Abstract
Common bile duct (CBD) cancer is a relatively rare malignancy that arises from the biliary epithelium and is associated with a poor prognosis. Here, we report a case of advanced metastatic CBD cancer successfully treated by chemotherapy with gemcitabine combined with S-1 (tegafur+gimeracil+oteracil). A 65-year-old male presented with pyogenic liver abscess. After antibiotic therapy and percutaneous drainage, follow-up computed tomography (CT) showed an enhanced nodule in the CBD. Biopsy was performed at the CBD via endoscopic retrograde cholangiopancreatography, which showed adenocarcinoma. Additional CT and magnetic resonance imaging showed multiple small nodules in the right hepatic lobe, which were confirmed as metastatic adenocarcinoma by sono-guided liver biopsy. The patient underwent combination chemotherapy with gemcitabine and S-1. After nine courses of chemotherapy, the hepatic lesion disappeared radiologically. Pylorus-preserving pancreaticoduodenectomy was performed, and no residual tumor was found in the resected specimen. Three weeks after the operation, the patient was discharged with no complications. Through 3 months of follow-up, no sign of recurrence was observed on CT scan. Gemcitabine combined with S-1 may be a highly effective treatment for advanced cholangiocarcinoma.
Keywords: Cholangiocarcinoma, Gemcitabine, S-1
INTRODUCTION
Common bile duct (CBD) cancer is a relatively rare but increasing malignancy worldwide that arises from biliary epithelium, known as a cancer with chemoresistance and poor prognosis. Complete resection is the most effective and the only potentially curative treatment.1 However, at the time of diagnosis most patients are presented with advanced diseases.2 Even the patients who had undergone curative resection show high recurrence rates. Thus systemic chemotherapy is the mainstay of the treatment but CBD cancer is highly resistant to most chemotherapeutic agents.3 Currently, there is no standard chemotherapeutic regimen established for CBD cancer. Gemcitabine appears to be the most effective single agent.4 Target therapy to avoid multidrug resistance is now under investigation. Here, we report a case of unresectable CBD cancer with liver metastasis, which completely responded to combination chemotherapy of gemcitabine and S-1. To our knowledge, this is the first case reporting a patient with advanced CBD cancer which showed pathologic complete remission after chemotherapy.
CASE REPORT
A 65-year-old male was admitted for abdominal pain and fever, which started 2 weeks ago. He was previously healthy without any specific past medical history. Abdominal physical examination revealed tenderness without rebound tenderness on epigastrium. Laboratory finding demonstrated white blood cells 12.3×103/µL, hemoglobin 11.6 g/dL, platelets 309×103/µL, total bilirubin 0.7 mg/dL, alkaline phosphatase 117 IU/L, aspartate aminotransferase 26 IU/L, alanine aminotransferase 33 IU/L, high sensitivity C-reactive protein 22.1 mg/dL, carcinoembryonic antigen 2.0 ng/mL, carbohydrate antigen 19-9 7.6 U/mL, and alphafetoprotein 1.3 ng/mL. Hepatitis B surface antigen was negative and hepatitis C virus antibody was positive. Abdominal computed tomography (CT) showed about 9 cm lobulating low attenuating mass with peripheral rim enhancement in the left lobe of the liver, which was thought to be mature pyogenic liver abscess (Fig. 1). He started antibiotics therapy with ceftriaxone and metronidazole, and underwent percutaneous drainage tube insertion using 10.2 Fr pigtail catheter into the liver abscess. Two days later fever subsided and a week later he was discharged with oral antibiotics. One month later, follow-up liver CT scan showed 1.5 cm enhancing lesion in distal CBD with progression of biliary dilatation, suggesting distal CBD cancer (Fig. 2). Thus biopsy was performed at CBD via endoscopic retrograde cholangiopancreatography (Fig. 3), which revealed adenocarcinoma (Fig. 4). Additional CT scan (Fig. 5) and magnetic resonance imaging (MRI) (Fig. 6) showed multiple small nodules in right hepatic lobe and metastatic adenocarcinoma was confirmed from frozen slide of sono-guided liver biopsy (Fig. 7). Since the patient was inoperable, he underwent combination chemotherapy with gemcitabine 1,600 mg intravenous infusion on day 1 and S-1 (tegafur+gimeracil+oteracil) 500 mg twice a day for 14 days with 1-week rest as one course. Serial CT scans checked every three cycles showed gradual regression of the metastatic nodules in liver (Fig. 8). After nine courses of chemotherapy the hepatic lesion completely disappeared on MRI (Fig. 9). Three weeks later, pylorus preserving pancreaticoduodenectomy was performed. Grossly the resected CBD showed a 1×1 cm sized gray solid mass infiltrating into adjacent soft tissue. Microscopically there were no residual tumor but chronic active cholangitis with multifocal erosion and subepithelial fibrosis in the resected CBD (Fig. 10). Then, 22 days after the operation he was discharged without any complication. For 3 months of follow-up, he did not develop any signs of recurrence and no evidence of recurrence was found in the 3-month postoperative CT scan (Fig. 11).
