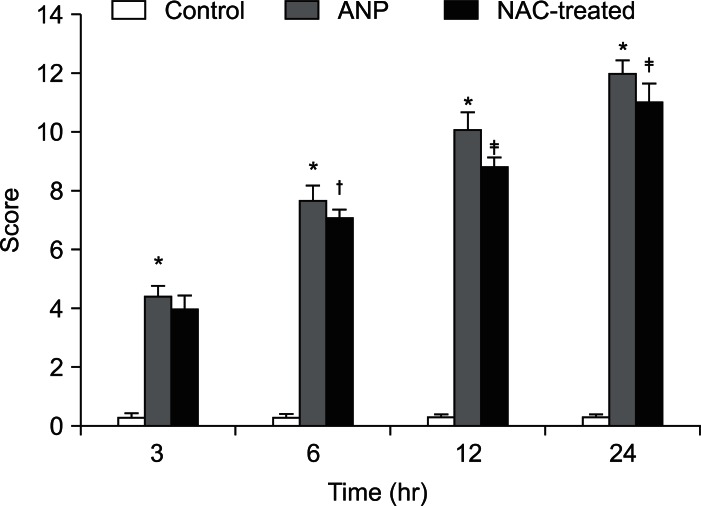
Pancreatic microvascular perfusion in differenct groups.
*p<0.01, the acute necrotizing pancreatitis (ANP) group vs the control group; †p<0.01, the N-acetylcysteine (NAC)-treated group vs the ANP group.
2. Serum AMS level
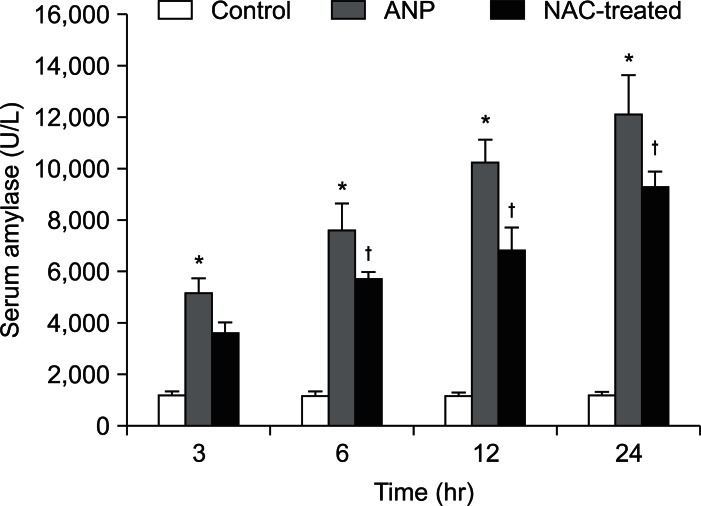
The serum AMS level increased significantly in both the ANP and NAC-treated groups compared to the control group (p<0.01). The NAC treatment reduced the AMS level at 6 hours (p<0.01) compared to the ANP group. The AMS level increased further at consecutive time points in the ANP group, but the NAC treatment inhibited this increase in the AMS level (Fig. 3).
Fig. 3.
Serum amylase levels in different groups.
*p<0.01, the acute necrotizing pancreatitis (ANP) group vs the control group; †p<0.01, the N-acetylcysteine (NAC)-treated group vs the ANP group.
3. MPO level in the pancreas
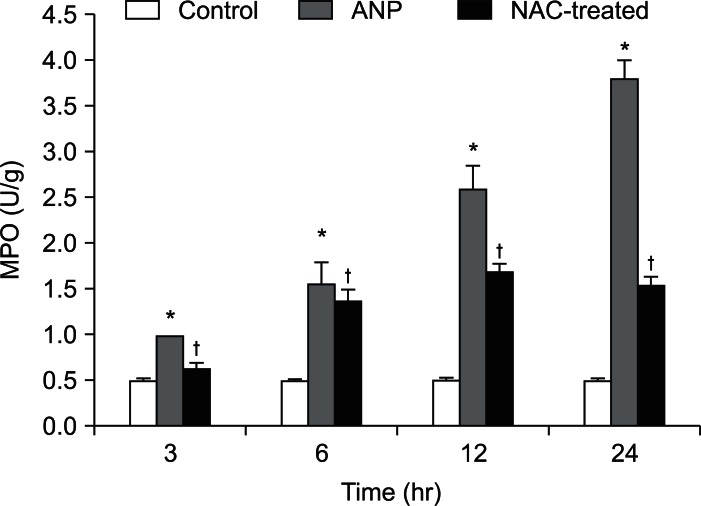
The MPO level in the pancreas was higher in the ANP group than the control group at 3 hours (p<0.01) and reached a maximum at 24 hours. The MPO level, which reached a peak value at 12 hours and then decreased after NAC treatment, was significantly lower (p<0.01) than that of the ANP group (Fig. 4).
