Abstract
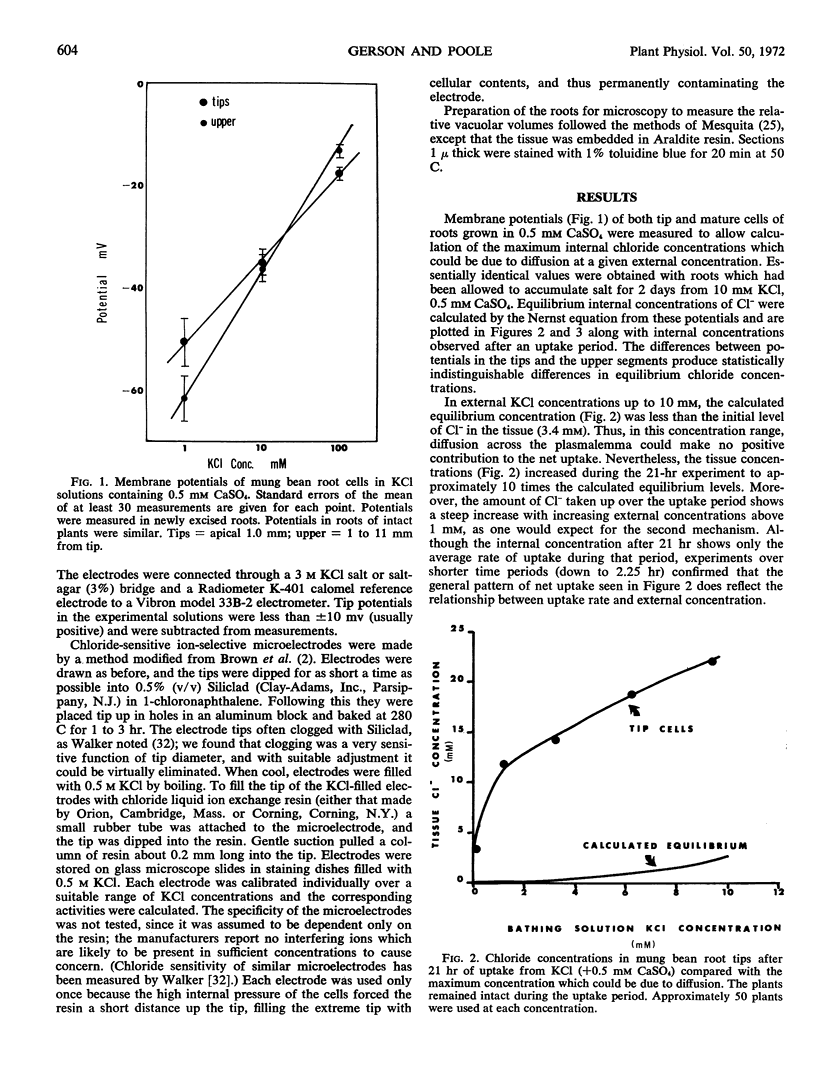
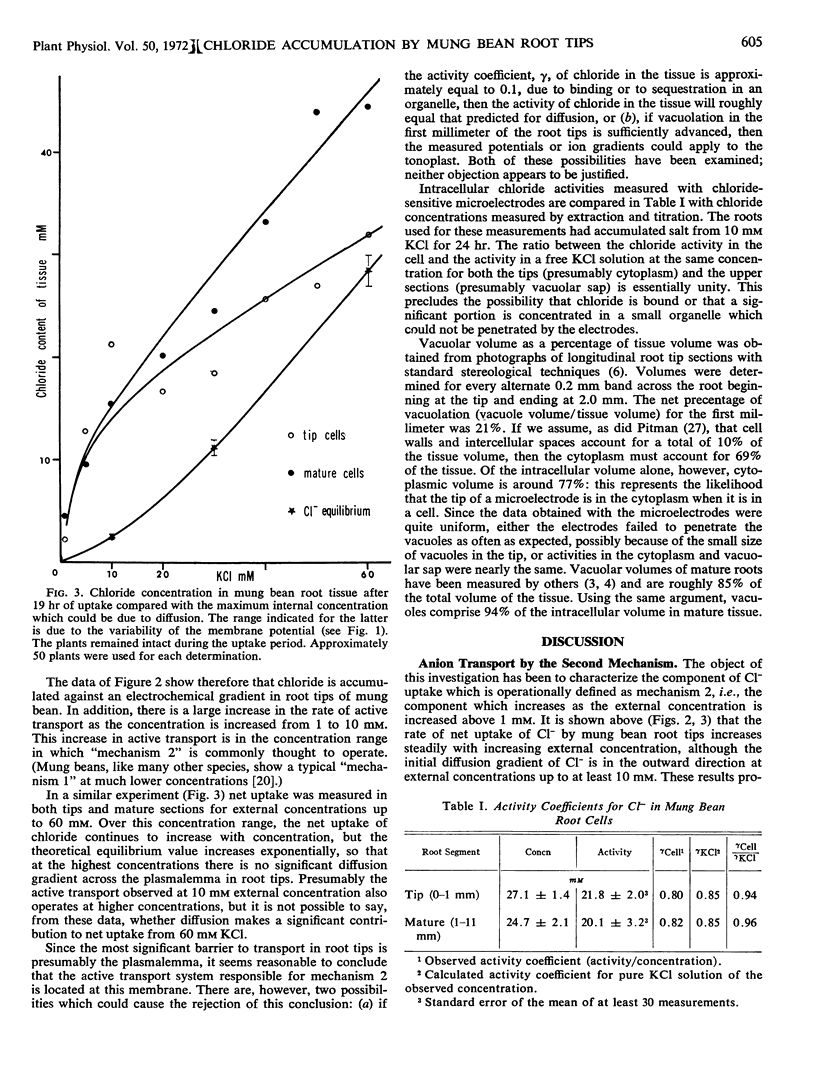
Net uptake of Cl− into root tips of mung bean (Phaseolus aureus) increases steadily with increasing external concentrations from 1 to 60 mm. Membrane potentials were measured to determine the equilibrium concentration of Cl− in the tissue which could be due to diffusion. This concentration was readily exceeded in both the relatively nonvacuolate tips (0 to 1 mm) and the vacuolate, mature upper sectons (1 to 11 mm) of the roots. The activity coefficient of both cytoplasmic and vacuolar Cl−, measured with Cl− sensitive microelectrodes, was approximately the same as that of a pure KCl solution of the same concentration. It is concluded that the “second mechanism” of ion uptake involves a large increase in the rate of active transport at the plasmalemma as the external concentration is increased above 1 mm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 11;150(4):618–625. doi: 10.1016/0005-2736(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Sutton R. B., Walker J. L., Jr Increased chloride conductance as the proximate cause of hydrogen ion concentration effects in Aplysia neurons. J Gen Physiol. 1970 Nov;56(5):559–582. doi: 10.1085/jgp.56.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYES F. DETERMINATION OF RELATIVE VOLUME BY SECTIONAL ANALYSIS. Lab Invest. 1965 Jun;14:987–995. [PubMed] [Google Scholar]
- Cram W. J. Compartmentation and exchange of chloride in carrot root tissue. Biochim Biophys Acta. 1968 Nov 5;163(3):339–353. doi: 10.1016/0005-2736(68)90119-3. [DOI] [PubMed] [Google Scholar]
- Cram W. J. Short term influx as a measure of influx across the plasmalemma. Plant Physiol. 1969 Jul;44(7):1013–1015. doi: 10.1104/pp.44.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzam O. E., Rains D. W., Epstein E. Ion transport kinetics in plant tissue: complexity of the chloride absorption isotherm. Biochem Biophys Res Commun. 1964 Mar 26;15(3):273–276. doi: 10.1016/0006-291x(64)90159-7. [DOI] [PubMed] [Google Scholar]
