Abstract
Purpose
This study illustrates how network meta-analysis techniques (meta-analysis, adjusted indirect comparison, mixed treatment comparison [MTC]) can provide comparisons of the relative efficacy of postmenopausal osteoporosis therapies in the absence of comprehensive head-to-head trials.
Methods
Source articles were identified in MEDLINE; EMBASE; Cochrane Central Register of Controlled Trials (CENTRAL) via Wiley Interscience; and Cumulative Index to Nursing and Allied Health Literature (CINAHL) between April 28, 2009 and November 4, 2009. Two reviewers identified English-language articles reporting randomized controlled trials (RCTs) with on-label dosing of marketed osteoporosis agents and fracture endpoints. Trial design, population characteristics, intervention and comparator, fracture outcomes and adverse events were abstracted for analysis. Primary analyses included data from RCTs with fracture endpoints. Sensitivity analyses also included studies with fractures reported through adverse event reports. Meta-analysis compared fracture outcomes for pharmacologic therapies versus placebo (fixed and random effects models); adjusted indirect comparisons and MTC assessed fracture risk in postmenopausal women treated with denosumab versus other agents.
Results
Using data from 33 studies, random effects meta-analysis showed that all agents except etidronate significantly reduced risk of new vertebral fractures compared with placebo; denosumab, risedronate, and zoledronic acid significantly reduced non-vertebral and hip fracture while alendronate, strontium ranelate, and teriparatide significantly reduced risk for non-vertebral fractures. MTC showed denosumab as more effective than strontium ranelate, raloxifene, alendronate, and risedronate in preventing new vertebral fractures.
Conclusions
The conditional estimates of relative treatment efficacy indicate that there are important differences in fracture risk reduction profiles for marketed pharmacologic therapies for postmenopausal osteoporosis.
Keywords: osteoporosis, treatment efficacy, postmenopausal women, meta-analysis, mixed treatment comparison
Introduction
In randomized controlled trials (RCTs), treatments for osteoporosis in postmenopausal women have demonstrated the level of efficacy required to obtain regulatory approval [1, 2].
In this population, fracture risk reduction was demonstrated primarily in placebo-controlled trials, and head-to-head comparisons are rare. Osteoporosis trials may also use surrogate markers, such as bone mineral density (BMD) or biochemical markers of bone turnover. These markers are not appropriate for comparing treatment-related fracture reductions across agents, since the relationship between changes in surrogate markers and anti-fracture effect is different for each agent and fracture location [3, 4]. Thus, it is not possible to compare the fracture reduction value of a new agent, such as denosumab, to other available therapies directly from the published study results or product labeling.
Network meta-analysis techniques can be used to estimate differences in fracture risk reduction between treatments and classes of treatment, in the absence of head-to-head RCTs. With this approach, treatments are linked in a network of trials through common comparators [5, 6]. In the current study, meta-analysis, adjusted indirect comparison, and mixed treatment comparison (MTC) techniques were used to assess the relative efficacy of denosumab and other osteoporosis therapies to reduce fractures in postmenopausal women.
Material and Methods
The study was conducted in three phases: a systematic review to identify relevant RCTs; a meta-analysis comparing fracture outcomes for pharmacologic osteoporosis therapies versus placebo using fixed and random effects models; and the use of adjusted indirect comparisons and MTC methods to assess the risk of fracture in postmenopausal women treated with denosumab versus patients receiving other osteoporosis therapies.
Data sources and searches
The evidence reviews for osteoporosis agents developed by the National Collaborating Centre for Nursing & Supportive Care (NCCNSC) and published on the National Institute for Health and Clinical Excellence (NICE) website (www.nice.org.uk) in 2008 was the basis for the systematic review. Search criteria and strings from the NCCNSC review, with the addition of denosumab and bazedoxifene which were approved in the interim, were used to update the original literature review with articles published through November 4, 2009.
The agents included in both the NCCNSC review and in our study were alendronate, risedronate, ibandronate, zoledronic acid, etidronate, strontium ranelate, teriparatide, and raloxifene. Calcitonin, hormone replacement therapy, calcium and/or vitamin D, mineral supplements, nandrolone, and combination therapy were included in the NCCNSC review, but excluded from our review. Since bazedoxifene was not marketed in the United Kingdom or a number of other European countries at the time of our analysis, it was excluded from the meta-analyses.
Searches were run between April 28, 2009 and November 4, 2009 on MEDLINE, 1950 to present; EMBASE, 1980 to 2009 Week 16; Cochrane Central Register of Controlled Trials (CENTRAL); and Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Study selection
The primary systematic review identified English-language articles reporting results from RCTs evaluating the impact of the osteoporosis agents listed above on mixed trauma (excluding major trauma) and non-traumatic fracture among individuals at risk of osteoporotic fractures, with/without previous fracture, including patients with osteoporosis, osteopenia or normal BMD, and those with glucocorticoid-induced osteoporosis. Studies with BMD endpoints that reported fractures only as adverse events were also included. The search excluded studies of individuals with elevated fracture risk due to other underlying conditions; dose finding and formulation studies without a placebo or an active control arm; in vitro studies, in vivo studies, and quasi-randomized studies; studies with fewer than 10 patients in each arm; and studies with less than 12 months of follow-up after the intervention. Review articles and editorials were also excluded. This strategy resulted in inclusion of all the pivotal trials for each drug of interest.