Fig. 4.
MPO levels in the pancreas of different groups.
*p<0.01, the acute necrotizing pancreatitis (ANP) group vs the control group; †p<0.01, the N-acetylcysteine (NAC)-treated group vs the ANP group.
4. Pathological scores and histopathology
Extensive edema, inflammatory infiltration, hemorrhaging, and necrosis were observed in the pancreatic tissue of the ANP group from 3 to 24 hours. These changes were similar in the NAC-treated group, but slightly and less hemorrhaging and necrosis were observed. The pathological score declined significantly at 6 hours (p<0.05) and further decreased at 24 hours (p<0.01) after NAC treatment (Figs. 5 and 6).
Fig. 5.
Pathological scores in different groups.
*p<0.01, the acute necrotizing pancreatitis (ANP) group vs the control group; †p<0.05, the N-acetylcysteine (NAC)-treated group vs the ANP group; ‡p<0.01, the NAC-treated group vs the ANP group.
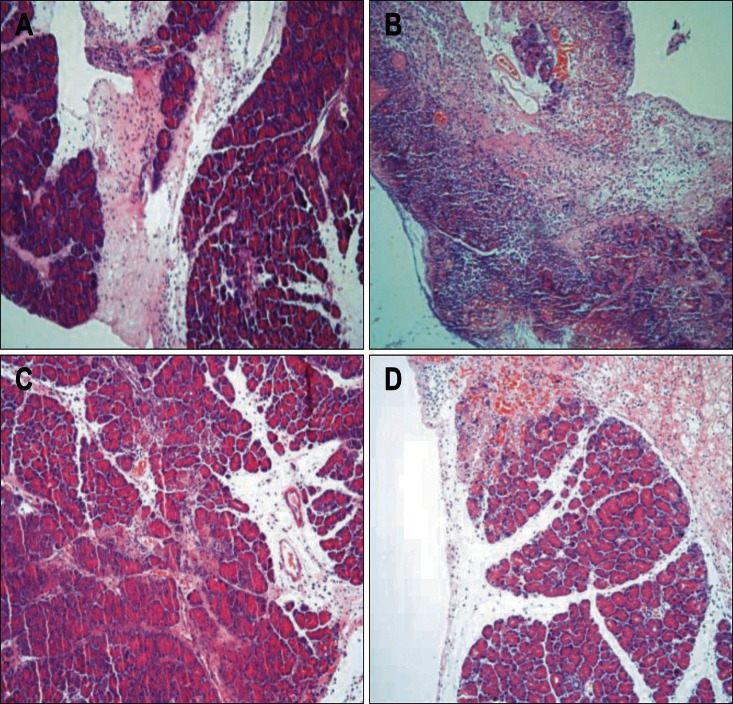
Fig. 6.
Light microscopic photograph of the histopathologic changes in different groups (×100). (A) Extensive edema, inflammatory infiltration, hemorrhaging and necrosis were observed at 6 hours after the induction of acute necrotizing pancreatitis (ANP). (B) Only a small amount of residual pancreatic tissue was observed at 24 hours after the induction of ANP, rather than large areas of hemorrhaging and necrosis. (C) Edema and inflammatory infiltration decreased significantly, and hemorrhaging and necrosis were significantly reduced at 6 hours after N-acetylcysteine (NAC) treatment. (D) Hemorrhaging were observed only slightly, and more residual pancreatic tissue at 24 hours after NAC treatment was noted.
DISCUSSION
Acute pancreatitis is a complicated disorder with several causative factors. We chose the severe process of ANP as a model and focused on microcirculation as a determining factor in the pathogenesis of acute pancreatitis.17-19 Many mediators play important roles in causing microcirculatory disturbances. TNF-α, NF-κB, and oxygen free radicals constitute the upstream signal of the inflammatory network and were shown to be the key trigger of inflammatory cytokines in ANP. Other factors, including nitric oxide, endothelin (ET), and intercellular adhesion molecule-1 (ICAM-1), are part of the downstream network.20 NAC is a free radical scavenger capable of stimulating glutathione synthesis, and many recent studies have confirmed that it could inhibit inflammatory factors such as IL-6, TNF, and NF-κB, in addition to blocking ICAM-1 related pathways.21 Therefore, NAC is thought to mediate anti-inflammatory, antioncogenic, and antioxidant activities to improve the microcirculatory disturbance.