Fig. 1.
Initial abdomen computed tomography shows about 9 cm lobulating low attenuating mass with peripheral rim enhancement with enhancing septum like structures in the left lobe of the liver.
Fig. 2.
Follow-up computed tomography scan 1 month after the treatment for pyogenic abscess shows 1.5 cm enhancing portion in the intrapancreatic common bile duct (CBD) with progression of biliary dilatation suggesting distal CBD cancer (arrow).
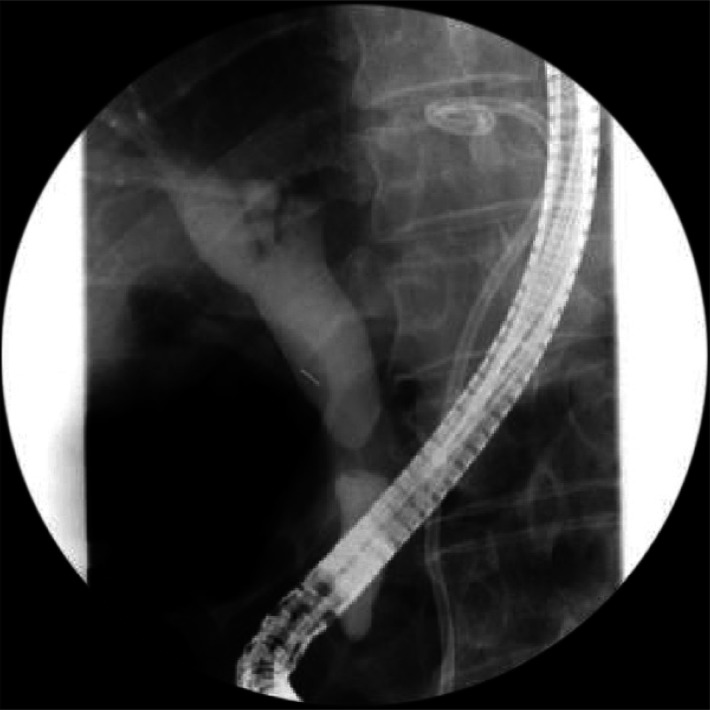
Fig. 3.
Endoscopic retrograde cholangiopancreatography shows concentric narrowing at the mid-common bile duct (CBD) with tapering of both proximal and distal CBD.
Fig. 4.
Hematoxyline-eosin staining of CBD biopsy slide shows a few atypical cells in lymphocytic background, suspicious for adenocarcinoma (×200).
Fig. 5.
Abdominal computed tomography scan for preoperative evaluation shows small nodular lesion with peripheral enhancement in S7, suggesting liver metastasis (arrow).
Fig. 6.
T1 magnetic resonance imaging for preoperative evaluation shows multifocal low signal intensity nodular lesions in the right lobe (arrows).
Fig. 7.
Frozen slide of liver biopsy shows poorly differentiated adenocarcinoma (H&E stain, ×200).
Fig. 8.
Serial follow-up computed tomography scan shows regression of hepatic lesion in S7 segment.
Fig. 9.
Liver magnetic resonance imaging shows complete regression of multifocal hepatic nodules after nine cycles of chemotherapy.
Fig. 10.
Hematoxyline-eosin staining of resected common bile duct specimen shows no residual tumor cells but lymphoid aggregation with subepithelial fibrosis suggesting chronic active cholangitis (×200).
Fig. 11.
Three-month postoperative computed tomography scan shows no evidence of tumor recurrence.