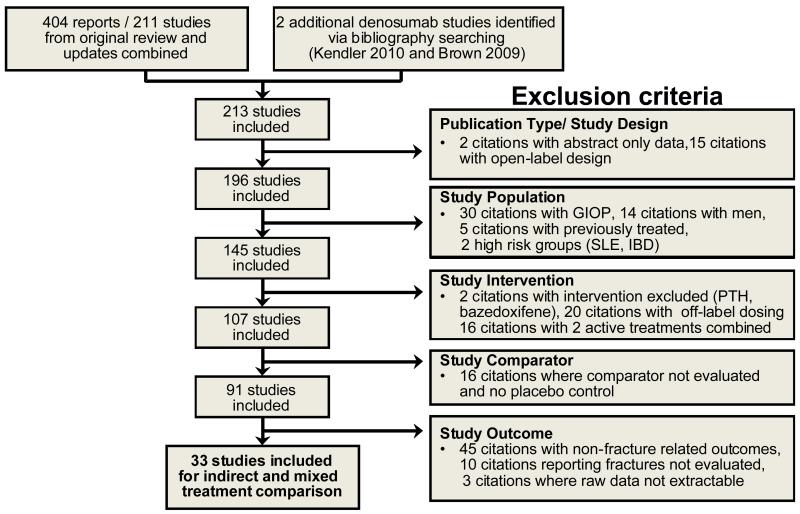
Secondary selection of studies to be included in the meta-analysis, adjusted indirect comparison and MTC was conducted after the primary systematic review. This process identified articles reporting raw fracture data for blinded RCTs of oral and IV bisphosphonates, strontium ranelate, raloxifene, teriparatide, denosumab in postmenopausal women meeting the World Health Organization criteria for osteoporosis as part or all of the trial population [7]. Data were obtained for new radiologically-identified vertebral; clinical vertebral; non-vertebral; hip; and wrist. Only trials examining on-label dosing with a single agent for postmenopausal osteoporosis as defined by the British National Formulary and European Medicines Agency were included with one exception; trials comparing ibandronate 2.5mg/day against placebo were included, which is consistent with the NCCNSC methodology. Open-label trials and abstract only publications were excluded. Additional details of the study selection process and the yield at each step are provided in Figure 1.
Fig. 1.
Study selection process and results. Bazedoxifene was not marketed in the UK and a number of other European countries at the time of analysis; therefore it was excluded from the indirect and mixed treatment comparison. GIOP glucocorticoid-induced osteoporosis, PTH parathyroid hormone.
Data extraction and quality assessment
At each screening stage, citations were assessed independently by two reviewers, with a third independent reviewer resolving any discrepancies. The full-text of all references that met initial inclusion based on title and abstract were screened further. Data on study design, including the intervention and comparator; characteristics of study patients, including mean age, percent with prevalent vertebral fracture, and mean BMD at the femoral neck or hip; intent-to-treat sample sizes; analysis sample sizes; number of fracture events in treatment and control arms; fracture endpoint (primary, or adverse event); vertebral fracture definition used (20% change in vertebral height, 15% change, or undefined); and follow-up time were abstracted from qualifying studies. All RCTs were scored for quality using the Jadad method[8] with scores determined by the presence of three key items considered directly related to bias reduction; randomization, blinding, and description of withdrawals and dropouts.
Data synthesis and statistical analysis
Primary analyses used data from RCTs with explicit fracture endpoints (excluding trials with fractures captured only through adverse event reports). Sensitivity analyses also included studies in which fractures were only reported through adverse event reports. Network diagrams were constructed to display the network of available evidence. Direct comparisons for each active treatment with placebo were obtained by pooling similar studies using meta-analysis with the inverse-variance of each study as the weight. An unconditional maximum likelihood fixed effects model and random effects model using method of moments were performed with the relative risks (RR) based on the raw events for the number of patients with a fracture during the follow-up period [9, 10]. Cochran’s Q test and the I2 statistic were used to assess heterogeneity. A continuity correction for zero fracture events was applied by adding 0.5 to the fracture count in both arms. Trials with zero events in both arms were excluded from the analyses. Analyses were carried out within SAS v9.1 (SAS Institute Inc, Cary, NC).
Adjusted indirect comparisons were performed using the approach of Bucher et al.[5] adapted to use RR as the measure of treatment effect. The indirect estimates of denosumab versus each active comparator were adjusted according to the results of their direct comparisons with a common control (ie, placebo) from the fixed effect and random effects meta-analysis.