In our study, we used laser Doppler flowmetry to measure real-time pancreatic microvascular perfusion, which could reflect pancreatic microcirculatory impairment in acute pancreatitis. In vivo microscopy with fluorescein isothiocyanate-labeled erythrocytes may be another way to measure pancreatic microcirculation, but the process is complicated and cannot provide measurements in real time. Laser Doppler flowmetry is ideal for monitoring changes in tissue perfusion over time and has successfully been applied to numerous organs in experimental and human studies.22-24 We circumnavigated some factors such as respiration and small changes in probe angle by using an artificial probe holder that allows the tissue to be maintained by gravity.
To our best of knowledge, this is the first experimental study showing the beneficial effect of NAC on pancreatic microvascular perfusion. The results showed an increase in microvascular perfusion over 24 hours in comparison to the ANP group. MPO is a major component of myeloid precursor cells, and the enzymatic activity of MPO is used to classify acute leukemias. In our study, the MPO level decreased significantly after NAC treatment. It reflected the anti-inflammatory and antioxidant effects of NAC in ANP. Furthermore, the serum AMS level was reduced and the histopathological scores were improved after NAC treatment. A pathological examination showed that the edema and inflammatory infiltrate decreased, and the hemorrhaging and pancreatic necrosis were significantly reduced. These results emphasize the severity of ANP and confirm the results of previous studies.25,26 However, we observed that the pancreatic microvascular perfusion in our study decreased significantly compared to the control group. The serum AMS and MPO levels, pathological scores and histopathology revealed similar results, which may because NAC could not completely inhibit coagulatory factors such as ET and platelet-activating factor. An ANP combination therapy may be required.
On the other hand, Leme et al.27 found that the severity of pancreatic necrosis and the increase in polymorphonuclear cells in alveolar septa induced by sodium taurocholate-induced pancreatitis were not reduced by the prior administration of NAC. This result may be related to the dose and NAC administration method. In our study, NAC (100 mg/kg) was administered twice: 2 hours before and 30 minutes after the induction of pancreatitis. In contrast, the Leme et al.27 study administered NAC (200 mg/kg) once 30 minutes before the induction of pancreatitis. Therefore, further research on the dose and NAC administration method are needed to confirm our results.
In conclusion, the current study has shown that NAC could improve pancreatic microvascular perfusion and alleviate the severity of sodium taurocholate-induced ANP in rats. While it could not completely inhibit ANP, the effective dose and administration method are a source of controversy. NAC may represent a new therapeutic approach to prevent the progression of ANP.
ACKNOWLEDGEMENTS
The authors thank the Laboratory of Functional Sciences of Preclinical and Forensic Medicine of Sichuan University for excellent technical assistance.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Slavin J, Ghaneh P, Sutton R, et al. Management of necrotizing pancreatitis. World J Gastroenterol. 2001;7:476–481. doi: 10.3748/wjg.v7.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis: an analysis of 279 cases. Gut. 1995;37:121–126. doi: 10.1136/gut.37.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider L, Pietschmann M, Hartwig W, et al. Inosine reduces microcirculatory disturbance and inflammatory organ damage in experimental acute pancreatitis in rats. Am J Surg. 2006;191:510–514. doi: 10.1016/j.amjsurg.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Peng XG, Liu CC, Liu H, Lu Y. Low-dose dopamine reduces inflammatory factors of acute pancreatitis in rats. Hepatobiliary Pancreat Dis Int. 2007;6:646–649. [PubMed] [Google Scholar]
- 5.Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933–936. doi: 10.3748/wjg.v8.i5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, Seo JY, Roh KH, Lim JW, Kim KH. Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med. 2000;29:674–683. doi: 10.1016/s0891-5849(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 7.Sunamura M, Yamauchi J, Shibuya K, et al. Pancreatic microcirculation in acute pancreatitis. J Hepatobiliary Pancreat Surg. 1998;5:62–68. doi: 10.1007/pl00009952. [DOI] [PubMed] [Google Scholar]