DISCUSSION
Although radical surgery is the most effective therapy for cure in patients with cholangiocarcioma, only 20% of patients are diagnosed with resectable diseases.5 Patients with unresectable cholangiocarcinoma have dismal prognosis with median survival of 6 to 12 months6 and 5-year survival rate of less than 5%.7 For these patients palliative therapy is important in terms of quality of life as well as the survival rate. The role of chemotherapy is not yet established. However chemotherapy has been reported to be more beneficial than the best supportive care.8 Gemcitabine has shown to be an effective therapy for cholangiocarcinoma in phase II trials.9,10 Fluorouracil also has shown activity in combination with gemcitabine.11 S-1 is a novel oral fluoropyrimidine agent containing tegafur, gimeracil and oteracil potassium. Gimeracil is a competitive inhibitor of dihydropyrimidine dehyrogenase, which achieves higher concentrations of 5-fluorouracil in plasma and tumor tissues.12 Yoshizawa et al.13 tested the combination of S-1 with other anticancer drugs (gemcitabine, cisplatin, irinotecan, mitomycin C, adriamycin, and paclitaxel) and reported that synergistic effect was most evident in gemcitabine/S-1 combination. A recent review reported phase II trials supporting the following combinations: gemcitabine/cisplatin, gemcitabine/oxaliplatin, gemcitabine/capecitabine, and 5-fluorouracil in unresectable or metastatic cholangiocarcinoma.14 Also there have been phase II trials of gemcitabine and S-1 combination chemotherapy showing a promising survival benefit with acceptable toxicity in patients with advanced biliary tract cancer.15,16
In our case, we used gemcitabine combined with S-1. Gemcitabine was chosen for its extensively evaluated data supporting effectiveness in advanced CBD cancer. S-1 was selected as an alternative to 5-fluorouracil for its convenience of administration and less toxicity.
So far, several cases have been reported, in which advanced cholangiocarcinoma was completely treated with gemcitabine chemotherapy in Japan,17-20 although only one of them has shown complete remission histopathologically.14 In our case, the metastatic lesion disappeared radiologically and primary tumor was confirmed to have disappeared histopathologically after the chemotherapy. Thus there are chances to have radiologically invisible remnant malignant cells in hepatic tissue and potential risk of recurrence. However, the 3-month postoperative follow-up CT scan showed no evidence of recurrence. It is remarkable that the combination chemotherapy intended for palliation played a role of curative treatment.
In conclusion, we experienced a case of advanced CBD cancer with liver metastasis completely responded to combination chemotherapy with gemcitabine and S-1. Adenocarcinoma was proven both in primary tumor and metastasis. After 12 cycles of chemotherapy, surgical resection for primary tumor was performed because the metastatic lesion had completely disappeared, from which no remnant cancer cells were found. We suggest that this combination chemotherapy would be an effective treatment in advanced cholangiocarcinoma. More clinical trials using gemcitabine and S-1 combination chemotherapy would be mandatory.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 4.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 5.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo S, Takada T, Miyazaki M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41–54. doi: 10.1007/s00534-007-1279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farley DR, Weaver AL, Nagorney DM. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 9.Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol. 2001;12:183–186. doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- 10.Tsavaris N, Kosmas C, Gouveris P, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193–198. doi: 10.1023/B:DRUG.0000011797.09549.53. [DOI] [PubMed] [Google Scholar]
- 11.Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol. 2001;19:4089–4091. doi: 10.1200/JCO.2001.19.20.4089. [DOI] [PubMed] [Google Scholar]
- 12.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer. 2004;91:1769–1774. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizawa J, Takizawa A, Takeuchi O, et al. Experimental study of combination therapy with S-1 against pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:1211–1219. doi: 10.1007/s00280-009-0990-0. [DOI] [PubMed] [Google Scholar]
- 14.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Isayama H, Nakai Y, et al. Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2010;65:1101–1107. doi: 10.1007/s00280-009-1115-5. [DOI] [PubMed] [Google Scholar]
- 16.Kanai M, Yoshimura K, Tsumura T, et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2011;67:1429–1434. doi: 10.1007/s00280-010-1443-5. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida R, Matsuda T, Watanabe T, Iwadou H, Hunabiki K, Kamikawa Y. A case of gallbladder cancer which completely responded to gemcitabine. Gan To Kagaku Ryoho. 2010;37:1771–1773. [PubMed] [Google Scholar]
- 18.Nitta S, Nagayama K, Sakai Y, Kusano F, Tazawa J. Biliary tract carcinoma in elderly patients in whom gemcitabine chemotherapy induced complete remission: a report of two cases. Gan To Kagaku Ryoho. 2007;34:941–943. [PubMed] [Google Scholar]
- 19.Matsuo R, Kondo T, Ohshiro Y, et al. A case of stage IVA intrahepatic biliary tract cancer successfully treated with gemcitabine. Gan To Kagaku Ryoho. 2006;33:1501–1504. [PubMed] [Google Scholar]
- 20.Nakayama A, Takasu K, Ogiwara H, et al. An effective case of gemcitabine therapy with post-operative peritoneal recurrence of intrahepatic cholangiocarcinoma. Gan To Kagaku Ryoho. 2010;37:1595–1598. [PubMed] [Google Scholar]