The MTC incorporated all available evidence by combining data from direct and indirect comparisons. A Bayesian approach was taken using the methodology outlined by Ades et al., 2006 [11] adapted to use RR as the measure of treatment effect with non-informative uniform priors used. The analyses were performed with WinBUGs 1.4 (MRC Biostatistics Unit Cambridge, Cambridge, UK). Comparisons of active treatment with placebo and denosumab with each active comparator were estimated.
Subgroup analyses were not performed because of limited data obtained in the systematic review. We used meta-regression techniques to investigate the relationship between the trial level covariates and trial level placebo fracture rate, risk difference and RR for all trials in the analysis set since characteristics of the study population, fracture definitions and other study-level variables may introduce bias by acting as effect modifiers for the treatment effect..
Results
The results of the primary systematic review identified 211 studies matching the NCCNSC search criteria. Of these, 34 studies met our criteria and were deemed to be adequately similar in terms of patient population and endpoints for inclusion in the meta-analysis and MTC of the efficacy of osteoporosis therapies in reducing fractures in postmenopausal women. The single bazedoxifene study was excluded prior to analysis since that agent was not marketed in Europe at the time our study was conducted. Key elements of these studies and network diagrams of the available evidence are summarized in Supplemental material (Table S1; Figure S1-S10).
Direct comparisons for each active comparator with placebo from the random effects meta-analysis showed that all agents demonstrated statistically significant reductions in the risk of new vertebral fractures, except etidronate (Table 1). Denosumab, risedronate, and zoledronic acid also showed statistically significant reductions for non-vertebral and hip fractures compared with placebo, while alendronate, strontium ranelate, and teriparatide only showed statistically significant differences versus placebo for non-vertebral fractures.
Table 1.
Random Effects Meta-Analysis and Mixed Treatment Comparison Results for Fracture Endpoints
| Meta-analysis: Active Comparator vs. Placebo |
New Vertebral RR (95% CI) |
Clinical Vertebral RR (95% CI) |
Non-Vertebral RR (95% CI) |
Hip RR (95% CI) | Wrist RR (95% CI) |
|---|---|---|---|---|---|
| Denosumab |
0.33
(0.26 to 0.41) |
0.32
(0.21 to 0.48) |
0.81
(0.69 to 0.96) |
0.61
(0.37 to 0.98) |
0.84 (0.64 to 1.11) |
| Strontium ranelate |
0.72
(0.57 to 0.90) |
0.65
(0.50 to 0.84) |
0.88
(0.78 to 0.99) |
0.89 (0.67 to 1.18) |
0.98 (0.73 to 1.31) |
| Raloxifene |
0.65
(0.54 to 0.78) |
0.45 (0.05 to 3.82) |
0.66 (0.16 to 2.65) |
||
| Teriparatide |
0.35
(0.22 to 0.55) |
0.47
(0.25 to 0.88) |
0.25 (0.03 to 2.24) |
0.29 (0.06 to 1.38) |
|
| Zoledronic acid |
0.30
(0.24 to 0.38) |
0.23
(0.14 to 0.37) |
0.75
(0.65 to 0.87) |
0.59
(0.42 to 0.83) |
|
| Alendronate |
0.56
(0.46 to 0.69) |
0.45
(0.28 to 0.74) |
0.85
(0.75 to 0.97) |
0.65 (0.41 to 1.03) |
0.81 (0.37 to 1.80) |
| Risedronate |
0.62
(0.50 to 0.77) |
0.81
(0.71 to 0.92) |
0.74
(0.59 to 0.94) |
0.68 (0.42 to 1.07) |
|
| Etidronate | 0.46 (0.17 to 1.31) |
3.96 (0.45 to 34.86) |
2.97 (0.12 to 72.11) |
4.95 (0.24 to 101.92) |
|
| Ibandronate oral (2.5 mg) |
0.51
(0.34 to 0.74) |
0.54
(0.32 to 0.89) |
1.11 (0.83 to 1.48) |
||
| Bisphosphonates (IV- includes ibandronate oral)a |
0.38
(0.23 to 0.63) |
0.35
(0.15 to 0.81) |
0.89 (0.61 to 1.31) |
0.59
(0.42 to 0.83) |
|
| Bisphosphonates (oral – includes ibandronate oral) |
0.58
(0.50 to 0.66) |
0.49