- 8.Andersson E, Axelsson J, Pedersen LC, Elm T, Andersson R. Treatment with anti-factor VIIa in acute pancreatitis in rats: blocking both coagulation and inflammation? Scand J Gastroenterol. 2007;42:765–770. doi: 10.1080/00365520701295632. [DOI] [PubMed] [Google Scholar]
- 9.Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration. 1999;66:495–500. doi: 10.1159/000029447. [DOI] [PubMed] [Google Scholar]
- 10.Ramudo L, Manso MA, Sevillano S, de Dios I. Kinetic study of TNF-alpha production and its regulatory mechanisms in acinar cells during acute pancreatitis induced by bile-pancreatic duct obstruction. J Pathol. 2005;206:9–16. doi: 10.1002/path.1747. [DOI] [PubMed] [Google Scholar]
- 11.Shi C, Zhao X, Wang X, Andersson R. Role of nuclear factor-kappaB, reactive oxygen species and cellular signaling in the early phase of acute pancreatitis. Scand J Gastroenterol. 2005;40:103–108. doi: 10.1080/00365520410009555. [DOI] [PubMed] [Google Scholar]
- 12.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275(6 Pt 1):G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 13.Virlos IT, Mason J, Schofield D, McCloy RF, Eddleston JM, Siriwardena AK. Intravenous n-acetylcysteine, ascorbic acid and selenium-based anti-oxidant therapy in severe acute pancreatitis. Scand J Gastroenterol. 2003;38:1262–1267. doi: 10.1080/00365520310006540. [DOI] [PubMed] [Google Scholar]
- 14.Narasimhan S, Khwaja HA, Dutta S, Mitchenere P. Use of N-acetylcysteine for postnecrosectomy peripancreatic collections in a patient with severe, acute pancreatitis. Can J Surg. 2008;51:E133–E134. [PMC free article] [PubMed] [Google Scholar]
- 15.Wittel UA, Wiech T, Chakraborty S, et al. Taurocholate-induced pancreatitis: a model of severe necrotizing pancreatitis in mice. Pancreas. 2008;36:e9–e21. doi: 10.1097/MPA.0b013e3181575103. [DOI] [PubMed] [Google Scholar]
- 16.Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214–218. doi: 10.1016/0002-9610(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 17.Xiping Z, Yan P, Xinmei H, et al. Effects of dexamethasone and Salvia miltiorrhizae on the small intestine and immune organs of rats with severe acute pancreatitis. Inflammation. 2010;33:259–266. doi: 10.1007/s10753-010-9180-9. [DOI] [PubMed] [Google Scholar]
- 18.Schneider L, Hackert T, Heck M, et al. Capsaicin reduces tissue damage in experimental acute pancreatitis. Pancreas. 2009;38:676–680. doi: 10.1097/MPA.0b013e3181a5ef3a. [DOI] [PubMed] [Google Scholar]
- 19.Panek J, Zasada J, Poźniczek M. Microcirculatory disturbance in the course of acute pancreatitis. Przegl Lek. 2007;64:435–437. [PubMed] [Google Scholar]
- 20.Zhang XP, Li ZJ, Zhang J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:351–357. [PubMed] [Google Scholar]
- 21.Ramudo L, De Dios I, Yubero S, Vicente S, Manso MA. ICAM-1 and CD11b/CD18 expression during acute pancreatitis induced by bile-pancreatic duct obstruction: effect of N-acetylcysteine. Exp Biol Med (Maywood) 2007;232:737–743. [PubMed] [Google Scholar]
- 22.Konturek PC, Dembinski A, Warzecha Z, et al. Pioglitazone, a specific ligand of peroxisome proliferator-activated receptor-gamma, protects pancreas against acute cerulein-induced pancreatitis. World J Gastroenterol. 2005;11:6322–6329. doi: 10.3748/wjg.v11.i40.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips AR, Farrant GJ, Abu-Zidan FM, Cooper GJ, Windsor JA. A method using laser Doppler flowmetry to study intestinal and pancreatic perfusion during an acute intestinal ischaemic injury in rats with pancreatitis. Eur Surg Res. 2001;33:361–369. doi: 10.1159/000049731. [DOI] [PubMed] [Google Scholar]
- 24.Briskin BS, Bukatko VN. Laser doppler flowmetry for assessment of acute pancreatitis treatment efficacy. Khirurgiia (Mosk) 2003:20–25. [PubMed] [Google Scholar]
- 25.Mumcu S, Alhan E, Türkyilmaz S, Kural BV, Erçin C, Kalyoncu NI. Effects of N-acetylcysteine on acute necrotizing pancreatitis in rats. Eur Surg Res. 2005;37:173–178. doi: 10.1159/000085965. [DOI] [PubMed] [Google Scholar]
- 26.Yagci G, Gul H, Simsek A, et al. Beneficial effects of N-acetylcysteine on sodium taurocholate-induced pancreatitis in rats. J Gastroenterol. 2004;39:268–276. doi: 10.1007/s00535-003-1287-4. [DOI] [PubMed] [Google Scholar]
- 27.Leme AS, Lichtenstein A, Arantes-Costa FM, Landucci EC, Martins MA. Acute lung injury in experimental pancreatitis in rats: pulmonary protective effects of crotapotin and N-acetylcysteine. Shock. 2002;18:428–433. doi: 10.1097/00024382-200211000-00007. [DOI] [PubMed] [Google Scholar]