- Epstein E., Rains D. W. CARRIER-MEDIATED CATION TRANSPORT IN BARLEY ROOTS: KINETIC EVIDENCE FOR A SPECTRUM OF ACTIVE SITES. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1320–1324. doi: 10.1073/pnas.53.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Steady State Sodium and Rubidium Effluxes in Pisum sativum Roots. Plant Physiol. 1967 May;42(5):685–690. doi: 10.1104/pp.42.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G., Macdonald I. R., Dainty J. Influence of the Counter-ion on the Absorption Isotherm for Chloride at Low Temperature. Plant Physiol. 1964 Mar;39(2):254–262. doi: 10.1104/pp.39.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J. F. Electron microscope study of the origin and development of the vacuoles in root-tip cells of Lupinus albus L. J Ultrastruct Res. 1969 Feb;26(3):242–250. doi: 10.1016/s0022-5320(69)80004-3. [DOI] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiol. 1967 Mar;42(3):319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Sodium absorption by barley roots: role of the dual mechanisms of alkali cation transport. Plant Physiol. 1967 Mar;42(3):314–318. doi: 10.1104/pp.42.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. M., Epstein E. The dual mechanisms of alkali cation absorption by plant cells: their parallel operation across the plasmalemma. Proc Natl Acad Sci U S A. 1968 Oct;61(2):447–453. doi: 10.1073/pnas.61.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. M., Epstein E. The plasmalemma: seat of the type 2 mechanisms of ion absorption. Plant Physiol. 1969 Feb;44(2):301–304. doi: 10.1104/pp.44.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]