(0.35 to 0.70) |
0.85
(0.76 to 0.94) |
0.73
(0.59 to 0.90) |
0.79 (0.51 to 1.22) |
| Bisphosphonates (oral and IV) |
0.52
(0.42 to 0.66) |
0.38
(0.23 to 0.64) |
0.83
(0.75 to 0.91) |
0.69
(0.57 to 0.82) |
0.79 (0.51 to 1.22) |
|
Adjusted Indirect Comparison:
Denosumab vs. Comparator |
New Vertebral
RR (95% CI) |
Clinical Vertebral
RR (95% CI) |
Non-vertebral
RR (95% CI) |
Hip
RR (95% CI) |
Wrist
RR (95% CI) |
| Denosumab vs. Strontium ranelate |
0.45
(0.32 to 0.63) |
0.49
(0.30 to 0.80) |
0.93 (0.76 to 1.14) |
0.68 (0.39 to 1.19) |
0.86 (0.58 to 1.29) |
| Denosumab vs. Raloxifene |
0.50
(0.37 to 0.68) |
0.70 (0.08 to 6.17) |
1.24 (0.30 to 5.03) |
||
| Denosumab vs. Teriparatide | 0.94 (0.55 to 1.58) |
1.73 (0.91 to 3.30) |
2.41 (0.26 to 22.64) |
2.93 (0.60 to 14.39) |
|
| Denosumab vs. Zoledronic acid | 1.08 (0.78 to 1.51) |
1.40 (0.73 to 2.67) |
1.08 (0.87 to 1.35) |
1.03 (0.57 to 1.86) |
|
| Denosumab vs. Alendronate |
0.58
(0.42 to 0.79) |
0.70 (0.37 to 1.32) |
0.95 (0.78 to 1.17) |
0.93 (0.48 to 1.81) |
1.04 (0.45 to 2.40) |
| Denosumab vs. Risedronate |
0.53
(0.38 to 0.73) |
1.01 (0.82 to 1.24) |
0.82 (0.48 to 1.40) |
1.25 (0.73to 2.14) |
|
| Denosumab vs. Etidronate | 0.70 (0.24 to 2.02) |
0.21 (0.02 to 1.82) |
0.20 (0.01 to 5.13) |
0.17 (0.01 to 3.54) |
|
| Denosumab vs. Ibandronate oral (2.5 mg) |
0.64 (0.41 to 1.01) |
0.59 (0.31 to 1.14) |
0.73 (0.53to 1.02) |
||
| Denosumab vs. Bisphosphonates (IV, includes ibandronate oral)a |
0.85 (0.49 to 1.50) |
0.91 (0.35 to 2.34) |
0.91 (0.60 to 1.38) |
1.03 (0.57 to 1.86) |
|
| Denosumab vs. Bisphosphonates (oral, includes ibandronate oral) |
0.57
(0.43 to 0.74) |
0.64 (0.37 to 1.11) |
0.96 (0.79 to 1.17) |
0.83 (0.49 to 1.41) |
1.07 (0.64 to 1.79) |
| Denosumab vs. Bisphosphonates (oral and IV) |
0.62
(0.44 to 0.87) |
0.83 (0.43 to 1.62) |
0.98 (0.81 to 1.19) |
0.88 (0.53 to 1.48) |
1.07 (0.64 to 1.79) |
| Mixed Treatment Comparison: | New Vertebral | Clinical Vertebral | Non-vertebral | Hip | Wrist |
|---|---|---|---|---|---|
| Active Comparator vs. Placebo |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
| Denosumab vs. Placebo |
0.32 (0.22 to 0.46)
[1.00] |
0.31 (0.04 to 2.77) [0.90] |
0.81 (0.60 to 1.11) [0.93] |
0.60 (0.27 to 1.36) [0.92] |
0.84 (0.17, 4.00) [0.64] |
| Strontium ranelate vs. Placebo |
0.72 (0.57 to 0.90)
[0.99] |
0.65 (0.08 to 5.52) [0.75] |
0.88 (0.71 to 1.11) [0.90] |
0.89 (0.43 to 1.83) [0.70] |
0.98 (0.20, 4.97) [0.52] |
| Raloxifene vs. Placebo |
0.63 (0.48 to 0.80)
[1.00] |
0.40 (0.04 to 1.89) [0.87] |
0.87 (0.58, 1.25) [0.77] |
||
| Teriparatide vs. Placebo |
0.34 (0.20 to 0.58)
[1.00] |
0.47 (0.22 to 0.90)
[0.99] |
0.16 (0.01 to 1.55) [0.93] |
0.24 (0.02, 2.01) [0.91] |
|
| Zoledronic acid vs. Placebo |
0.30 (0.21 to 0.43)
[1.00] |
0.22 (0.02 to 1.95) [0.94] |
0.75 (0.55 to 1.01) [0.97] |
0.58 (0.28 to 1.22) [0.95] |
|
| Alendronate vs. Placebo |
0.57 (0.44 to 0.75)
[1.00] |
0.45 (0.05 to 4.07) [0.84] |
0.83 (0.65 to 1.02) [0.97] |
0.63 (0.33 to 1.19) [0.93] |
0.82 (0.25, 2.53) [0.68] |
| Risedronate vs. Placebo |
0.62 (0.46 to 0.83)
[1.00] |
0.80 (0.65 to 0.95)
[0.99] |
0.75 (0.50 to 1.15) [0.93] |
0.67 (0.20, 2.22) [0.80] |
|
| Etidronate vs. Placebo | 0.43 (0.14 to 1.19) [0.95] |
5.31 (0.58 to 172) [0.07] |
146 (0.49 to 17712) [0.06] |
198 (0.83, 23155) [0.03] |
|
| Ibandronate Oral (2.5 mg) vs. Placebo |
0.50 (0.31 to 0.80)
[1.00] |
0.54 (0.06 to 4.85) [0.80] |
1.11 (0.76 to 1.63) [0.28] |
||
| Bisphosphonates (IV- includes ibandronate oral)a |
0.38 (0.12 to 1.25) [0.96] |
0.34 (0.08 to 1.5) [0.95] |
0.90 (0.28 to 3.06) [0.65] |
0.59 (0.30 to 1.14) [0.96] | |
| Bisphosphonates (oral – includes ibandronate oral) |
0.57 (0.49 to 0.68)
[1.00] |
0.49 (0.16 to 1.47) [0.94] |
0.84 (0.73 to 0.96)
[0.99] |
0.73 (0.53 to 1.01) [0.97] |
0.84 (0.43 to 1.85) [0.76] |
| Bisphosphonates (oral and IV) |
0.52 (0.41 to 0.66)
[1.00] |
0.37 (0.16 to 0.89)
[0.98] |
0.82 (0.73 to 0.93)
[1.00] |
0.69 (0.54 to 0.89)
[0.99] |
0.84 (0.43 to1.85) [0.76] |
| Mixed Treatment Comparison: | New Vertebral | Clinical Vertebral | Non-vertebral | Hip | Wrist |
|---|---|---|---|---|---|
| Denosumab vs. Comparator |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
RR (95% CrI)
[P(RR<1)] |
| Denosumab vs. Strontium ranelate |
0.45 (0.29 to 0.68)
[1.00] |
0.48 (0.02 to 9.90) [0.77] |
0.92 (0.61 to 1.36) [0.70] |
0.68 (0.23 to 2.09) [0.80] |
0.85 (0.09 to 8.40) [0.59] |
| Denosumab vs. Raloxifene |
0.51 (0.33 to 0.81)
[1.00] |
0.77 (0.06 to 20.91) [0.65] |
0.93 (0.58 to 1.61) [0.64] |
||
| Denosumab vs. Teriparatide | 0.95 (0.50 to 1.80) [0.57] |
1.74 (0.83 to 3.93) [0.07] |
3.71 (0.33 to 108) [0.17] |
3.41 (0.24 to 59.50) [0.16] |
|
| Denosumab vs. Zoledronic acid | 1.08 (0.65 to 1.77) [0.38] |
1.42 (0.06 to 31.63) [0.35] |
1.08 (0.69 to 1.70) [0.32] |
1.03 (0.34 to 3.24) [0.48] |
|
| Denosumab vs. Alendronate |
0.56 (0.36 to 0.86)
[0.99] |
0.70 (0.03 to 15.23) [0.65] |
0.98 (0.67 to 1.49) [0.58] |
0.96 (0.33 to 2.82) [0.54] |
1.02 (0.14 to 7.74) [0.50] |
| Denosumab vs. Risedronate | 0.52 (0.33 to 0.82) [0.99] |
1.02 (0.71 to 1.51) [0.47] |
0.81 (0.33 to 2.09) [0.70] |
1.25 (0.16 to 9.42) [0.37] |
|
| Denosumab vs. Etidronate | 0.76 (0.25 to 2.37) [0.69] |
0.12 (0.00 to 1.24) [0.96] |
0.005 (0.00 to 1.82) [0.95] |
0.004 (0.00 to 1.20) [0.97] |
|
| Denosumab vs. Ibandronate oral (2.5 mg) |
0.64 (0.36 to 1.16) [0.94] |
0.59 (0.03 to 12.45) [0.71] |
0.72 (0.43 to 1.21) [0.92] |
||
| Denosumab vs. Bisphosphonates (IV - includes ibandronate oral)a |
0.86 (0.11 to 6.35) [0.61] |
0.93 (0.08 to 10.87)y [0.54] |
0.92 (0.12 to 7.16) [0.57] |
1.02 (0.39 to 2.64) [0.48] |
|
| Denosumab vs. Bisphosphonates (oral – includes ibandronate oral) |
0.56 (0.37 to 0.82)
[1.00] |
0.65 (0.11 to 4.15) [0.78] |
0.96 (0.68 to 1.39) [0.62] |
0.82 (0.37 to 1.81) [0.71] |
1.02 (0.19 to 4.89) |
| Denosumab vs. Bisphosphonates (oral and IV) |
0.62 (0.32 to 1.18) [0.93] |
0.84 (0.15 to 4.66) [0.63] |
0.98 (0.71 to 1.36) [0.54] |
0.88 (0.44 to 1.79) [0.65] |
[0.48] 1.02 (0.19 to 4.89) [0.48] |
RR relative risk, CI confidence interval, CrI credible interval, P(RR<1) probability that RR is less than 1
Comparisons with the CI or Crl excluding 1 are highlighted in bold
IV includes ibandronate oral as proxy for ibandronate IV
In the MTC of each active comparator with placebo, the RR for new vertebral fractures were consistent with those obtained directly from the meta-analyses (Table 1). For the other fracture types, the credible intervals of the RR from the MTC were wider than the confidence intervals obtained in the direct comparisons, reflecting extra binomial variability in the data set (systematic differences in the populations included in different trials). The only treatments that showed a reduction in non-vertebral fracture risk were teriparatide and risedronate. The most recently approved agent for postmenopausal osteoporosis, denosumab, was compared with other approved agents, and was more effective than strontium ranelate (P(RR<1 = 100%), raloxifene (P(RR<1 = 100%), alendronate (P(RR<1 = 99%), and risedronate (P(RR<1 = 99%) in preventing new vertebral fractures. In these four comparisons, the point estimates for the RR ranged from 0.56 for alendronate to 0.45 for strontium ranelate. These results were consistent with those obtained in adjusted indirect comparisons (Table 1).
In the MTC, comparing denosumab to three categories of bisphosphonate (intravenous [IV]; oral; oral and IV), the only statistically significant difference was for new vertebral fractures where denosumab was associated with a RR of 0.56 (95% CI: 0.37, 0.82) compared with bisphosphonates (Table 1). In the adjusted indirect comparisons, denosumab was shown to be more effective than oral bisphosphonates and oral/IV bisphosphonates (Table 1).
The results of the sensitivity analyses, which also included studies that reported fractures only through adverse event reports, are provided in Supplemental Material (Table S2). For the direct comparisons, all statistically significant differences remained significant. The hip fracture risk reduction for alendronate compared with placebo was also statistically significant, For the adjusted indirect comparison and MTC, all statistically significant differences observed in the primary analyses were still significant in the sensitivity analyses. The wrist fracture risk reduction for denosumab compared with etidronate was also statistically significant in the MTC. Overall the results of the sensitivity analyses were in line with the primary analyses, although differences were observed in the level of heterogeneity and size of relative treatment effect.
Discussion
In general, clinical practice guidelines use the best available published evidence (RCTs and meta-analysis of high-quality RCTs) on treatment efficacy and safety to inform clinical decision making [12]. This suggests that the most appropriate method to assess the comparative efficacy of pharmacologic therapies would be to run a RCT that includes all agents approved for a particular clinical indication [13]. Unfortunately, this type of trial is rarely undertaken, and would be prohibitively expensive and difficult to execute [14, 15]. The lack of data on direct comparisons of multiple agents in a given therapeutic area complicates policy and clinical decision-making, and highlights the need for other sources of comparative information. Our study was undertaken to illustrate how adjusted indirect comparisons and MTC can be used to help fill this information gap and, in our case, to compare fracture reduction efficacy for the most frequently used pharmacologic osteoporosis agents by combining published RCT results.
In reviewing these results, it is important to note that while helpful, network meta-analysis methods are not a substitute for landmark RCTs asking the relevant question [6]. However, in the absence of such trials they may represent the best available comparison of the relative (conditional) evidence of treatment effects of agents.
Network meta-analysis has been used previously to compare the efficacy of bisphosphonates in the prevention of vertebral fractures [16]. However, our study is the first, to our knowledge, to include all key osteoporotic fracture locations and pharmacologic agents from multiple therapeutic classes, including bisphosphonates, a selective estrogen receptor modulator, a full-length or peptide derivatives of parathyroid hormone, strontium ranelate, and a RANKL inhibitor. Results from our meta-analysis indicate that all therapies assessed demonstrated statistically significant reductions in new vertebral fractures compared with placebo, except etidronate. Denosumab, zoledronic acid, and risedronate also significantly reduced the risk of non-vertebral and hip fractures compared with placebo. Strontium ranelate, teriparatide and alendronate reduced the risk of non-vertebral fractures, but not hip fractures, compared with placebo. Adjusted indirect comparisons and MTC results for placebo comparisons were generally similar for vertebral fractures. For active comparators, denosumab showed a superior reduction of vertebral fracture risk compared with strontium ranelate, raloxifene, risedronate, and alendronate. The heterogeneity of the underlying data in class-level comparisons (ie, denosumab versus oral, IV, and oral/IV bisphosphonates) likely led to wide confidence intervals, and fewer statistically significant results for those comparisons.
An earlier MTC analyzed data from seven RCTs with over 20,000 postmenopausal patients and found that zoledronic acid provided greater risk reduction for vertebral fractures than ibandronate, alendronate or risedronate [16]. Specifically, in that study, a fixed effects model comparing zoledronic acid with the other agents resulted in the following odds ratios for vertebral fractures (95% credible intervals): 0.57 (0.36, 0.92) for ibandronate, 0.54 (0.39, 0.75) for alendronate, and 0.49 (0.34, 0.69) for risedronate. A random effects model reported in the same paper produced similar point estimates, although the credibility intervals all included 1, describing considerable uncertainty as to the true effects of treatment.
As the results from our study show, adjusted indirect comparisons and MTC provide a method of estimating the conditional relative treatment efficacy of one agent versus another, in the absence of head-to-head trials. These analytic approaches are particularly useful in osteoporosis, where the treatment options have increased significantly over the last decade, and will likely continue to expand [17, 18]. While effective treatments offer potential clinical benefit to patients, their availability also means that it is no longer ethical to conduct a placebo-controlled study in a high-risk population. These placebo-arm studies can be conducted in patients with low risk only, ie, patients with a low incidence of fracture, which dramatically increases the sample size required to adequately assess fracture efficacy and the cost of the trial.
Osteoporosis therapies differ in terms of safety profiles, mechanisms of action, dosing, dosing frequency, modes of administration (oral, IV infusion, subcutaneous injections), and efficacy across fracture locations, yet it is rare for multiple active comparators to be included in a single fracture trial [19]. This is due in part to the regulatory process, which favors placebo-controlled trials in appropriate populations in order to obtain an accurate safety profile, and in part to cost and sample size constraints, since a comparative study of fracture incidence for multiple active agents would require a large number of patients. Indeed over 100,000 patients would need to be randomized between two active treatments to have 90% power (1-β) to find a 10% relative risk reduction statistically significant at a conventional two-sided α of 5%, presuming a cumulative fracture rate of 3.5% in the comparator therapy. In addition, given the lengthy follow-up required for a study of this type (10 years or longer), it is possible that a study may become irrelevant before the trial is completed. Not surprisingly, sponsors are generally unwilling and unable to afford this investment of time or money.
The issues of large sample sizes and long follow-up required for fracture endpoints are amplified with multiple treatment arms, making it unlikely that an all-inclusive head-to-head trial will be conducted. For the reasons described above, the therapeutic area of osteoporosis provides a ready example to illustrate the value of MTC and other similar statistical approaches for providing important comparative assessments. These techniques may offer similar value in any number of other therapeutic areas where multiple competing treatment options exist and there is a lack of direct comparisons across the available agents. These comparisons can provide important information to support clinical, reimbursement and policy decisions.
There are important considerations that may impact each phase of a network meta-analysis. Even though it is based on the results of RCTs, the MTC approach may be considered observational or quasi-randomized since it provides conditional estimates of treatment effects across a network of linked trials, with inference made using non-randomized between-trial information through the link of a common comparator [13]. The heterogeneity of the study populations included in published RCTs also presents a methodological challenge for MTCs. Issues such as differences in disease severity between trial populations, for example, lead to over dispersion (extra binomial variability) where clustering of patient characteristics in different trials means that each individual patient may not be considered independent. This phenomenon is addressed using the mixed effects model, which can lead to wide confidence intervals. Thus estimation across the network can result in wider confidence intervals for treatments than those estimated in individual trials.
The results of the meta-regression to assess potential bias and to evaluate the validity of our key assumptions indicated that although study level covariates may have influenced individual trial event rates within a treatment group, they did not influence trial level RR between treatment groups. The RRs appeared to be invariant to mean age, mean BMD, and proportion of patients with a prevalent vertebral fracture at baseline. In addition, less heterogeneity was observed within the RR meta-analyses than within the risk difference meta-analyses, enhancing the applicability of the exchangeability assumption required for indirect comparisons. Sensitivity analyses were also conducted to assess the influence of specific RCTs and to investigate further trials that were excluded from the primary analyses.
Another consideration is assessing the results of a network meta-analysis is the selection of comparator agents. In our study, for example, etidronate was included as its use is reimbursable under the NICE guidelines and it was included in the NCCNSC review, which served as the starting point for data collection for our study. While the efficacy data are not particularly strong for this agent, excluding it from analysis would introduce bias through the exclusion of neutral results. In the absence of trials of IV ibandronate, inference on this treatment was made from trials of oral ibandronate therapy. This provides some challenges as IV administration may independently confer advantages over oral administration, even for the same therapeutic agent. However, such advantages would need to be confirmed and weighed against potential complications (eg, difficulties with port installation or injection site reactions) in appropriately designed outcome trials to justify modifying our assumptions and analytic approach.
The consistency of results obtained from the adjusted indirect comparison and the MTC highlights the robustness of these two methodologies. It is important to note, however, that differences in the design, outcome measurement and populations included in the underlying studies must be carefully considered in interpreting the results of these analyses. Between trial heterogeneity, as assessed by the Cochran’s Q test and I2 statistic from the meta-analysis, was observed for some fracture endpoints and comparators, although it is well recognized that these statistics are limited in their ability to assess heterogeneity due to low statistical power [20]. The meta-analysis and MTC employed an appropriate method (ie, random effects model), which assumes that differences in treatment effect exist between studies, and the individual study results are exchangeable. The exchangeability assumption assumes that the effect of any given treatment included in the model should be exchangeable across the other trials included in the analyses; however, the strength of the exchangeability assumption is difficult to assess and quantify [14]. Limited amounts of heterogeneity among trials and estimates were observed, indicating no major departure from the exchangeability assumption.
The study is limited as a complete data set of all fracture endpoints for all comparators was not available. In particular, no on-label ibandronate trials were identified and it was necessary to include an off-label dose (ibandronate 2.5mg/day) in the primary analysis for completeness. In addition, trials that had zero events in both arms were excluded from the analyses, albeit such trials include little statistical information. Only a small number of trials were excluded for this reason, and the exclusion of these studies does not detract from the overall estimates from these analyses.
The results of our study reflect real-life effectiveness provided that the risk of fracture, adherence and persistence to therapy were similar to the original RCTs. The trial setting differs substantially from usual care, and it is unlikely that the population of patients using osteoporosis therapies outside an RCT will achieve the efficacy levels that were reached under the optimal and artificial conditions maintained within the RCT. Real-world effectiveness can be improved by considering efficacy and side-effect profiles, in conjunction with patient preference, comorbidities, potential barriers to adherence, and cost of therapy in the selection of the most appropriate therapy for each patient [5, 21].
Conclusions
Our study indicates differences in fracture risk reduction profiles for the available pharmacologic therapies for postmenopausal osteoporosis, and provides results that may assist therapy choice.
Network meta-analysis techniques (meta-analysis, adjusted indirect comparison, mixed treatment comparison) allow for treatment comparisons in the absence of head-to-head trials. In this study, conditional estimates of relative treatment efficacy derived through these techniques show important differences in the fracture risk reduction profiles of marketed pharmacologic therapies for postmenopausal osteoporosis.
Acknowledgements
The authors would like to acknowledge James Matcham for technical statistical support, and Sally Wade, and Mandy Suggitt on behalf of Amgen Inc., for writing and editorial support.
Footnotes
Conflicts of interest
This study was funded by Amgen Inc.
NF has received research grants from Amgen, Inc. and has served as a consultant for Amgen, Inc., Sanofi-Aventis, Pfizer, Wyeth, and Eli Lilly. CC has received consulting and lecture fees from Amgen, Inc., GSK, Eli Lilly, Novartis, Servier, and Alliance for Bone Health. AD-P has received honoraria or consulted for Amgen, Inc., Novartis, Eli Lilly and MSD; and received research grants from the Alliance of Bone Health, and Amgen, Inc. CR has received research grants, and/or honoraria from Amgen, MSD, Servier, Novartis, and Lilly. tter Bone Health, Shire, Wyeth, and Pfizer.
MG, HR and SS are employees of and have stock ownership in Amgen, Inc.
Contributor Information
N. Freemantle, University College London, London, UK
C. Cooper, University of Southampton, Southampton, UK
A. Diez-Perez, Autonomous University of Spain, Barcelona, Spain
M. Gitlin M, Amgen Inc., Thousand Oaks, CA, USA
H. Radcliffe, Amgen Ltd., Cambridge, UK
S. Shepherd, Amgen Ltd., Uxbridge, UK
C. Roux, Paris Descartes University, Paris, France
References
- 1.Moen MD, Keam SJ. Denosumab: a review of its use in the treatment of postmenopausal osteoporosis. Drugs Aging. 2011;28:63–82. doi: 10.2165/11203300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnick SL, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med. 2006;119:S25–31. doi: 10.1016/j.amjmed.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 5.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead A. Meta-analysis of controlled clinical trials. John Wiley & Sons; Chichester: 2002. [Google Scholar]
- 11.Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, Lu G. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1–19. doi: 10.2165/00019053-200624010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Binkley N. Evidence-based medicine, clinical practice guidelines, and common sense in the management of osteoporosis. Endocr Pract. 2009;15:573–579. doi: 10.4158/EP09107.RA. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, C B, Cappelleri JC. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision-making: report of the ISPOR task force on indirect treatment comparison good research practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26:753–767. doi: 10.2165/00019053-200826090-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect evidence: indirect treament comparisons in meta-analysis. Canadian Agency for Drugs and Technologies in Health; Ottawa: 2009. [Google Scholar]
- 16.Jansen JP, Bergman GJ, Huels J, Olson M. Prevention of vertebral fractures in osteoporosis: mixed treatment comparison of bisphosphonate therapies. Curr Med Res Opin. 2009;25:1861–1868. doi: 10.1185/03007990903035281. [DOI] [PubMed] [Google Scholar]
- 17.Brewer L, Williams D, Moore A. Current and future treatment options in osteoporosis. Eur J Clin Pharmacol. 2011;67:321–331. doi: 10.1007/s00228-011-0999-2. [DOI] [PubMed] [Google Scholar]
- 18.Lewiecki EM. Current and emerging pharmacologic therapies for the management of postmenopausal osteoporosis. J Womens Health (Larchmt) 2009;18:1615–1626. doi: 10.1089/jwh.2008.1086. [DOI] [PubMed] [Google Scholar]
- 19.Brown JP, Prince RL, Deal C, et al. Comparison of the Effect of Denosumab and Alendronate on Bone Mineral Density and Biochemical Markers of Bone Turnover in Postmenopausal Women With Low Bone Mass: A Randomized, Blinded, Phase 3 Trial. J Bone Miner Res. 2009;24:1–34. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]